Entomotoxic Activity of the Extracts from the Fungus, Alternaria tenuissima and Its Major Metabolite, Tenuazonic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Fermentation
2.2. Extraction of Secondary Metabolites
2.3. Analysis of Extracts
2.4. Isolation of Tenuazonic Acid
2.5. Bioassays
2.5.1. Entomotoxic Activity of Extracts
2.5.2. Phytotoxic Activity of Extracts
2.5.3. Cytotoxic Activity of Extracts
2.5.4. Antimicrobial Activity of Extracts
2.5.5. Insect Models and TeA
2.5.6. Acute Contact Toxicity of TeA
2.5.7. Oral Toxicity of TeA
2.5.8. Contact Intestinal Toxicity of TeA
2.5.9. Cytotoxic Activity of TeA
2.6. Statistical Analysis
3. Results
3.1. The Yield of A. tenuissima Extracts
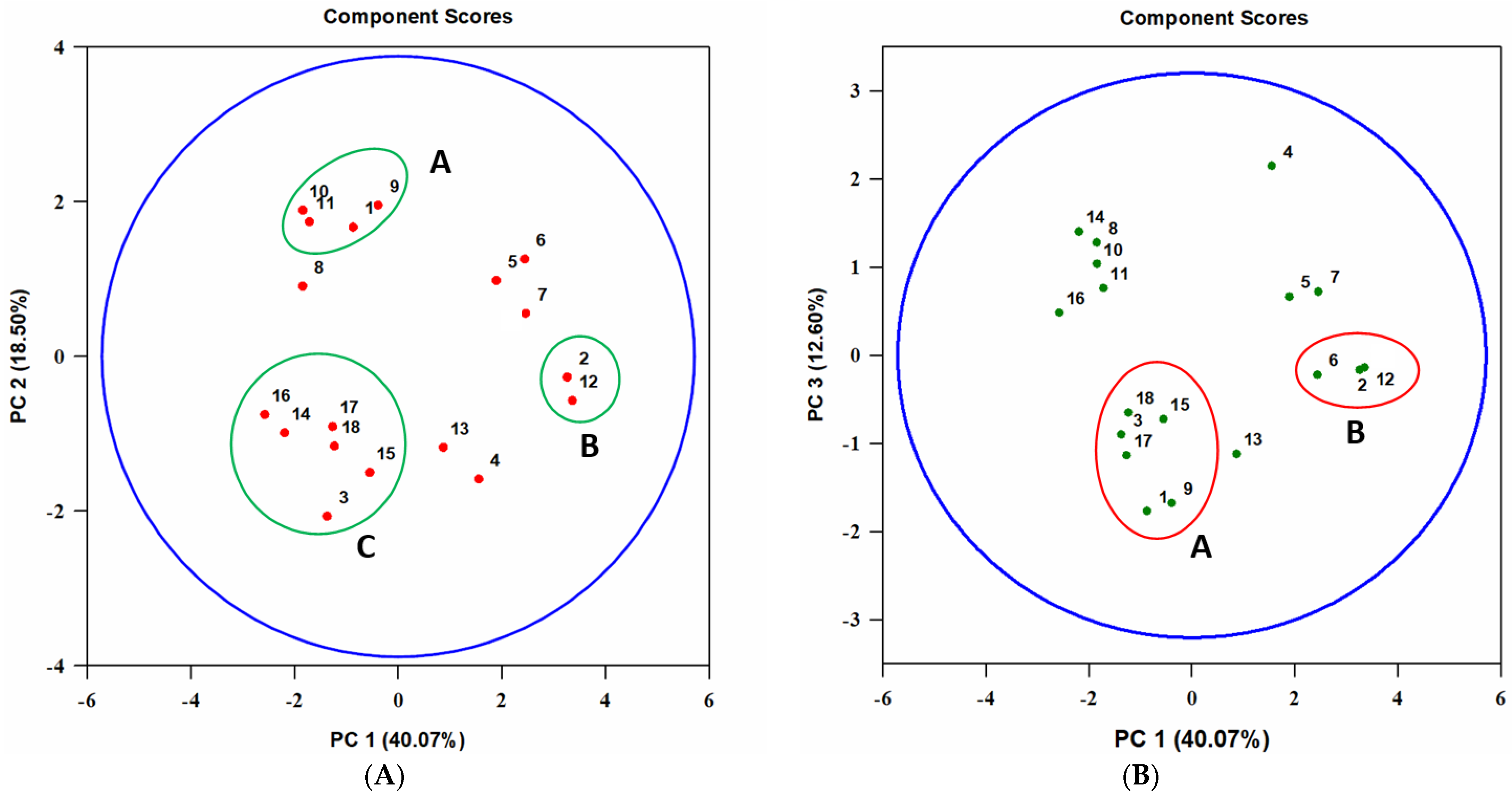
3.2. The Spectrum of Biological Activity of A. tenuissima Extracts
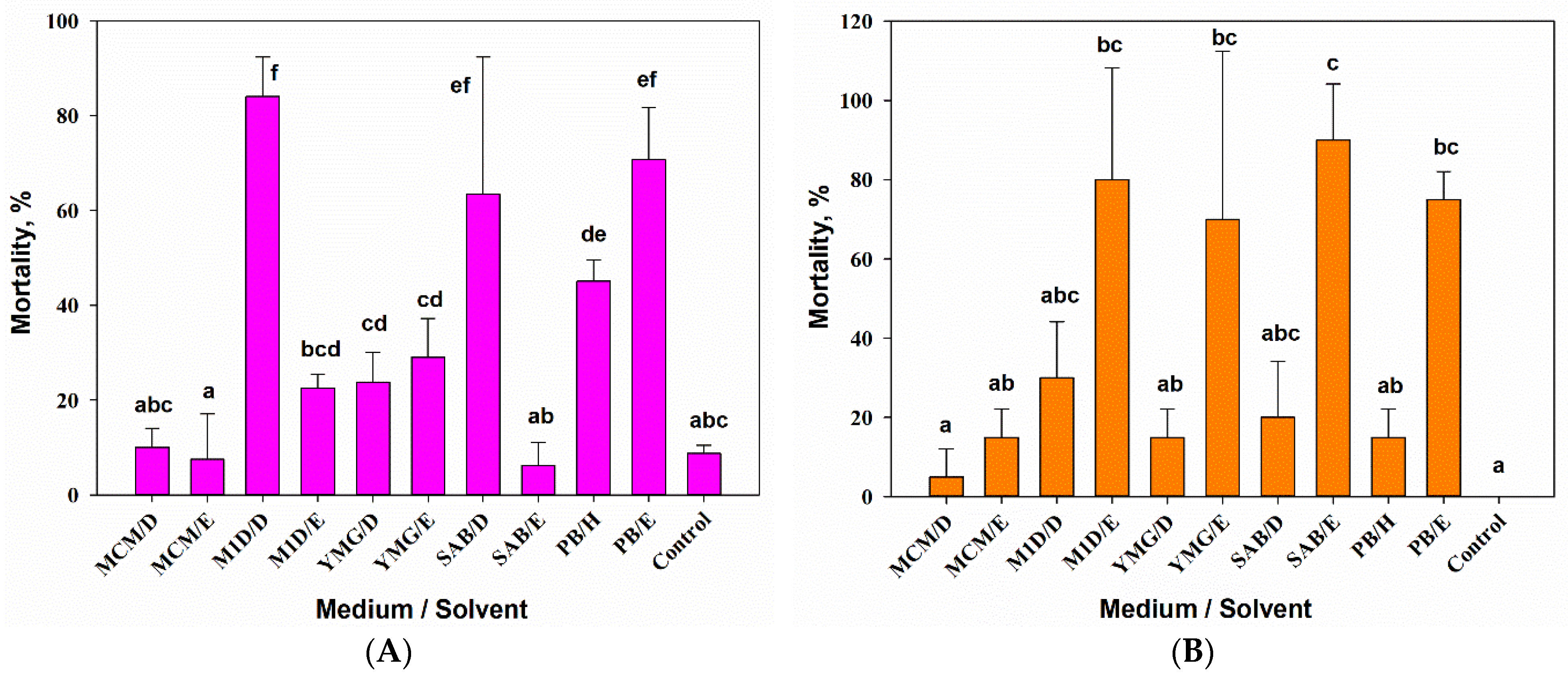
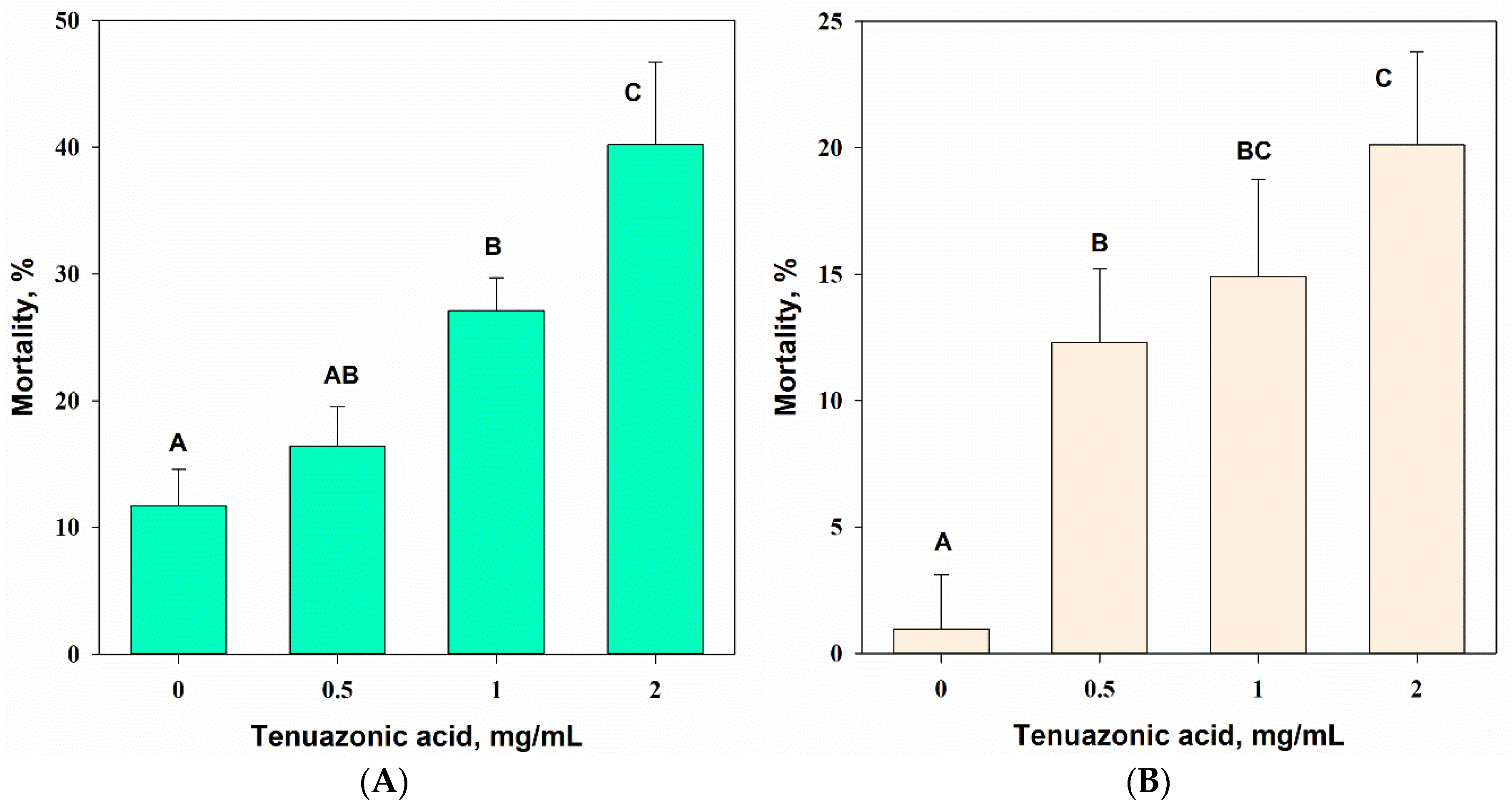
3.2.1. Entomotoxic Activity
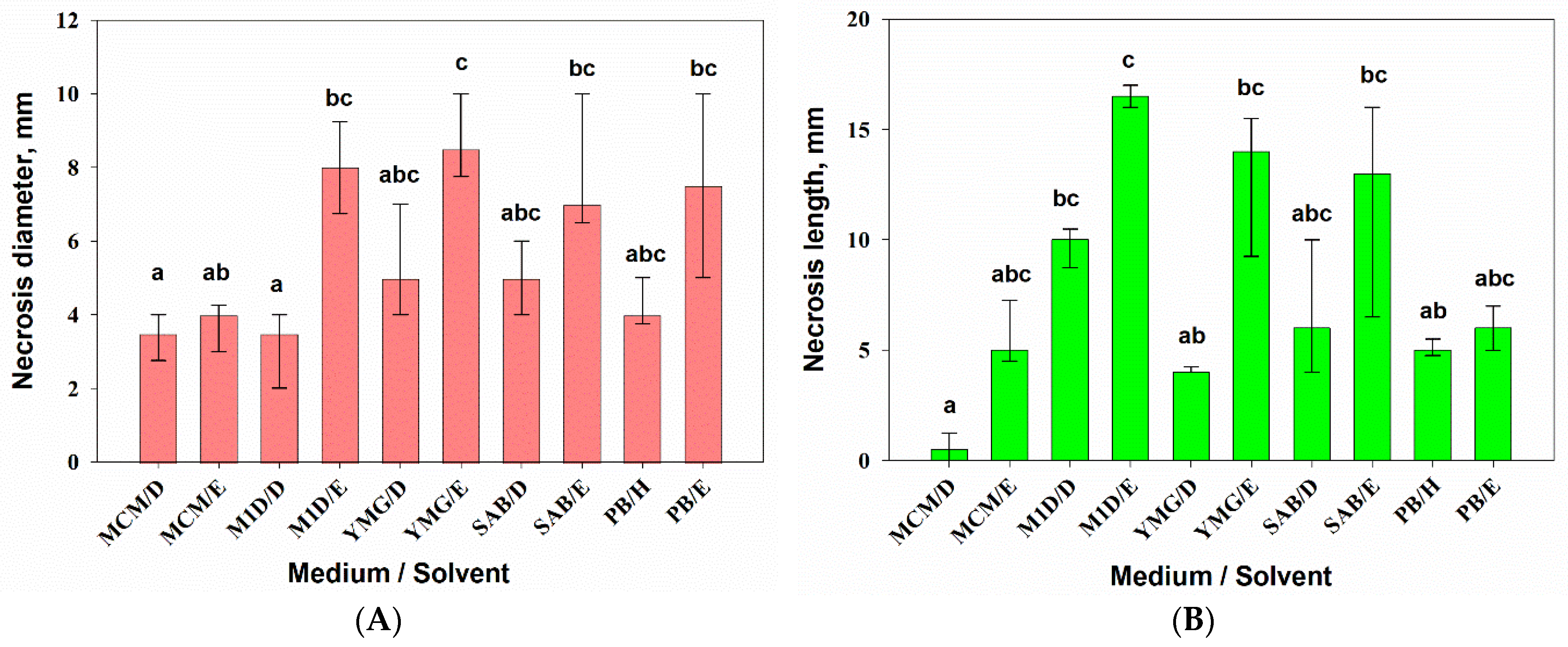
3.2.2. Phytotoxic Activity
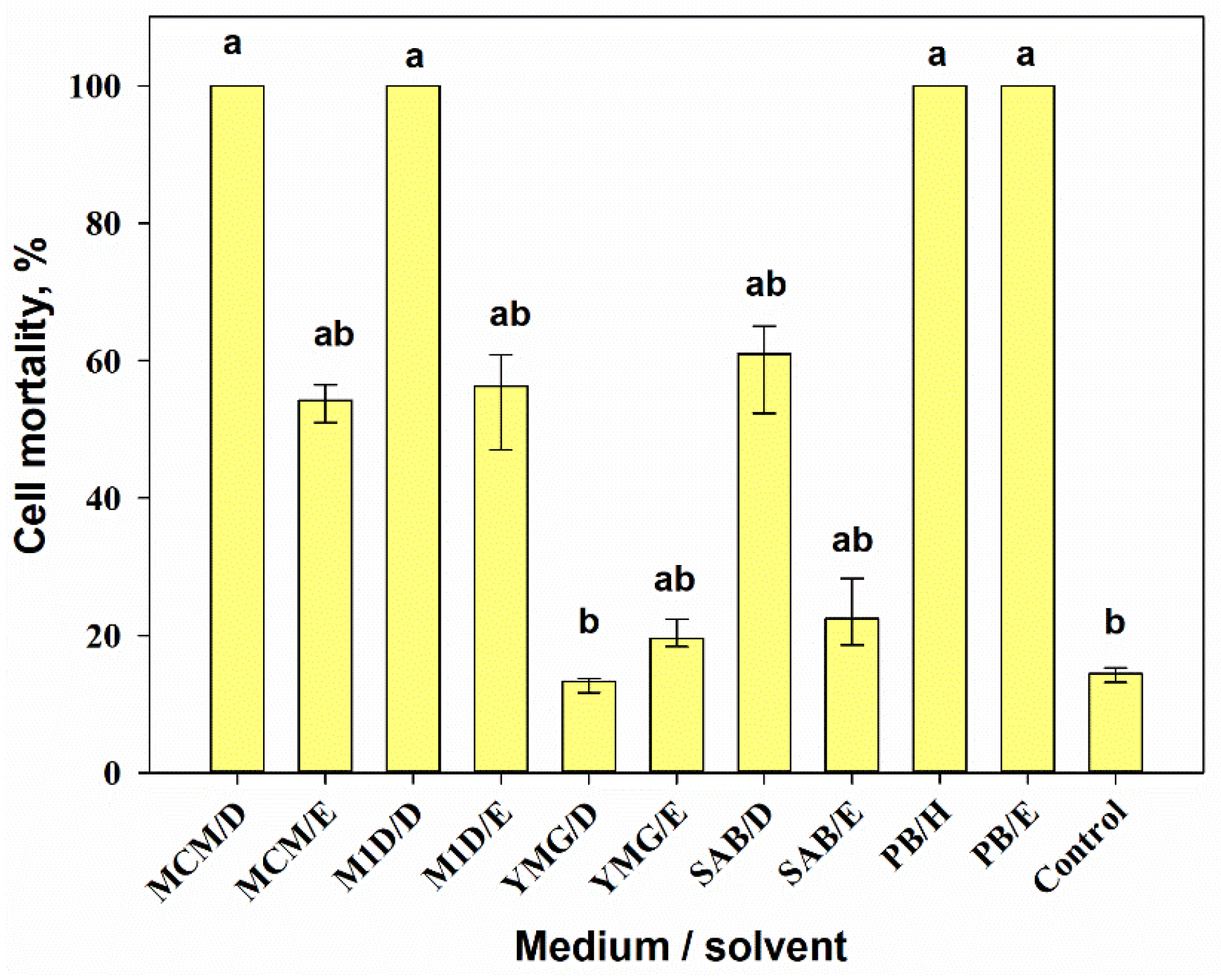
3.2.3. Cytotoxic Activity
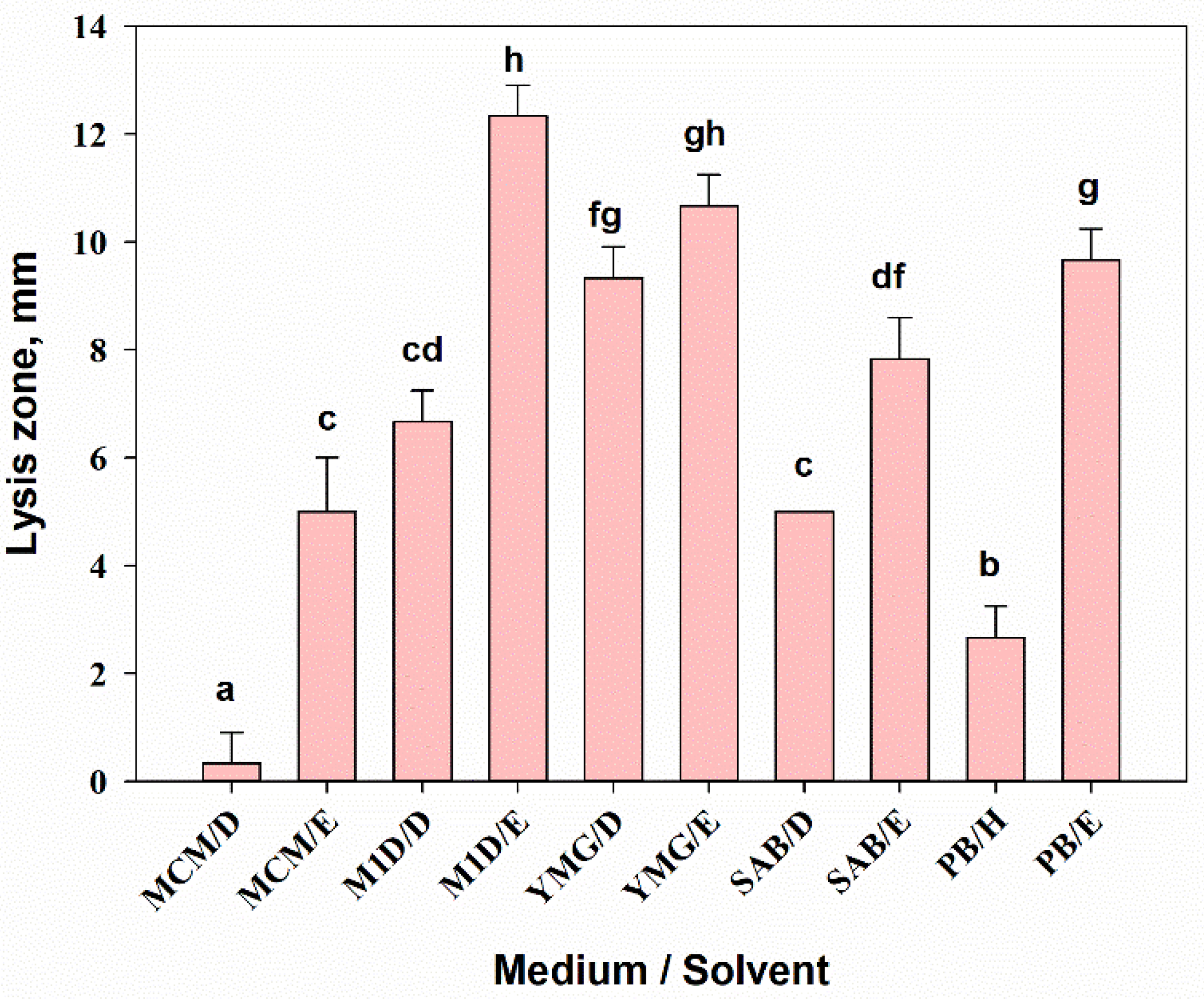
3.2.4. Antimicrobial Activity
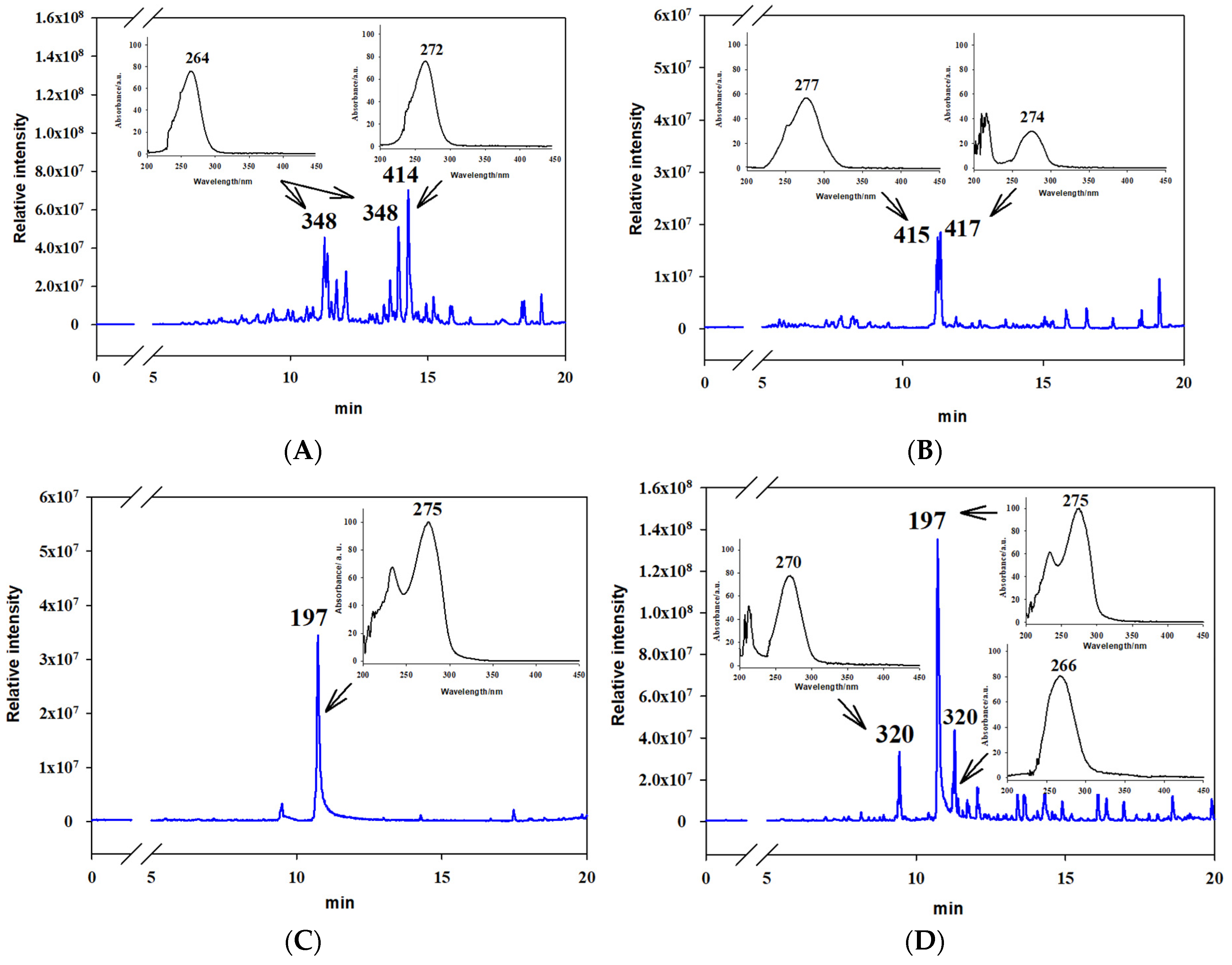
3.3. Analysis of Extracts
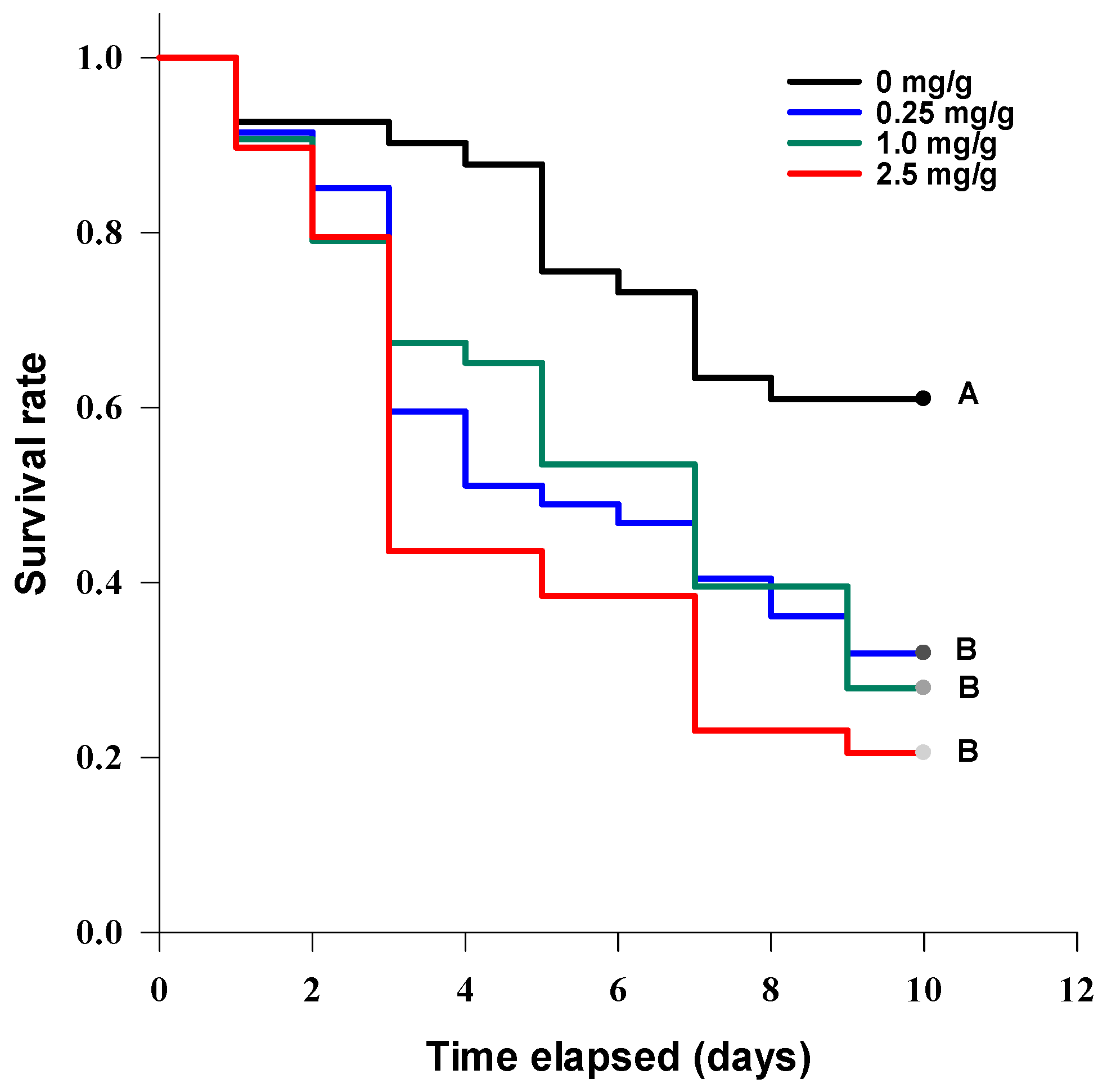
3.4. Entomotoxic Activity of Tenuazonic Acid
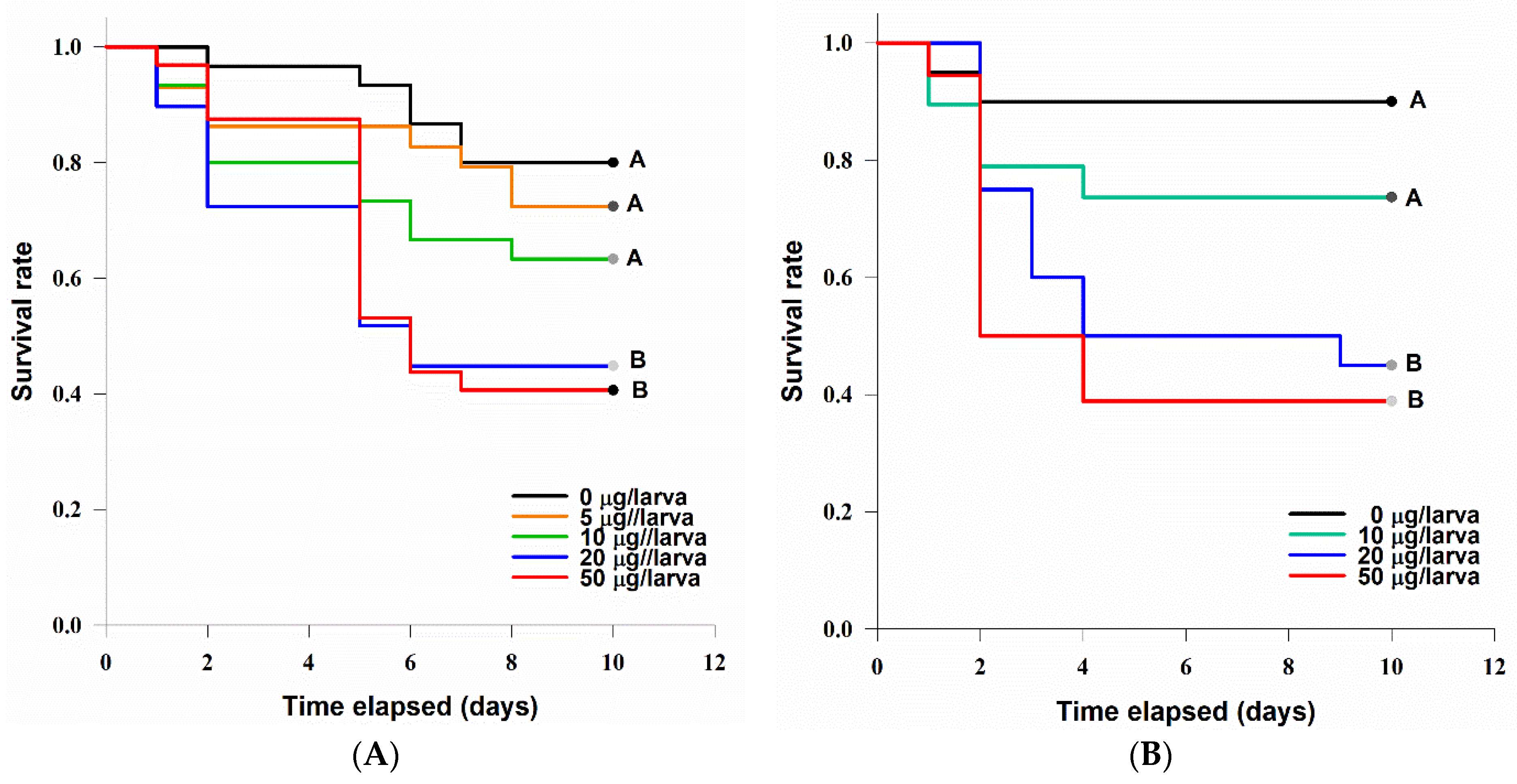
3.4.1. Acute Contact Larvicidal Toxicity
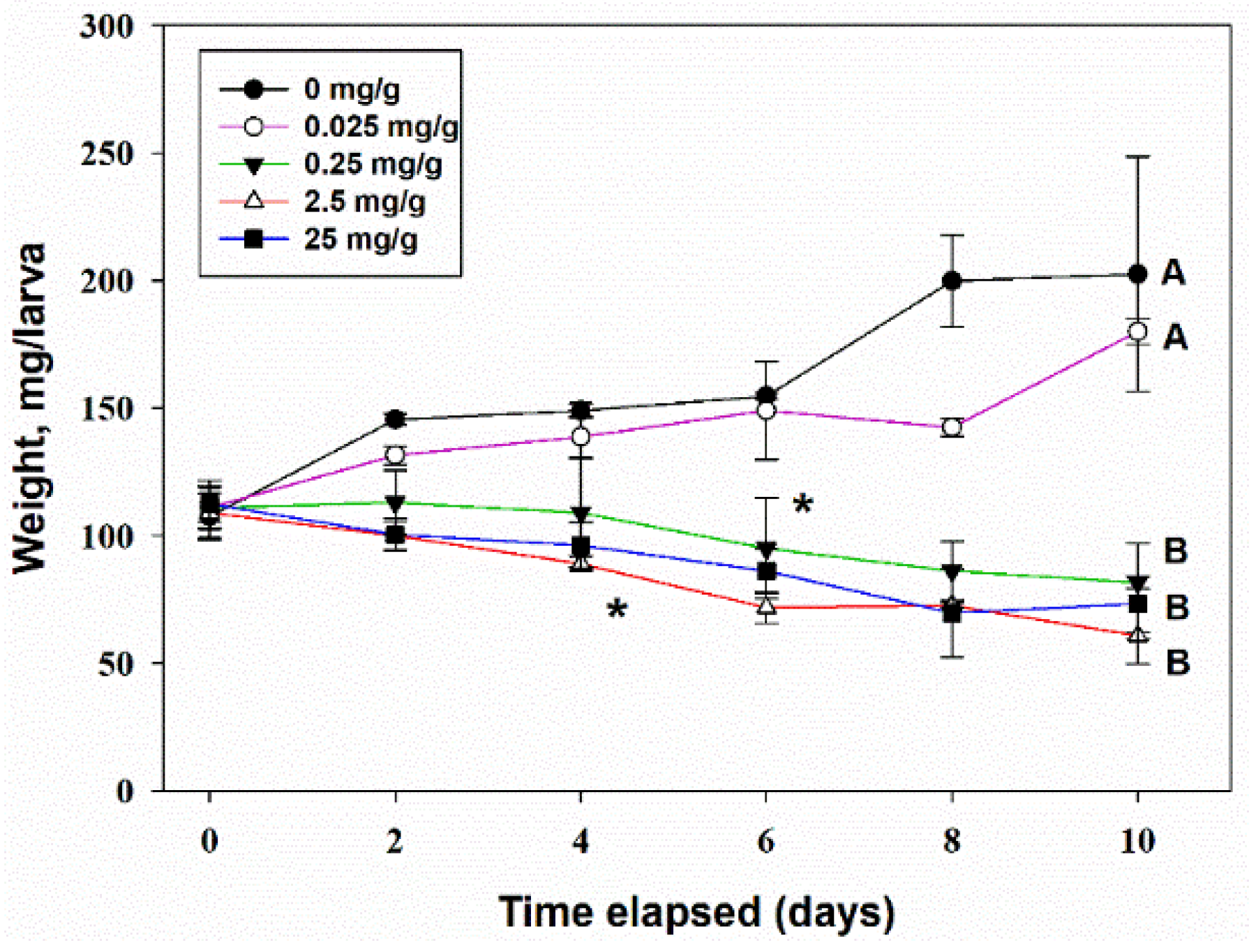
3.4.2. Oral Toxicity
3.4.3. Contact Intestinal Activity
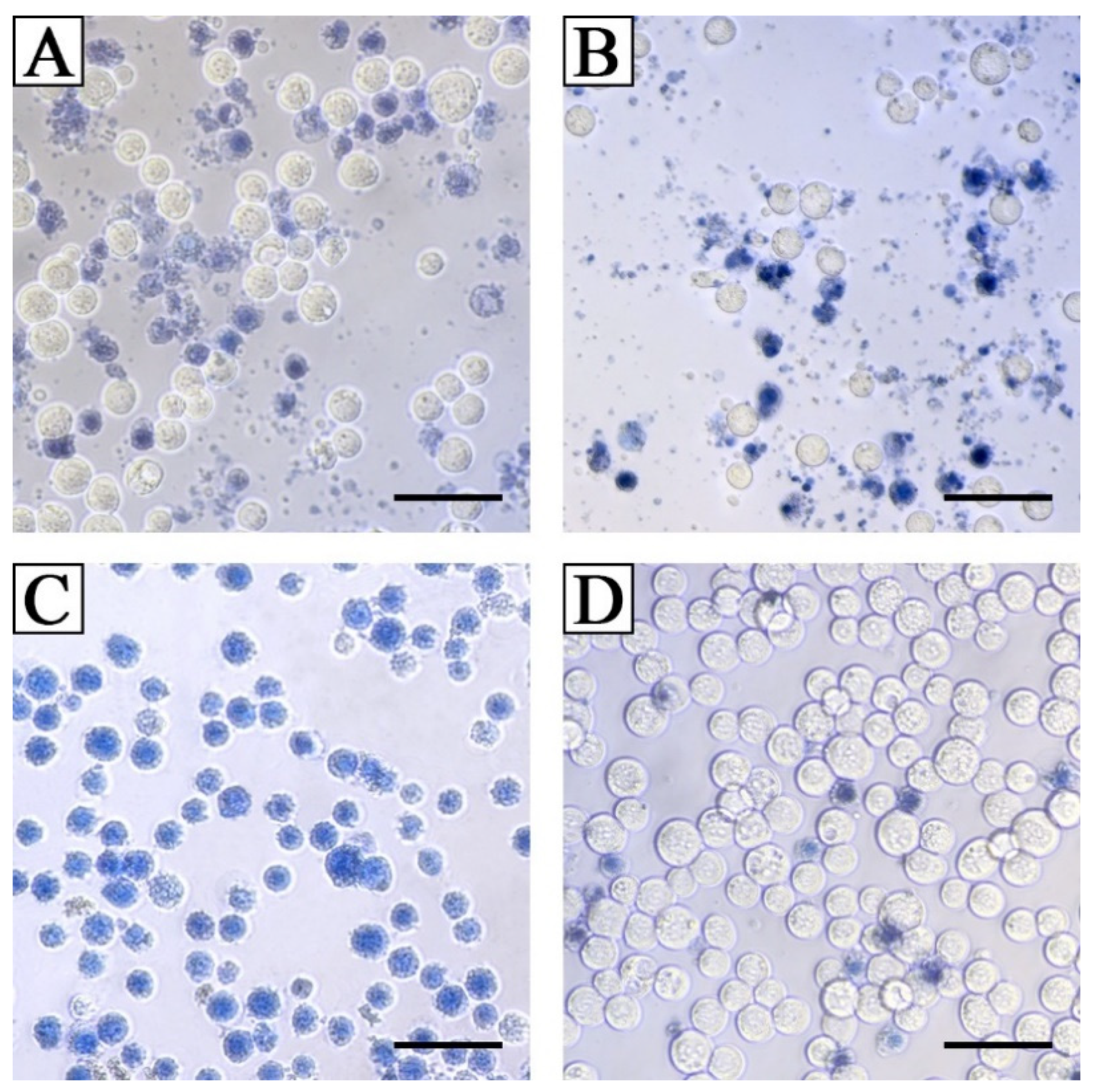
3.4.4. Cytotoxic Activity
4. Discussion
4.1. Detection of Entomotoxic Metabolites in A. tenuissima Extracts
4.2. Entomotoxicity of Tenuazonic Acid
4.3. Practical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berestetskiy, A.; Hu, Q. The chemical ecology approach to reveal fungal metabolites for arthropod pest management. Microorganisms 2021, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Berestetskiy, A.O.; Gannibal, F.B.; Minkovich, E.V.; Osterman, I.A.; Salimova, D.R.; Sergiev, P.V.; Sokornova, S.V. Spectrum of biological activity of the Alternaria fungi isolated from the phyllosphere of herbaceous plants. Microbiology 2018, 87, 806–816. [Google Scholar] [CrossRef]
- Berestetskiy, A.O.; Apollonova, L.S.; Sokornova, S.V.; Chermenskaya. T., D. Insecticidal properties of phytopathogenic ascomycetes. Vestn. Zashchity Rasteniy 2015, 85, 52–54. (In Russian) [Google Scholar]
- Singh, B.; Thakur, A.; Kaur, S.; Chadha, B.S.; Kaur, A. Acetylcholinesterase inhibitory potential and insecticidal activity of an endophytic Alternaria sp. from Ricinus communis. Appl. Biochem. Biotechnol. 2012, 168, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.P.; Singh, B.; Thakur, A.; Kaur, A.; Kau, S. Studies on immunomodulatory effect of endophytic fungus Alternaria alternate on Spodoptera litura. J. Asia-Pac. Entomol. 2015, 18, 67–75. [Google Scholar] [CrossRef]
- Kaur, J.; Sharma, A.; Sharma, M.; Manhas, R.K.; Kaur, S.; Kaur, A. Effect of α-glycosidase inhibitors from endophytic fungus Alternaria destruens on survival and development of insect pest Spodoptera litura Fab. and fungal phytopathogens. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Buchwaldt, L.; Green, H. Phytotoxicity of destruxin B and its possible role in the pathogenesis of Alternaria brassicae. Plant Pathol. J. 1992, 41, 55–63. [Google Scholar] [CrossRef]
- Sowjanya Sree, K.; Padmaja, V.; Murthy, Y.L. Insecticidal activity of destruxin, a mycotoxin from Metarhizium anisopliae (Hypocreales), against Spodoptera litura (Lepidoptera: Noctuidae) larval stages. Pest Manag. Sci. 2008, 64, 119–125. [Google Scholar] [CrossRef]
- Dalinova, A.; Chisty, L.; Kochura, D.; Garnyuk, V.; Petrova, M.; Prokofieva, D.; Yurchenko, A.; Dubovik, V.; Ivanov, A.; Smirnov, S.; et al. Isolation and bioactivity of secondary metabolites from solid culture of the fungus, Alternaria sonchi. Biomolecules 2020, 10, 81. [Google Scholar] [CrossRef]
- Bhagat, J.; Kaur, A.; Kaur, R.; Yadav, A.K.; Sharma, V.; Chadha, B.S. Cholinesterase inhibitor (Altenuene) from an endophytic fungus Alternaria alternata: Optimization, purification and characterization. J. Appl. Microbiol. 2016, 121, 1015–1025. [Google Scholar] [CrossRef]
- La Croix, E.A.S.; Mhasalkar, S.E.; Mamalis, P.; Harrington, F.P. Insecticidal activity of some tenuazonic acid analogues. Pestic. Sci. 1975, 6, 491–496. [Google Scholar] [CrossRef]
- Reid, W.R.; Parker, B.L.; Gouli, S.Y.; Skinner, M.; Gouli, V.V.; Teillon, H.B. Fungi associated with the hemlock woolly adelgid, Adelges tsugae, and assessment of entomopathogenic isolates for management. J. Insect Sci. 2010, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Sharma, A. Use of Alternaria spp. as a pest control agent: A review. World App. Sci. J. 2014, 31, 1869–1872. [Google Scholar] [CrossRef]
- Wakil, W.; Usman Ghazanfar, M.; Yasin, M. Naturally occurring entomopathogenic fungi infecting stored grain insect species in Punjab, Pakistan. J. Insect Sci. 2014, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Shabana, Y.M.; Ragab, M.E. Alternaria infectoria, a promising biological control agent for the fig wax scale, Ceroplastes rusci (Homoptera: Coccidae), in Egypt. Biocontrol Sci. Technol. 1997, 7, 553–564. [Google Scholar] [CrossRef]
- Christias, C.H.; Hatzipapas, P.; Dara, A.; Kaliafas, A.; Chrysanthis, G. Alternariaalternata, a new pathotype pathogenic to aphids. BioControl 2001, 46, 105–124. [Google Scholar] [CrossRef]
- Hatzipapas, P.; Kalosak, K.; Dara, A.; Christias, C. Spore germination and appressorium formation in the entomopathogenic Alternaria alternata. Mycol. Res. 2002, 106, 1349–1359. [Google Scholar] [CrossRef]
- Gannibal, P.B. Species of the genus Alternaria revealed in Russia and some neighboring territories. Mikol. Fitopatol. 2015, 49, 374–385. (In Russian) [Google Scholar]
- Yang, F.Z.; Yang, B.; Li, B.B.; Xiao, C. Alternaria toxin-induced resistance in rose plants against rose aphid (Macrosiphum rosivorum): Effect of tenuazonic acid. J. Zhejiang Univ. Sci. B 2015, 16, 264–274. [Google Scholar] [CrossRef]
- Basit, A.; Farhan, M.; Abbas, M.; Wang, Y.; Zhao, D.G.; Mridha, A.U.; Al-tawaha, A.R.M.S.; Bashir, M.A.; Arif, M.; Ahmed, S.; et al. Do microbial protein elicitors PeaT1 obtained from Alternaria tenuissima and PeBL1 from Brevibacillus laterosporus enhance defense response against tomato aphid (Myzus persicae)? Saudi J. Biol. Sci. 2021, 28, 3242–3248. [Google Scholar] [CrossRef]
- Salimova, D.R.; Kochura, D.S.; Sokornova, S.V.; Orina, A.S.; Hannibal, F.B.; Berestetskiy, A.O. Identification and toxigenic properties of Alternaria japonica strains. Mikol. Fitopatol. 2021, 55, 203–218. (In Russian) [Google Scholar] [CrossRef]
- Mikula, H.; Horkel, E.; Hans, P.; Hametner, C.; Fröhlich, J. Structure and tautomerism of tenuazonic acid—A synergetic computational and spectroscopic approach. J. Hazard. Mater. 2013, 250, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Chermenskaya, T.D.; Stepanycheva, E.A.; Shchenikova, A.V.; Savelieva, E.I.; Chakaeva, A.S. Insecticidal effects of Ungernia severtzovii bulb extracts against the grain aphid Schizaphis graminum (Rondani). Ind. Crops Prod. 2012, 36, 122–126. [Google Scholar] [CrossRef]
- Allegra, E.; Titball, R.W.; Carter, J.; Champion, O.L. Galleria mellonella larvae allow the discrimination of toxic and non-toxic chemicals. Chemosphere 2018, 198, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Berestetskii, A.O.; Yuzikhin, O.S.; Katkova, A.S.; Dobrodumov, A.V.; Sivogrivov, D.E.; Kolombet, L.V. Isolation, identification, and characteristics of the phytotoxin produced by the fungus Alternaria cirsinoxia. Appl. Biochem. Microbiol. 2010, 46, 75–79. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Rincón, R.A.; Rodríguez, D.; Coy-Barrera, E. Botanicals against Tetranychus urticae Koch under laboratory conditions: A survey of alternatives for controlling pest mites. Plants 2019, 8, 272. [Google Scholar] [CrossRef]
- Kono, Y.; Gardner, J.M.; Takeuchi, S. Structure of the host-selective toxins produced by a pathotype of Alternaria citri causing brown spot disease of mandarins. Agr. Biol. Chem. 1986, 50, 801–804. [Google Scholar] [CrossRef]
- Liebermann, B.; Ellinger, R.; Günther, W.; Ihn, W.; Gallander, H. Tricycloalternarenes produced by Alternaria alternata related to ACTG-toxins. Phytochemistry 1997, 46, 297–303. [Google Scholar] [CrossRef]
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Gotthardt, M.; Kanawati, B.; Schmidt, F.; Asam, S.; Hammerl, R.; Frank, O.; Hofmann, T.; Schmitt-Kopplin, P.; Rychlik, M. Comprehensive analysis of the Alternaria mycobolome using mass spectrometry based metabolomics. Mol. Nutr. Food Res. 2019, 64, 1900558. [Google Scholar] [CrossRef]
- Wang, J.T.; Ma, Z.H.; Wang, G.K.; Xu, F.Q.; Chen, L.; Yang, Y.; Wang, G.; Liu, J.S. Four meroterpenoids from Alternaria alternata isolated from Paeonia lactiflora. Phytochem. Lett. 2019, 31, 1–4. [Google Scholar] [CrossRef]
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from Alternaria fungi and their bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef]
- Pinto, V.E.; Patriarca, A. Alternaria species and their associated mycotoxins. In Methods in Molecular Biology, 2nd ed.; Moretti, A., Susca, A., Eds.; Humana Press: New York, NY, USA, 2017; Chapter 1542; pp. 13–32. [Google Scholar] [CrossRef]
- Dalinova, A.A.; Salimova, D.R.; Berestetskiy, A.O. Fungi of the genera Alternaria as producers of biological active compounds and mycoherbicides. Appl. Biochem. Microbiol. 2020, 56, 256–272. [Google Scholar] [CrossRef]
- DesRochers, N.; Walsh, J.P.; Renaud, J.B.; Seifert, K.A.; Yeung, K.K.-C.; Sumarah, M.W. Metabolomic profiling of fungal pathogens responsible for root rot in American ginseng. Metabolites 2020, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, M.M.; Sayed, A.M.; AboulMagd, A.M.; Hassan, H.M.; El Amir, D.; Abouzid, S.F.; El-Gendy, A.O.; Rateb, M.E.; Abdelmohsen, U.R.; Alhadrami, H.; et al. Metabolomic profiling, biological evaluation of Aspergillus awamori, the river Nile-derived fungus using epigenetic and OSMAC approaches. RSC Adv. 2021, 11, 6709–6719. [Google Scholar] [CrossRef]
- Hessel-Pras, S.; Kieshauer, J.; Roenn, G.; Luckert, C.; Braeuning, A.; Lampen, A. In vitro characterization of hepatic toxicity of Alternaria toxins. Mycotoxin Res. 2019, 35, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Abdou, R.; Dawoud, M. Cytotoxic metabolites of Alternaria alternata, an endophyte of the medicinal plant Bidens bipinnata. Int. J. Pharm. Pharm. Sci. 2020, 12, 42–48. [Google Scholar] [CrossRef][Green Version]
- Shen, L.; Tian, S.J.; Song, H.L.; Chen, X.; Guo, H.; Wan, D.; Wang, Y.R.; Wang, F.W.; Liu, L.J. Cytotoxic tricycloalternarene compounds from endophyte Alternaria sp. W-1 associated with Laminaria japonica. Mar. Drugs 2018, 16, 402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, L.; Jiao, J.; Liu, D.; Zhang, X.; Li, J.; Che, Q.; Zhu, T.; Li, D. Cytotoxic meroterpenoids from the sponge-associated fungus Alternaria sp. JJY-32. Chem. Biodivers. 2020, 17, e2000226. [Google Scholar] [CrossRef]
- Klotz, M.G.; Erdei, L. Effect of tentoxin on K+ transport in winter wheatseedlings of different K+-status. Physiol. Plant. 1988, 72, 298–304. [Google Scholar] [CrossRef]
- Sugawara, F.; Uzawa, J.; Esumi, Y.; Suzuki, M.; Yoshida, S.; Strobel, G.; Steiner, J.L.R.; Clardy, J. Phytotoxins from the Septoria spp. plant pathogenic fungus on leafy spurge. Biosci. Biotechnol. Biochem. 1998, 62, 638–642. [Google Scholar] [CrossRef]
- Aichinger, G.; Del Favero, G.; Warth, B.; Marko, D. Alternaria toxins—Still emerging? Compr. Rev. Food Sci. Food Saf. 2021, 1–17. [Google Scholar] [CrossRef]
- Cutuli, M.A.; Petronio, G.P.; Vergalito, F.; Magnifico, I.; Pietrangelo, L.; Venditti, N.; Di Marco, R. Galleria mellonella as a consolidated in vivo model hosts: New developments in antibacterial strategies and novel drug testing. Virulence 2019, 10, 527–541. [Google Scholar] [CrossRef]
- Kinoshita, T.; Renbutsu, Y.; Khan, I.D.; Kohmojo, K.; Nishimura, S. Distribution of tenuazonic acid production in the genus Alternaria and its pathological evaluation. Jpn. J. Phytopathol. 1972, 38, 397–404. [Google Scholar] [CrossRef]
- Davis, N.D.; Diener, U.L.; Morgan-Jones, G. Tenuazonic acid production by Alternaria alternata and Alternaria tenuissima isolated from cotton. Appl. Environ. Microbiol. 1977, 34, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Brzonkalik, K.; Herrling, T.; Syldatk, C.; Neumann, A. Process development for the elucidation of mycotoxin formation in Alternaria alternata. AMB Express 2011, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Brzonkalik, K.; Hümmer, D.; Syldatk, C.; Neumann, A. Influence of pH and carbon to nitrogen ratio on mycotoxin production by Alternaria alternata in submerged cultivation. AMB Express 2012, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Swapnil, P.; Upadhyay, R.S. Isolation, characterization and toxicological potential of Alternaria-mycotoxins (TeA, AOH and AME) in different Alternaria species from various regions of India. Sci. Rep. 2017, 7, 8777. [Google Scholar] [CrossRef] [PubMed]
- Zwickel, T.; Kahl, S.M.; Klaffke, H.; Rychlik, M.; Müller, M.E.H. Spotlight on the underdogs—An analysis of underrepresented Alternaria mycotoxins formed depending on varying substrate, time and temperature conditions. Toxins 2016, 8, 344. [Google Scholar] [CrossRef]
- Coates, C.J.; Lim, J.; Harman, K.; Rowley, A.F.; Griffiths, D.J.; Emery, H.; Layton, W. The insect, Galleria mellonella, is a compatible model for evaluating the toxicology of okadaic acid. Cell Biol. Toxicol. 2019, 35, 219–232. [Google Scholar] [CrossRef]
- Bandani, A.R.; Khambay, B.P.S.; Faull, J.L.; Newton, R.; Deadman, M.; Butt, T.M. Production of efrapeptins by Tolypocladium species and evaluation of their insecticidal and antimicrobial properties. Mycol. Res. 2000, 104, 537–544. [Google Scholar] [CrossRef]
- Safavi, S.A. In vitro and in vivo induction, and characterization of beauvericin isolated from Beauveria bassiana and its bioassay on Galleria mellonella larvae. J. Agric. Sci. Technol. 2013, 15, 1–10. [Google Scholar]
- Woolley, V.C.; Teakle, G.R.; Prince, G.; de Moor, C.H.; Chandler, D. Cordycepin, a metabolite of Cordyceps militaris, reduces immune-related gene expression in insects. J. Invertebr. Pathol. 2020, 177, 107480. [Google Scholar] [CrossRef] [PubMed]
- Benzertiha, A.; Kierończyk, B.; Kołodziejski, P.; Pruszyńska–Oszmałek, E.; Rawski, M.; Józefiak, D.; Józefiak, A. Tenebrio molitor and Zophobas morio full-fat meals as functional feed additives affect broiler chickens’ growth performance and immune system traits. Poult. Sci. 2020, 99, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Mulè, G.; Logrieco, A.; Bottalico, A.; Stea, G. Screening for Fusarium toxigenic isolates using larvae of Galleria mellonella. Mycotoxin Res. 1994, 10, 41–46. [Google Scholar] [CrossRef]
- Mulè, G.; D’Ambrosio, A.; Logrieco, A.; Bottalico, A. Toxicity of mycotoxins of Fusarium sambucinum for feeding in Galleria mellonella. Entomol. Exp. Appl. 1992, 62, 17–22. [Google Scholar] [CrossRef]
- Abendstein, D.; Schweigkofler, W.; Strasser, H. Effect of the fungal metabolite oosporein on feeding behavior and survival of larvae of Melolontha melolontha L. and Galleria mellonella L. Laimburg J. 2001, 1, 1–4. [Google Scholar]
- Wrońska, A.K.; Boguś, M.I.; Kaczmarek, A.; Kazek, M. Harman and norharman, metabolites of entomopathogenic fungus Conidiobolus coronatus (Entomopthorales), disorganize development of Galleria mellonella (Lepidoptera) and affect serotonin-regulating enzymes. PLoS ONE 2018, 13, e0204828. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cassi, X.; Supeanu, A.; Jansson, A.; Boqvist, S.; Vagsholm, I. Novel foods: A risk profile for the house cricket (Acheta domesticus). EFSA J. 2018, 16, e16082. [Google Scholar] [CrossRef]
- Ganassi, S.; Moretti, A.; Pagliai, M.B.; Logrieco, A.; Sabatini, M.A. Effects of beauvericin on Schizaphis graminum (Aphididae). J. Invertebr. Pathol. 2002, 80, 90–96. [Google Scholar] [CrossRef]
- Berestetskiy, A.O.; Dalinova, A.A.; Dubovik, V.R.; Grigoryeva, E.N.; Kochura, D.M.; Senderskiy, I.V.; Smirnov, S.N.; Stepanycheva, E.A.; Turaeva, S.M. Analysis and isolation of secondary metabolites of Bipolaris sorokiniana by different chromatography techniques and the spectrum of their biological activity. Appl. Biochem. Microbiol. 2020, 56, 569–582. [Google Scholar] [CrossRef]
- Aeinehchi, P.; Naseri, B.; Rafiee Dastjerdi, H.; Nouri-Ganbalani, G.; Golizadeh, A. Lethal and sublethal effects of thiacloprid on Schizaphis graminum (Rondani) (Hemiptera: Aphididae) and its predator Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae). Toxin Rev. 2019, 1–11. [Google Scholar] [CrossRef]
- Gao, J.R.; Zhu, K.Y. Comparative toxicity of selected organophosphate insecticides against resistant and susceptible clones of the greenbug, Schizaphis graminum (Homoptera: Aphididae). J. Agric. Food Chem. 2000, 48, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Al Khoury, C.; Guillot, J.; Nemer, N. Lethal activity of beauvericin, a Beauveria bassiana mycotoxin, against the two-spotted spider mites, Tetranychus urticae Koch. J. Appl. Entomol. 2019, 143, 974–983. [Google Scholar] [CrossRef]
- Osman, M.E.; Elnasr, A.A.A.; Nawar, M.A.; Hefnawy, G.A. Myco-metabolites as biological control agents against the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). Egypt J. Biol. Pest Control 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Mak, M.; Beattie, K.D.; Basta, A.; Randall, D.; Chen, Z.; Spooner-Hart, R. Triangulation of methods using insect cell lines to investigate insecticidal mode-of-action. Pest Manag. Sci. 2021, 77, 492–501. [Google Scholar] [CrossRef]
- Boguś, M.I.; Wrońska, A.K.; Kaczmarek, A.; Boguś-Sobocińska, M. In vitro screening of 65 mycotoxins for insecticidal potential. PLoS ONE 2021, 16, e0248772. [Google Scholar] [CrossRef]
- Novak, B.; Rainer, V.; Sulyok, M.; Haltrich, D.; Schatzmayr, G.; Mayer, E. Twenty-eight fungal secondary metabolites detected in pig feed samples: Their occurrence, relevance and cytotoxic effects in vitro. Toxins 2019, 11, 537. [Google Scholar] [CrossRef]
- Chen, S.; Qiang, S. Recent advances in tenuazonic acid as a potential herbicide. Pestic. Biochem. Phys. 2017, 143, 252–257. [Google Scholar] [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging mycotoxins: Beyond traditionally determined food contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef] [PubMed]
- Muehlebach, M.; Buchholz, A.; Zambach, W.; Schaetzer, J.; Daniels, M.; Hueter, O.; Kloer, D.P.; Lind, R.; Maienfisch, P.; Pierce, A.; et al. Spiro N-methoxy piperidine ring containing aryldiones for the control of sucking insects and mites: Discovery of spiropidion. Pest Manag. Sci. 2020, 76, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, H.; Meng, B.; Wei, R.; Wang, L.; An, C.; Chen, S.; Yang, C.; Qiang, S. An evaluation of tenuazonic acid, a potential biobased herbicide in cotton. Pest Manag. Sci. 2019, 75, 2482–2489. [Google Scholar] [CrossRef]
- Fischer, R.; Lehr, S.; Arnold, C.; Auler, T.; Dittgen, J.; Feucht, D.; Franken, E.M.; Hempel, W.; Hills, M.J.; Kehne, H.; et al. Trifluoromethoxyphenyl-Substituted Tetramic Acid Derivatives as Pesticides and/or Herbicides. WO Patent N 2008067873A1, 12 June 2008. Available online: https://patents.google.com/patent/WO2008067873A1/da (accessed on 25 August 2021).
- Chen, M.; Geng, C.W.; Han, L.; Liu, Y.; Yu, Y.K.; Lu, A.M.; Yang, C.L.; Li, G.H. Design, synthesis, crystal structure, and herbicidal activity of novel pyrrolidine-2,4-dione derivatives incorporating an alkyl ether pharmacophore with natural tetramic acids as lead compounds. New J. Chem. 2021, 45, 5621–5630. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Gallone, T.; Garganese, F.; Caruso, A.G.; Amenduni, M.; Ippolito, A. Contamination of fresh and dried tomato by Alternaria toxins in southern Italy. Food Addit. Contam. A 2019, 36, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, C.; Savoy, M.C.; Baslé, Q.; Woo, P.M.; Ee, E.C.Y.; Mottier, P.; Bessaire, T. Levels of Alternaria toxins in selected food commodities including green coffee. Toxins 2020, 12, 595. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yin, C.; Qiang, S.; Zhou, F.; Dai, X. Chloroplastic oxidative burst induced by tenuazonic acid, a natural photosynthesis inhibitor, triggers cell necrosis in Eupatorium adenophorum Spreng. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Bjørk, P.K.; Rasmussen, S.A.; Gjetting, S.K.; Havshøi, N.W.; Petersen, T.I.; Ipsen, J.Ø.; Larsen, T.O.; Fuglsang, A.T. Tenuazonic acid from Stemphylium loti inhibits the plant plasma membrane H+-ATPase by a mechanism involving the C-terminal regulatory domain. New Phytol. 2020, 226, 770–784. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, M.; Gao, L.; Yang, Q.; Kalaji, H.M.; Qiang, S.; Strasser, R.J.; Chen, S. Tenuazonic acid-triggered cell death is the essential prerequisite for Alternaria alternata (Fr.) Keissler to infect successfully host Ageratina adenophora. Cells 2021, 10, 1010. [Google Scholar] [CrossRef]
- Piemontese, L.; Vitucci, G.; Catto, M.; Laghezza, A.; Perna, F.M.; Rullo, M.; Loiodice, F.; Capriati, V.; Solfrizzo, M. Natural scaffolds with multi-target activity for the potential treatment of Alzheimer’s disease. Molecules 2018, 23, 2182. [Google Scholar] [CrossRef] [PubMed]
- Poliseno, V.; Chaves, S.; Brunetti, L.; Loiodice, F.; Carrieri, A.; Laghezza, A.; Tortorella, P.; Magalhães, J.D.; Cardoso, S.M.; Santos, M.A.; et al. Derivatives of tenuazonic acid as potential new multi-target anti-Alzheimer’s disease agents. Biomolecules 2021, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Tirkey, N.N. Tenuazonic Acid: A potent mycotoxin. In Recent Trends in Human and Animal Mycology, 2nd ed.; Singh, K., Srivastava, N., Eds.; Springer Nature: Singapore, 2019; Chapter 8; pp. 203–211. [Google Scholar] [CrossRef]
- Jeschke, P. Status and outlook for acaricide and insecticide discovery. Pest Manag. Sci. 2021, 77, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Senthil-Nathan, S. A review of biopesticides and their mode of action against insect pests. In Environmental Sustainability; Thangavel, P., Sridevi, G., Eds.; Springer: New Delhi, India, 2015; pp. 49–63. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salimova, D.; Dalinova, A.; Dubovik, V.; Senderskiy, I.; Stepanycheva, E.; Tomilova, O.; Hu, Q.; Berestetskiy, A. Entomotoxic Activity of the Extracts from the Fungus, Alternaria tenuissima and Its Major Metabolite, Tenuazonic Acid. J. Fungi 2021, 7, 774. https://doi.org/10.3390/jof7090774
Salimova D, Dalinova A, Dubovik V, Senderskiy I, Stepanycheva E, Tomilova O, Hu Q, Berestetskiy A. Entomotoxic Activity of the Extracts from the Fungus, Alternaria tenuissima and Its Major Metabolite, Tenuazonic Acid. Journal of Fungi. 2021; 7(9):774. https://doi.org/10.3390/jof7090774
Chicago/Turabian StyleSalimova, Dilara, Anna Dalinova, Vsevolod Dubovik, Igor Senderskiy, Elena Stepanycheva, Oksana Tomilova, Qiongbo Hu, and Alexander Berestetskiy. 2021. "Entomotoxic Activity of the Extracts from the Fungus, Alternaria tenuissima and Its Major Metabolite, Tenuazonic Acid" Journal of Fungi 7, no. 9: 774. https://doi.org/10.3390/jof7090774
APA StyleSalimova, D., Dalinova, A., Dubovik, V., Senderskiy, I., Stepanycheva, E., Tomilova, O., Hu, Q., & Berestetskiy, A. (2021). Entomotoxic Activity of the Extracts from the Fungus, Alternaria tenuissima and Its Major Metabolite, Tenuazonic Acid. Journal of Fungi, 7(9), 774. https://doi.org/10.3390/jof7090774







