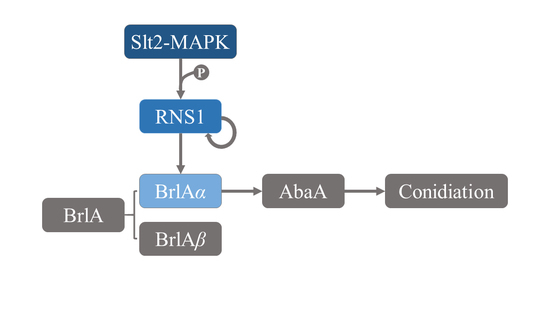
Slt2-MAPK/RNS1 Controls Conidiation via Direct Regulation of the Central Regulatory Pathway in the Fungus Metarhizium robertsii
Abstract
:1. Introduction
2. Material and Methods
2.1. Fungal and Bacteria Strains
2.2. Assays of Colony Growth and Conidiation
2.3. Assays of Tolerance to Abiotic Stresses
2.4. Yeast Two-Hybrid Assay
2.5. Co-IP Assays
2.6. Phos-Tag Assays
2.7. LC–MS/MS Analysis
2.8. EMSA, ChIP-qPCR and qRT-PCR Analysis
2.9. RACE
3. Results
3.1. RNS1 Regulates Conidiation in M. robertsii
3.2. RNS1 Positively Regulates the Central Regulatory Pathway for Conidiation
3.3. RNS1 Induces the Expression of the BrlAα Transcript
3.4. Slt2-MAPK Phosphorylates RNS1 during Conidiation
3.5. Phosphorylated RNS1 Upregulates Its Own Expression during Conidiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dagenais, T.R.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef] [Green Version]
- Ebbole, D.J. The conidium. In Cellular and Molecular Biology of Filamentous Fungi; ASM Press: Washington, DC, USA, 2010; pp. 577–590. [Google Scholar]
- Adams, T.H.; Wieser, J.K.; Yu, J.H. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 1998, 62, 35–54. [Google Scholar] [CrossRef] [Green Version]
- Guarro, J.; Gené, J.; Stchigel, A.M. Developments in fungal taxonomy. Clin. Microbiol. Rev. 1999, 12, 454–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boylan, M.T.; Mirabito, P.M.; Willett, C.E.; Zimmerman, C.R.; Timberlake, W.E. Isolation and physical characterization of three essential conidiation genes from Aspergillus nidulans. Mol. Cell. Biol. 1987, 7, 3113–3118. [Google Scholar] [PubMed]
- Park, H.S.; Yu, J.H. Genetic control of asexual sporulation in filamentous fungi. Curr. Opin. Microbiol. 2012, 15, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Adams, T. Complex control of the developmental regulatory locus brlA in Aspergillus nidulans. Mol. Genet. Genom. 2001, 266, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Prade, R.A.; Timberlake, W.E. The Aspergillus nidulans brlA regulatory locus encodes two functionally redundant polypeptides that are individually required for conidiophore development. EMBO J. 1993, 12, 2439–2447. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Navarro, J.; Greve, R.A.; Adams, T.H. Translational repression of brlA expression prevents premature development in Aspergillus. EMBO J. 1993, 12, 2449–2457. [Google Scholar] [CrossRef]
- Mooney, J.L.; Yager, L.N. Light is required for conidiation in Aspergillus nidulans. Genes Dev. 1990, 4, 1473–1482. [Google Scholar] [CrossRef] [Green Version]
- Chae, K.S.; Kim, J.H.; Choi, Y.; Han, D.M.; Jahng, K.Y. Isolation and characterization of a genomic DNA fragment complementing an nsdD mutation of Aspergillus nidulans. Mol. Cells 1995, 5, 146–150. [Google Scholar]
- Sun, X.; Yu, L.; Lan, N.; Wei, S.; Yu, Y.; Zhang, H.; Zhang, X.; Li, S. Analysis of the role of transcription factor VAD-5 in conidiation of Neurospora crassa. Fungal Genet. Biol. 2012, 49, 379–387. [Google Scholar] [CrossRef]
- Carmen, R.H.; Luis, M.C. Conidiation in Neurospora crassa: Vegetative reproduction by a model fungus. Int. Microbiol. 2019, 23, 97–105. [Google Scholar]
- Aguirre, J. Spatial and temporal controls of the Aspergillus brlA developmental regulatory gene. Mol. Microbiol. 1993, 8, 211–218. [Google Scholar] [CrossRef]
- Liu, K.; Dong, Y.; Wang, F.; Jiang, B.; Wang, M.; Fang, X. Regulation of cellulase expression, sporulation, and morphogenesis by velvet family proteins in Trichoderma reesei. Appl. Microbiol. Biotechnol. 2016, 100, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Mouhoumed, A.Z.; Tong, S.; Ying, S.; Feng, M. BrlA and AbaA Govern Virulence-Required Dimorphic Switch, Conidiation, and Pathogenicity in a Fungal Insect Pathogen. mSystems 2019, 4, e00140-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Lovett, B.; Fang, W. Genetically Engineering Entomopathogenic Fungi. Adv. Genet. 2016, 94, 137–163. [Google Scholar]
- Fang, W.; Azimzadeh, P.; St. Leger, R.J. Strain improvement of fungal insecticides for controlling insect pests and vector-borne diseases. Curr. Opin. Microbiol. 2012, 15, 232–238. [Google Scholar] [CrossRef]
- Roberts, D.W.; St. Leger, R.J. Metarhizium spp., cosmopolitan insect-pathogenic fungi: Mycological aspects. Adv. Appl. Microbiol. 2004, 54, 1–70. [Google Scholar]
- Guo, N.; Qian, Y.; Zhang, Q.; Chen, X.; Zeng, G.; Zhang, X.; Mi, W.; Xu, C.; St. Leger, R.J.; Fang, W. Alternative transcription start site selection in Mr-OPY2 controls lifestyle transitions in the fungus Metarhizium robertsii. Nat. Commun. 2017, 8, 1565. [Google Scholar] [CrossRef] [Green Version]
- Zeng, G.; Chen, X.; Zhang, X.; Zhang, Q.; Xu, C.; Mi, W.; Guo, N.; Zhao, H.; You, Y.; Dryburgh, F.J.; et al. Genome-wide identification of pathogenicity, conidiation and colony sectorization genes in Metarhizium robertsii. Environ. Microbiol. 2017, 19, 3896–3908. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Bidochka, M.J. Expression of genes involved in germination, conidiogenesis and pathogenesis in Metarhizium anisopliae using quantitative real-time RT-PCR. Mycol. Res. 2006, 110 Pt 10, 1165–1171. [Google Scholar] [CrossRef]
- Chen, X.; Xu, C.; Qian, Y.; Liu, R.; Zhang, Q.; Zeng, G. MAPK cascade mediated regulation of pathogenicity, conidiation and tolerance to abiotic stresses in the entomopathogenic fungus Metarhizium robertsii. Environ. Microbiol. 2016, 18, 1048–1062. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, X.; Qian, Y.; Chen, X.; Liu, R.; Zeng, G.; Zhao, H.; Fang, W. A high-throughput gene disruption methodology for the entomopathogenic fungus Metarhizium robertsii. PLoS ONE 2014, 9, e107657. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, X.; Fang, W. Increasing Pyruvate Concentration Enhances Conidial Thermotolerance in the Entomopathogenic Fungus Metarhizium robertsii. Front. Microbiol. 2019, 10, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.; Zhang, X.; Guo, N.; Fang, W. MrSt12 implicated in the regulation of transcription factor AFTF1 by Fus3-MAPK during cuticle penetration by the entomopathogenic fungus Metarhizium robertsii. Fungal Genet. Biol. 2019, 131, 103244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Meng, Y.; Huang, Y.; Zhang, D.; Fang, W. A novel cascade allows Metarhizium robertsii to distinguish cuticle and hemocoel microenvironments during infection of insects. PLoS Biol. 2021, 19, e3001360. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, X.; Tang, D.; Chen, J.; Fang, W. A fungal novel nitrogen and carbon metabolism regulatory cascade is implicated in cuticle penetration by the entomopathogenic fungus Metarhizium robertsii. mSystems 2021, 6, e00499-21. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mims, C.W.; Richardson, E.A.; Timberlake, W.E. Ultrastructural analysis of conidiophore development in the fungus Aspergillus nidulans using freeze-substitution. Protoplasma 1988, 44, 132–141. [Google Scholar] [CrossRef]
- Barton, L.M.; Prade, R.A. Inducible RNA Interference of brlA in Aspergillus nidulans. Eukaryot. Cell 2005, 7, 2004–2007. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; He, Z.; Zhang, S.; Keyhani, N.; Song, Y.; Yang, Z.; Jiang, Y.; Zhang, W.; Pei, Y.; Zhang, Y. Interplay between calcineurin and the Slt2 MAP-kinase in mediating cell wall integrity, conidiation and virulence in the insect fungal pathogen Beauveria bassiana. Fungal Genet. Biol. 2015, 83, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Barelli, L.; Moonjely, S.; Behie, S.W.; Bidochka, M.J. Fungi with multifunctional lifestyles: Endophytic insect pathogenic fungi. Plant Mol. Biol. 2016, 90, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Valiante, V.; Jain, R.; Heinekamp, T.; Brakhage, A.A. The MpkA MAP kinase module regulates cell wall integrity signaling and pyomelanin formation in Aspergillus fumigatus. Fungal Genet. Biol. 2009, 46, 909–918. [Google Scholar] [CrossRef] [PubMed]






| Name | Description | Ref |
|---|---|---|
| Fusion proteins | ||
| RNS1::FLAG | RNS1 tagged with FLAG | [28] |
| RNS1-DBD | A section of RNS1 containing DNA binding domain | [28] |
| RNS1S306A::FLAG | A mutated RNS1::FLAG with Ser-306 substituted to Ala | This study |
| Slt2::MYC | Slt2 tagged with MYC | This study |
| Plasmids | ||
| pPK2-bar-Ptef-MYC | Expression of a protein tagged with MYC | [28] |
| pPK2-sur-Ptef-FLAG | Expression of a protein tagged with FLAG | [28] |
| pPK2-sur-Ptef-RNS1-FLAG | Expression of the protein RNS1::FLAG | This study |
| pPK2-bar-Ptef-Slt2-MYC | Expression of the protein Slt2::MYC | This study |
| pPK2-sur-Ptef-RNS1S306A-FLAG | Expression of the protein RNS1S306A::FLAG | This study |
| Promoters | ||
| PRns1 | The promoter region (1724 bp upstream of the ORF) of the gene Rns1 | [28] |
| PRns1∆BM2 | The mutated PRns1 with all 7 nt in the BM2 motif substituted to A | [28] |
| PbrlA PAbaA | The promoter region (2000 bp upstream of the ORF) of the gene BrlAThe promoter region (1020 bp upstream of the ORF) of the gene AbaA | This study This study |
| Genomic clones | ||
| gRns1 | The genomic clone of the Rns1 gene | [28] |
| gRns1S306A | A mutated gRns1 with Ser-306 substituted to Ala in the RNS1 protein | This study |
| gRns1∆BM2 | A mutated gRns1 with all 7 nt in the BM2 motif in PRns1 substituted to A | [28] |
| Fungal strains | ||
| WT | The wild-type strain of M. robertsii ARSEF2575 | [28] |
| ∆Rns1 | The deletion mutant of the Rns1 gene | [28] |
| C-∆Rns1 | The complemented strain of the mutant ∆Rns1 | [28] |
| WT-FLAG | Expressing FLAG tag in the WT strain | [28] |
| WT-RNS1-FLAG | Expressing RNS1::FLAG in the WT strain | [28] |
| ∆Slt2-RNS1-FLAG | Expressing RNS1::FLAG in the mutant ∆Slt2 | This study |
| WT-RNS1S306A-FLAG | Expressing RNS1S306A::FLAG in the WT strain | This study |
| ∆Slt2-RNS1S306A-FLAG | Expressing RNS1S306A::FLAG in the mutant ∆Slt2 | This study |
| WT-PRns1-gfp | The gfp gene driven by PRns1 in the WT strain | [28] |
| WT-PRns1∆BM2-gfp | The gfp gene driven by PRns1∆BM2 in the WT strain | [28] |
| ∆Rns1-PRns1-gfp | The gfp gene driven by PRns1 in the mutant ∆Rns1 | [28] |
| ∆Slt2-PRns1-gfp | The gfp gene driven by PRns1 in the mutant ∆Slt2 | This study |
| RNS1-FLAG/Slt2-MYC | Expressing RNS1::FLAG and Slt2::MYC in the WT strain | This study |
| RNS1-FLAG/MYC | Expressing RNS1::FLAG and MYC tag in the WT strain | This study |
| ∆Rns1-gRns1S306A | Complementation of ∆Rns1 using the genomic clone gRns1 S306A | This study |
| ∆Rns1-gRns1∆BM2 | Complementation of ∆Rns1 using the genomic clone gRns1∆BM2 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Tang, X.; Bao, Y.; Zhang, M.; Tang, D.; Zhang, X.; Chen, X.; Fang, W. Slt2-MAPK/RNS1 Controls Conidiation via Direct Regulation of the Central Regulatory Pathway in the Fungus Metarhizium robertsii. J. Fungi 2022, 8, 26. https://doi.org/10.3390/jof8010026
Meng Y, Tang X, Bao Y, Zhang M, Tang D, Zhang X, Chen X, Fang W. Slt2-MAPK/RNS1 Controls Conidiation via Direct Regulation of the Central Regulatory Pathway in the Fungus Metarhizium robertsii. Journal of Fungi. 2022; 8(1):26. https://doi.org/10.3390/jof8010026
Chicago/Turabian StyleMeng, Yamin, Xingyuan Tang, Yuting Bao, Mingxiang Zhang, Dan Tang, Xing Zhang, Xiaoxuan Chen, and Weiguo Fang. 2022. "Slt2-MAPK/RNS1 Controls Conidiation via Direct Regulation of the Central Regulatory Pathway in the Fungus Metarhizium robertsii" Journal of Fungi 8, no. 1: 26. https://doi.org/10.3390/jof8010026