Antifungal Activity of the Phenolic Compounds Ellagic Acid (EA) and Caffeic Acid Phenethyl Ester (CAPE) against Drug-Resistant Candida auris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Strains and Culture Conditions
2.3. Minimum Inhibitory Concentration
2.4. Time-Kill Curves
2.5. Checkerboard Microdilution Assay
2.6. Mechanism of Action
2.6.1. Sorbitol Protection Assay
2.6.2. Ergosterol Binding Assay
2.7. Virulence Factors
2.7.1. Proteinase
2.7.2. Phospholipase
2.7.3. Induction of Fungal Filamentation
2.7.4. Biofilm Inhibition and Disruption
Quantification of Biofilm Biomass
Quantification of Metabolic Activity
Live/Dead Staining Assay
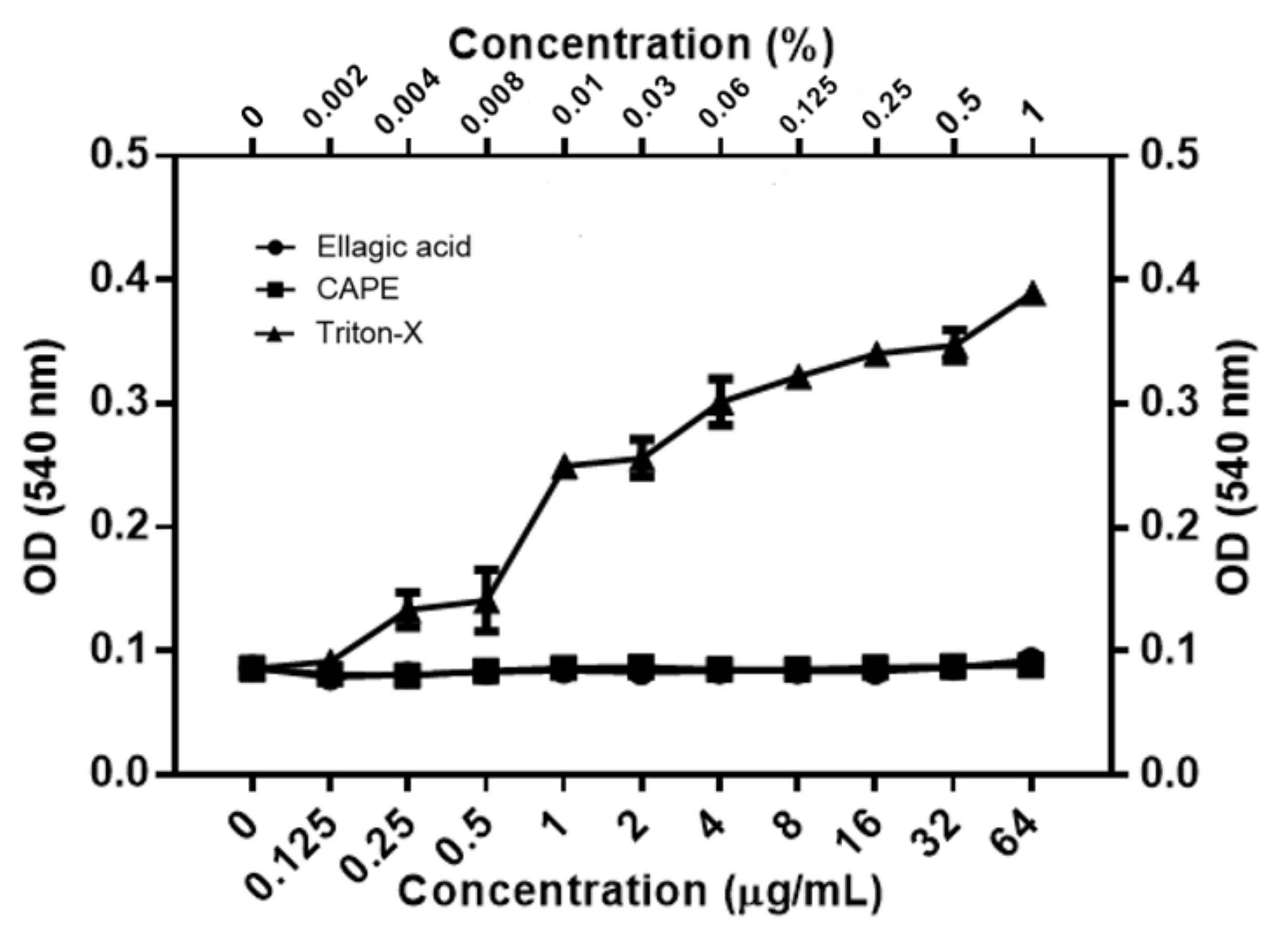
2.8. Hemolysis Assay
2.9. Adherence Assay with A549 Cells
2.10. Galleria mellonella Assays
2.10.1. Toxicity
2.10.2. Infection Rescue Assay
2.11. C. elegans-C. albicans Infection Assay
2.12. Statistical Analysis
3. Results
3.1. CAPE and EA Show Antifungal Activity against C. auris
3.2. CAPE Is Fungicidal and EA Is Fungistatic toward C. auris
3.3. EA and CAPE May Affect Fungal Cell Wall
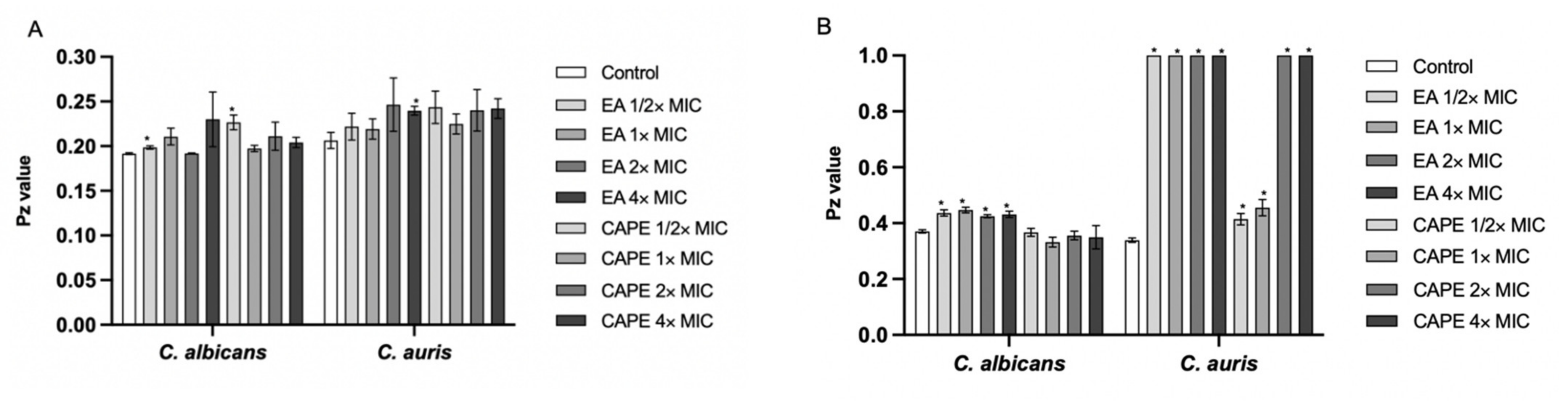
3.4. Effect of EA and CAPE on Proteinase and Phospholipase Production
3.5. CAPE Inhibits Hyphal Development in C. albicans
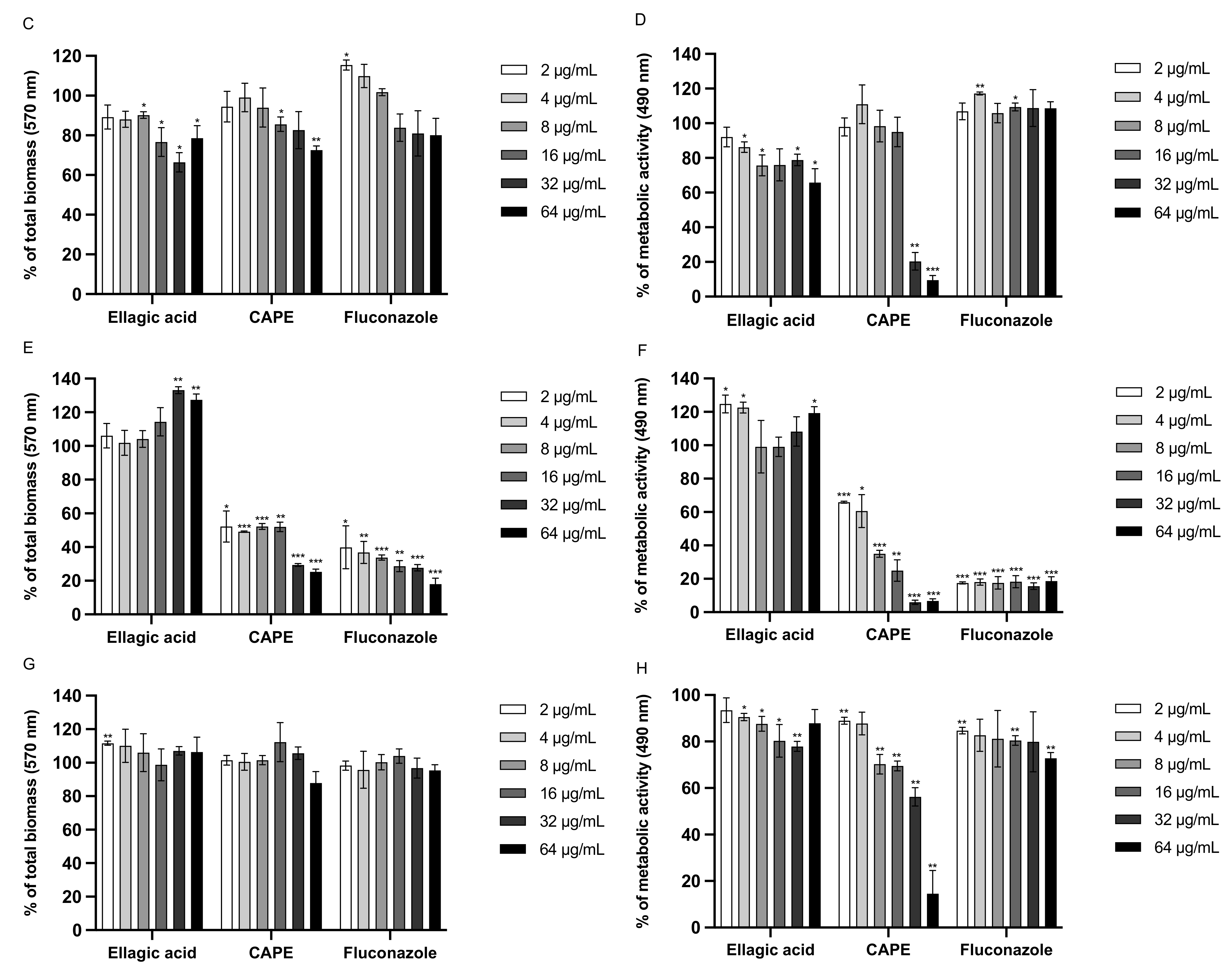
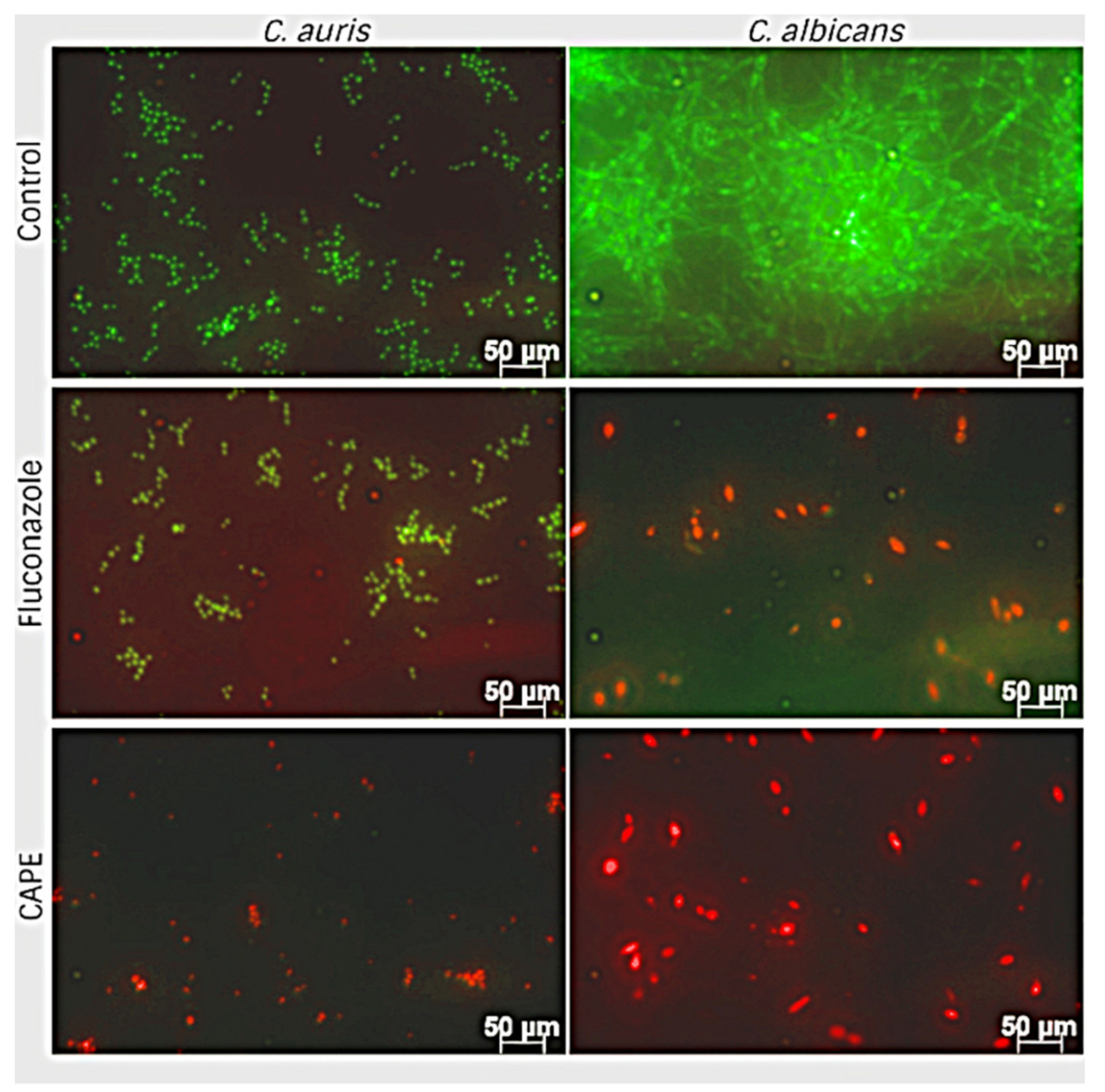
3.6. Effect of EA and CAPE on C. auris and C. albicans Biofilms
3.7. Toxicity of EA and CAPE on Human Red Blood Cells (hRBC)
3.8. CAPE Inhibits Adhesion of C. auris and C. albicans on A549 Cells
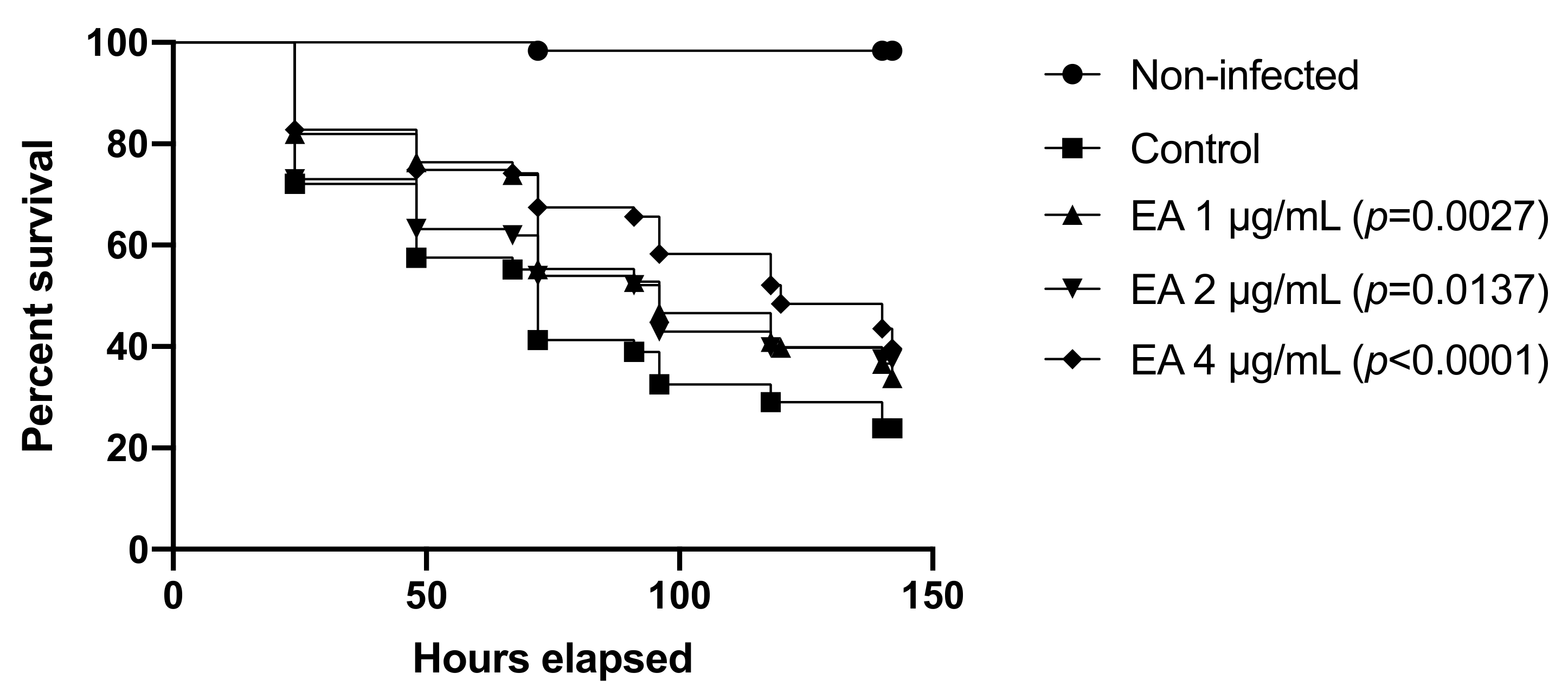
3.9. EA and CAPE Protect Galleria mellonella Larvae from Candida spp. Infection
3.10. EA Protects C. elegans from C. albicans Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dixon, D.M.; McNeil, M.M.; Cohen, M.L.; Gellin, B.G.; la Montagne, J.R. Fungal infections: A growing threat. Public Health Rep. 1996, 111, 226–235. [Google Scholar]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Rueping, M.J.; Vehreschild, J.-J.; Cornely, O.A. Invasive candidiasis and candidemia: From current opinions to future perspectives. Expert Opin. Investig. Drugs 2009, 18, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [Green Version]
- Paramythiotou, E.; Frantzeskaki, F.; Flevari, A.; Armaganidis, A.; Dimopoulos, G. Invasive fungal infections in the ICU: How to approach, how to treat. Molecules 2014, 19, 1085–1119. [Google Scholar] [CrossRef] [Green Version]
- Dubey, A.K.; Singla, R.K. Perspectives on anti-Candida drug development. Curr. Top. Med. Chem. 2019, 19, 2375–2376. [Google Scholar] [CrossRef]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Kim, M.; Shin, J.H.; Sung, H.; Lee, K.; Kim, E.; Ryoo, N.; Lee, J.; Jung, S.; Park, K.H.; Kee, S.J.; et al. Candida haemulonii and closely related species at 5 university hospitals in Korea: Identification, antifungal susceptibility, and clinical features. Clin. Infect. Dis. 2009, 48, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Van Schalkwyk, E.; Mpembe, R.S.; Thomas, J.; Shuping, L.; Ismail, H.; Lowman, W.; Karstaedt, A.S.; Chibabhai, V.; Wadula, J.; Avenant, T.; et al. Epidemiologic shift in candidemia driven by Candida auris, South Africa, 2016–2017. Emerg. Infect. Dis. 2019, 25, 1698–1707. [Google Scholar] [CrossRef] [Green Version]
- Vallabhaneni, S.; Kallen, A.; Tsay, S.; Chow, N.; Welsh, R.; Kerins, J.; Kemble, S.K.; Pacilli, M.; Black, S.R.; Landon, E.; et al. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus-United States, May 2013-August 2016. Am. J. Transpl. 2017, 17, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative pathogenicity of United Kingdom isolates of the emerging pathogen Candida auris and other key pathogenic Candida species. mSphere 2016, 1, e00189-16. [Google Scholar] [CrossRef] [Green Version]
- Cortegiani, A.; Misseri, G.; Fasciana, T.; Giammanco, A.; Giarratano, A.; Chowdhary, A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Spivak, E.S.; Hanson, K.E. Candida auris: An emerging fungal pathogen. J. Clin. Microbiol. 2017, 56, e01588-17. [Google Scholar] [CrossRef] [Green Version]
- Kean, R.; Brown, J.; Gulmez, D.; Ware, A.; Ramage, G. Candida auris: A decade of understanding of an enigmatic pathogenic yeast. J. Fungi 2020, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.-C. First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossato, L.; Colombo, A.L. Candida auris: What have we learned about its mechanisms of pathogenicity? Front. Microbiol. 2018, 9, 3081. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Cavalheiro, M.; Teixeira, M.C. Candida biofilms: Threats, challenges, and promising strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Priya, A.; Pandian, S.K. Piperine impedes biofilm formation and hyphal morphogenesis of Candida albicans. Front. Microbiol. 2020, 11, 756. [Google Scholar] [CrossRef]
- Breger, J.; Fuchs, B.B.; Aperis, G.; Moy, T.I.; Ausubel, F.M.; Mylonakis, E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007, 3, e18. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Ramage, G.; Saville, S.P.; Thomas, D.P.; López-Ribot, J.L. Candida biofilms: An update. Eukaryot. Cell 2005, 4, 633–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, V.; Singla, R.K.; Dubey, A.K. Emerging virulence, drug resistance and future antifungal drugs for Candida pathogens. Curr. Top. Med. Chem. 2018, 18, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Gauwerky, K.; Borelli, C.; Korting, H.C. Targeting virulence: A new paradigm for antifungals. Drug Discov. Today 2009, 14, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Molepo, J.; Patel, M. Challenges in the development of antifungal agents against Candida: Scope of phytochemical research. Curr. Pharm. Des. 2016, 22, 4135–4150. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.J.; Komura, T.; Munro, J.; Wu, M.P.; Busanelli, R.R.; Koehler, A.N.; Thomas, M.; Wagner, F.F.; Holson, E.B.; Mylonakis, E. Activity of caffeic acid phenethyl ester in Caenorhabditis elegans. Future Med. Chem. 2016, 8, 2033–2046. [Google Scholar] [CrossRef]
- Pukkila-Worley, R.; Holson, E.; Wagner, F.; Mylonakis, E. Antifungal drug discovery through the study of invertebrate model hosts. Curr. Med. Chem. 2009, 16, 1588–1595. [Google Scholar] [CrossRef] [Green Version]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.v.; Espín, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef]
- Da Graça Sampaio, A.; Gontijo, A.V.L.; Araujo, H.M.; Koga-Ito, C.Y. In vivo efficacy of ellagic acid against Candida albicans in a Drosophila melanogaster infection model. Antimicrob. Agents Chemother. 2018, 62, e01716-18. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Zhai, L.; Arendrup, M.C. In vitro activity of 23 tea extractions and epigallocatechin gallate against Candida species. Med. Mycol. 2015, 53, 194–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houillé, B.; Papon, N.; Boudesocque, L.; Bourdeaud, E.; Besseau, S.; Courdavault, V.; Enguehard-Gueiffier, C.; Delanoue, G.; Guérin, L.; Bouchara, J.-P.; et al. Antifungal activity of resveratrol derivatives against Candida species. J. Nat. Prod. 2014, 77, 1658–1662. [Google Scholar] [CrossRef]
- Ning, Y.; Ling, J.; Wu, C.D. Synergistic effects of tea catechin epigallocatechin gallate and antimycotics against oral Candida species. Arch. Oral Biol. 2015, 60, 1565–1570. [Google Scholar] [CrossRef]
- De Vita, D.; Friggeri, L.; D’Auria, F.D.; Pandolfi, F.; Piccoli, F.; Panella, S.; Palamara, A.T.; Simonetti, G.; Scipione, L.; di Santo, R.; et al. Activity of caffeic acid derivatives against Candida albicans biofilm. Bioorg. Med. Chem. Lett. 2014, 24, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, R.K.; Lee, J.-H.; Kim, Y.-G.; Lee, J. Alizarin and chrysazin inhibit biofilm and hyphal formation by Candida albicans. Front. Cell. Infect. Microbiol. 2017, 7, 447. [Google Scholar] [CrossRef]
- Zhong, H.; Hu, D.-D.; Hu, G.-H.; Su, J.; Bi, S.; Zhang, Z.-E.; Wang, Z.; Zhang, R.-L.; Xu, Z.; Jiang, Y.-Y.; et al. Activity of sanguinarine against Candida albicans biofilms. Antimicrob. Agents Chemother. 2017, 61, e02259-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Liao, K.; Wang, D. Effects of magnolol and honokiol on adhesion, yeast-hyphal transition, and formation of biofilm by Candida albicans. PLoS ONE 2015, 10, e0117695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Shan, M.; Li, S.; Wang, Y.; Yang, H.; Chen, Y.; Gu, B.; Zhu, Z. Teasaponin suppresses Candida albicans filamentation by reducing the level of intracellular cAMP. Ann. Transl. Med. 2020, 8, 175. [Google Scholar] [CrossRef]
- Muthamil, S.; Prasath, K.G.; Priya, A.; Precilla, P.; Pandian, S.K. Global proteomic analysis deciphers the mechanism of action of plant derived oleic acid against Candida albicans virulence and biofilm formation. Sci. Rep. 2020, 10, 5113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evensen, N.A.; Braun, P.C. The effects of tea polyphenols on Candida albicans: Inhibition of biofilm formation and proteasome inactivation. Can. J. Microbiol. 2009, 55, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Seleem, D.; Pardi, V.; Murata, R.M. Review of flavonoids: A diverse group of natural compounds with anti-Candida albicans activity in vitro. Arch. Oral Biol. 2017, 76, 76–83. [Google Scholar] [CrossRef]
- Clinical & Laboratory Standards Institute. M27-A4: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; CLSI: Wayne, PA, USA, 2017; ISBN 1562388274. [Google Scholar]
- Rossoni, R.D.; de Barros, P.P.; do Carmo Mendonça, I.; Medina, R.P.; Silva, D.H.S.; Fuchs, B.B.; Junqueira, J.C.; Mylonakis, E. The postbiotic activity of Lactobacillus paracasei 28.4 against Candida auris. Front. Cell. Infect. Microbiol. 2020, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Scorneaux, B.; Angulo, D.; Borroto-Esoda, K.; Ghannoum, M.; Peel, M.; Wring, S. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob. Agents Chemother. 2017, 61, e01961. [Google Scholar] [CrossRef] [Green Version]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Pippi, B.; Lopes, W.; Reginatto, P.; Silva, F.É.K.; Joaquim, A.R.; Alves, R.J.; Silveira, G.P.; Vainstein, M.H.; Andrade, S.F.; Fuentefria, A.M. New insights into the mechanism of antifungal action of 8-hydroxyquinolines. Saudi Pharm. J. 2019, 27, 41–48. [Google Scholar] [CrossRef]
- Yousuf, S.; Ahmad, A.; Khan, A.; Manzoor, N.; Khan, L.A. Effect of garlic-derived allyl sulphides on morphogenesis and hydrolytic enzyme secretion in Candida albicans. Med. Mycol. 2011, 49, 444–448. [Google Scholar] [CrossRef] [Green Version]
- De Barros, P.P.; Scorzoni, L.; de Camargo Ribeiro, F.; de Oliveira Fugisaki, L.R.; Fuchs, B.B.; Mylonakis, E.; Jorge, A.O.C.; Junqueira, J.C.; Rossoni, R.D. Lactobacillus paracasei 28.4 reduces in vitro hyphae formation of Candida albicans and prevents the filamentation in an experimental model of Caenorhabditis elegans. Microb. Pathog. 2018, 117, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, H.; Eldesouky, H.E.; Hazbun, T.; Mayhoub, A.S.; Seleem, M.N. Identification of a phenylthiazole small molecule with dual antifungal and antibiofilm activity against Candida albicans and Candida auris. Sci. Rep. 2019, 9, 18941. [Google Scholar] [CrossRef] [Green Version]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- Khan, S.N.; Khan, S.; Iqbal, J.; Khan, R.; Khan, A.U. Enhanced killing and antibiofilm activity of encapsulated cinnamaldehyde against Candida albicans. Front. Microbiol. 2017, 8, 1641. [Google Scholar] [CrossRef]
- Tharmalingam, N.; Khader, R.; Fuchs, B.B.; Mylonakis, E. The anti-virulence efficacy of 4-(1,3-dimethyl-2,3-dihydro-1h-benzimidazol-2-yl)phenol against methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2019, 10, 1557. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-D.; Deng, L.; Hu, G.-H.; Zhao, L.-X.; Hu, D.-D.; Jiang, Y.-Y.; Wang, Y. Using Galleria mellonella–Candida albicans infection model to evaluate antifungal agents. Biol. Pharm. Bull. 2013, 36, 1482–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossoni, R.D.; Fuchs, B.B.; de Barros, P.P.; dos Santos Velloso, M.; Jorge, A.O.C.; Junqueira, J.C.; Mylonakis, E. Lactobacillus paracasei modulates the immune system of Galleria mellonella and protects against Candida albicans infection. PLoS ONE 2017, 12, e0173332. [Google Scholar] [CrossRef]
- Junior, I.F.S.; Raimondi, M.; Zacchino, S.; Filho, V.C.; Noldin, V.F.; Rao, V.S.; Lima, J.C.S.; Martins, D.T.O. Evaluation of the antifungal activity and mode of action of Lafoensia pacari A. St.-Hil., Lythraceae, stem-bark extracts, fractions and ellagic acid. Rev. Bras. Farmacogn. 2010, 20. [Google Scholar] [CrossRef]
- Pagano, M.; Faggio, C. The use of erythrocyte fragility to assess xenobiotic cytotoxicity. Cell. Biochem. Funct. 2015, 33, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Alagawany, M. Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem. Biol. Interact. 2018, 279, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Kutsumi, Y.; Sadanaga, J.; Ishikawa, M.; Sugita, D.; Ikeda, R. Adherence and cytotoxicity of Candida spp. to HaCaT and A549 cells. Med. Mycol. J. 2019, 60, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Arendrup, M.C.; Patterson, T.F. Multidrug-resistant Candida: Epidemiology, molecular mechanisms, and treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef] [Green Version]
- Brighenti, F.L.; Salvador, M.J.; Gontijo, A.V.L.; Delbem, A.C.B.; Delbem, Á.C.B.; Soares, C.P.; de Oliveira, M.A.C.; Girondi, C.M.; Koga-Ito, C.Y. Plant extracts: Initial screening, identification of bioactive compounds and effect against Candida albicans biofilms. Future Microbiol. 2017, 12, 15–27. [Google Scholar] [CrossRef]
- Sun, L.; Liao, K.; Hang, C. Caffeic acid phenethyl ester synergistically enhances the antifungal activity of fluconazole against resistant Candida albicans. Phytomedicine 2018, 40, 55–58. [Google Scholar] [CrossRef]
- Freires, I.A.; Queiroz, V.C.P.P.; Furletti, V.F.; Ikegaki, M.; de Alencar, S.M.; Duarte, M.C.T.; Rosalen, P.L. Chemical composition and antifungal potential of Brazilian propolis against Candida spp. J. Mycol. Med. 2016, 26, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Wang, D.-M.; Pu, W.-J.; Li, D.-W. Phytochemical profiles, antioxidant and antimicrobial activities of three Potentilla species. BMC Complement Altern. Med. 2013, 13, 321. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.R.; de Lima Silva, J.; Lima, K.R.R.; Rocha, M.I.; Barros, L.M.; da Costa, J.G.M.; Boligon, A.A.; Kamdem, J.P.; Carneiro, J.N.P.; Leite, N.F.; et al. Rhaphiodon echinus (Nees & Mart.) Schauer: Chemical, toxicological activity and increased antibiotic activity of antifungal drug activity and antibacterial. Microb. Pathog. 2017, 107, 280–286. [Google Scholar] [CrossRef]
- Fecker, R.; Buda, V.; Alexa, E.; Avram, S.; Pavel, I.Z.; Muntean, D.; Cocan, I.; Watz, C.; Minda, D.; Dehelean, C.A.; et al. Phytochemical and biological screening of Oenothera biennis L. hydroalcoholic extract. Biomolecules 2020, 10, 818. [Google Scholar] [CrossRef]
- Fogliani, B.; Raharivelomanana, P.; Bianchini, J.-P.; Bouraı¨ma-Madjèbi, S.; Hnawia, E. Bioactive ellagitannins from Cunonia macrophylla, an endemic Cunoniaceae from New Caledonia. Phytochemistry 2005, 66, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-C.; Joshi, A.S.; ElSohly, H.N.; Khan, S.I.; Jacob, M.R.; Zhang, Z.; Khan, I.A.; Ferreira, D.; Walker, L.A.; Broedel, S.E.; et al. Fatty acid synthase inhibitors from plants: Isolation, structure elucidation, and SAR studies. J. Nat. Prod. 2002, 65, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.N.M.; Le, T.T.; van Camp, J.; Raes, K. Evaluation of antimicrobial activity of rambutan (Nephelium lappaceum L.) peel extracts. Int. J. Food Microbiol. 2020, 321, 108539. [Google Scholar] [CrossRef] [PubMed]
- Rangkadilok, N.; Tongchusak, S.; Boonhok, R.; Chaiyaroj, S.C.; Junyaprasert, V.B.; Buajeeb, W.; Akanimanee, J.; Raksasuk, T.; Suddhasthira, T.; Satayavivad, J. In vitro antifungal activities of longan (Dimocarpus longan lour.) seed extract. Fitoterapia 2012, 83, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Gatto, L.J.; Veiga, A.; Gribner, C.; Moura, P.F.; Rech, K.S.; Murakami, F.S.; de Fatima Gaspari Dias, J.; Miguel, O.G.; Miguel, M.D. Myrcia Hatschbachii: Antifungal activity and structural elucidation of ellagic and 3-O-methyl ellagic acids. Nat. Prod. Res. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, A.R.; Iles, B.; de Melo Nogueira, K.; do Nascimento Dias, J.; Plácido, A.; Rodrigues, A.; Albuquerque, P.; Silva-Pereira, I.; Socodatto, R.; Portugal, C.C.; et al. Antifungal and anti-inflammatory potential of eschweilenol c-rich fraction derived from Terminalia fagifolia Mart. J. Ethnopharmacol. 2019, 240, 111941. [Google Scholar] [CrossRef]
- Gontijo, A.V.; da Sampaio, A.G.; Koga-Ito, C.Y.; Salvador, M.J. Biopharmaceutical and antifungal properties of ellagic acid-cyclodextrin using an in vitro model of invasive candidiasis. Future Microbiol. 2019, 14, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Da Graça Sampaio, A.; Gontijo, A.V.L.; de Morais Gouvêa Lima, G.; de Oliveira, M.A.C.; Lepesqueur, L.S.S.; Koga-Ito, C.Y. Ellagic acid–cyclodextrin complexes for the treatment of oral candidiasis. Molecules 2021, 26, 505. [Google Scholar] [CrossRef]
- Saelo, S.; Assatarakul, K.; Sane, A.; Suppakul, P. Fabrication of novel bioactive cellulose-based films derived from caffeic acid phenethyl ester-loaded nanoparticles via a rapid expansion process: RESOLV. J. Agric. Food Chem. 2016, 64, 6694–6707. [Google Scholar] [CrossRef]
- Savic, I.M.; Jocic, E.; Nikolic, V.D.; Popsavin, M.M.; Rakic, S.J.; Savic-Gajic, I.M. The effect of complexation with cyclodextrins on the antioxidant and antimicrobial activity of ellagic acid. Pharm. Dev. Technol. 2019, 24, 410–418. [Google Scholar] [CrossRef]
- De Vita, D.; Simonetti, G.; Pandolfi, F.; Costi, R.; di Santo, R.; D’Auria, F.D.; Scipione, L. Exploring the anti-biofilm activity of cinnamic acid derivatives in Candida albicans. Bioorg. Med. Chem. Lett. 2016, 26, 5931–5935. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Hang, C.; Liao, K. Synergistic effect of caffeic acid phenethyl ester with caspofungin against Candida albicans is mediated by disrupting iron homeostasis. Food Chem. Toxicol. 2018, 116, 51–58. [Google Scholar] [CrossRef]
- Ma, C.-M.; Abe, T.; Komiyama, T.; Wang, W.; Hattori, M.; Daneshtalab, M. Synthesis, Anti-Fungal and 1,3-β-D-glucan synthase inhibitory activities of caffeic and quinic acid derivatives. Bioorg. Med. Chem. 2010, 18, 7009–7014. [Google Scholar] [CrossRef]
- Yue, H.; Bing, J.; Zheng, Q.; Zhang, Y.; Hu, T.; Du, H.; Wang, H.; Huang, G. Filamentation in Candida auris, an emerging fungal pathogen of humans: Passage through the mammalian body induces a heritable phenotypic switch. Emerg. Microbes Infect. 2018, 7, 188. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Yue, H.; Zheng, Q.; Bing, J.; Tian, S.; Chen, J.; Ennis, C.L.; Nobile, C.J.; Huang, G.; Du, H. Filamentous growth is a general feature of Candida auris clinical isolates. Med. Mycol. 2021, 59, 734–740. [Google Scholar] [CrossRef]
- Garcia-Bustos, V.; Ruiz-Saurí, A.; Ruiz-Gaitán, A.; Sigona-Giangreco, I.A.; Cabañero-Navalon, M.D.; Sabalza-Baztán, O.; Salavert-Lletí, M.; Tormo, M.Á.; Pemán, J. Characterization of the differential pathogenicity of Candida auris in a Galleria mellonella infection model. Microbiol. Spectr. 2021, 9, e0001321. [Google Scholar] [CrossRef]
- De Barros, P.P.; Rossoni, R.D.; Garcia, M.T.; de Lima Kamisnski, V.; Loures, F.V.; Fuchs, B.B.; Mylonakis, E.; Junqueira, J.C. The anti-biofilm efficacy of caffeic acid phenethyl ester (CAPE) in vitro and a murine model of oral candidiasis. Front. Cell. Infect. Microbiol. 2021, 11, 700305. [Google Scholar] [CrossRef]
- Meng, L.; Sun, C.; Zhang, C.; Song, S.; Sun, X.; Ju, J.; Deng, Y. Efficacy of compounds isolated from Streptomyces olivaceus against the morphogenesis and virulence of Candida albicans. Mar. Drugs 2019, 17, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial effect of caffeic acid phenethyl ester on cariogenic bacteria and Streptococcus mutans biofilms. Antimicrob. Agents. Chemother. 2020, 64, e00251-20. [Google Scholar] [CrossRef]
- Fedeli, D.; Berrettini, M.; Gabryelak, T.; Falcioni, G. The Effect of some tannins on trout erythrocytes exposed to oxidative stress. Mutat. Res. 2004, 563, 89–96. [Google Scholar] [CrossRef]
- Yilmaz, H.; Uz, E.; Gökalp, O.; Özçelik, N.; Çiçek, E.; Özer, M. Protective role of caffeic acid phenethyl ester and erdosteine on activities of purine-catabolizing enzymes and level of nitric oxide in red blood cells of isoniazid-administered rats. Toxicol. Ind. Health 2008, 24, 519–524. [Google Scholar] [CrossRef]
- Colina, J.R.; Suwalsky, M.; Manrique-Moreno, M.; Petit, K.; Aguilar, L.F.; Jemiola-Rzeminska, M.; Strzalka, K. An in vitro study of the protective effect of caffeic acid on human erythrocytes. Arch. Biochem. Biophys. 2019, 662, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Coote, P.J. Utility of greater wax moth larva (Galleria mellonella) for evaluating the toxicity and efficacy of new antimicrobial agents. Adv. Appl. Microbiol. 2012, 78, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.B.; Eby, J.; Nobile, C.J.; el Khoury, J.B.; Mitchell, A.P.; Mylonakis, E. Role of filamentation in Galleria mellonella killing by Candida albicans. Microbes. Infect. 2010, 12, 488–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Strains | MIC Values in μg/mL | ||||||
|---|---|---|---|---|---|---|---|
| Phenolic Compounds | |||||||
| CA | CAPE | CF | EA | EGCG | ENG | PD | |
| C. auris CAU-01 | 4 | 1 | 128 | 0.125 | 1 | >128 | >128 |
| C. auris CAU-02 | 64 | 8 | >128 | 0.25 | 64 | >128 | >128 |
| C. auris CAU-03 | 8 | 8 | >128 | 0.25 | 16 | >128 | >128 |
| C. auris CAU-04 | 64 | 16 | >128 | 0.25 | 4 | >128 | >128 |
| C. auris CAU-05 | 128 | 16 | >128 | 0.25 | 32 | >128 | >128 |
| C. auris CAU-06 | 128 | 32 | >128 | 0.25 | 64 | >128 | >128 |
| C. auris CAU-07 | 64 | 16 | >128 | 0.25 | 32 | >128 | >128 |
| C. auris CAU-08 | 128 | 16 | >128 | 0.25 | 64 | >128 | >128 |
| C. auris CAU-09 | 128 | 64 | >128 | 0.25 | 64 | >128 | >128 |
| C. auris CAU-10 | 128 | 64 | >128 | 0.25 | 64 | >128 | >128 |
| C. albicans CAL | >128 | 8 | >128 | 0.5 | 16 | >128 | >128 |
| C. glabrata CG | 8 | 4 | 16 | 0.125 | 0.5 | >128 | >128 |
| C. krusei CK | 64 | 2 | >128 | 0.125 | 2 | >128 | >128 |
| C. parapsilosis CP | 16 | 16 | 128 | 0.25 | 4 | >128 | >128 |
| C. tropicalis CT | >128 | 32 | >128 | 0.25 | 128 | >128 | >128 |
| Strain | MIC EA | MIC CAPE | MIC CPF (Control) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 2 | Day 7 | Day 2 | Day 7 | Day 2 | Day 7 | |||||||
| (−) S | (+) S | (−) S | (+) S | (−) S | (+) S | (−) S | (+) S | (−) S | (+) S | (−) S | (+) S | |
| CAU | 0.25 | 32 | 2 | 256 | 16 | 32 | 32 | 64 | 16 | 64 | 64 | 256 |
| CAL | 0.5 | 0.5 | 0.5 | 64 | 8 | 16 | 32 | 64 | 0.125 | 8 | 0.25 | 8 |
| Strain | Drug | Ergosterol Concentration (μg/mL) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 150 | 200 | 250 | ||
| CAU | EA | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| CAPE | 16 | 16 | 16 | 16 | 16 | 16 | |
| AMB (control) | 0.5 | 4 | 8 | 16 | 32 | 64 | |
| EA | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| CAL | CAPE | 8 | 8 | 8 | 8 | 8 | 8 |
| AMB (control) | 0.5 | 2 | 4 | 8 | 16 | 32 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Possamai Rossatto, F.C.; Tharmalingam, N.; Escobar, I.E.; d’Azevedo, P.A.; Zimmer, K.R.; Mylonakis, E. Antifungal Activity of the Phenolic Compounds Ellagic Acid (EA) and Caffeic Acid Phenethyl Ester (CAPE) against Drug-Resistant Candida auris. J. Fungi 2021, 7, 763. https://doi.org/10.3390/jof7090763
Possamai Rossatto FC, Tharmalingam N, Escobar IE, d’Azevedo PA, Zimmer KR, Mylonakis E. Antifungal Activity of the Phenolic Compounds Ellagic Acid (EA) and Caffeic Acid Phenethyl Ester (CAPE) against Drug-Resistant Candida auris. Journal of Fungi. 2021; 7(9):763. https://doi.org/10.3390/jof7090763
Chicago/Turabian StylePossamai Rossatto, Fernanda Cristina, Nagendran Tharmalingam, Iliana E. Escobar, Pedro Alves d’Azevedo, Karine Rigon Zimmer, and Eleftherios Mylonakis. 2021. "Antifungal Activity of the Phenolic Compounds Ellagic Acid (EA) and Caffeic Acid Phenethyl Ester (CAPE) against Drug-Resistant Candida auris" Journal of Fungi 7, no. 9: 763. https://doi.org/10.3390/jof7090763
APA StylePossamai Rossatto, F. C., Tharmalingam, N., Escobar, I. E., d’Azevedo, P. A., Zimmer, K. R., & Mylonakis, E. (2021). Antifungal Activity of the Phenolic Compounds Ellagic Acid (EA) and Caffeic Acid Phenethyl Ester (CAPE) against Drug-Resistant Candida auris. Journal of Fungi, 7(9), 763. https://doi.org/10.3390/jof7090763







