Development of PCR-Based Race-Specific Markers for Differentiation of Indian Fusarium oxysporum f. sp. cubense, the Causal Agent of Fusarium Wilt in Banana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Foc Races
2.2. DNA Extraction and Quantification
2.3. Sequence Alignment and Primer Design
2.4. Primer Design and Polymerase Chain Reaction (PCR) Conditions
2.5. Optimization of the Developed Marker
2.6. In Planta Detection of Foc Races
3. Results
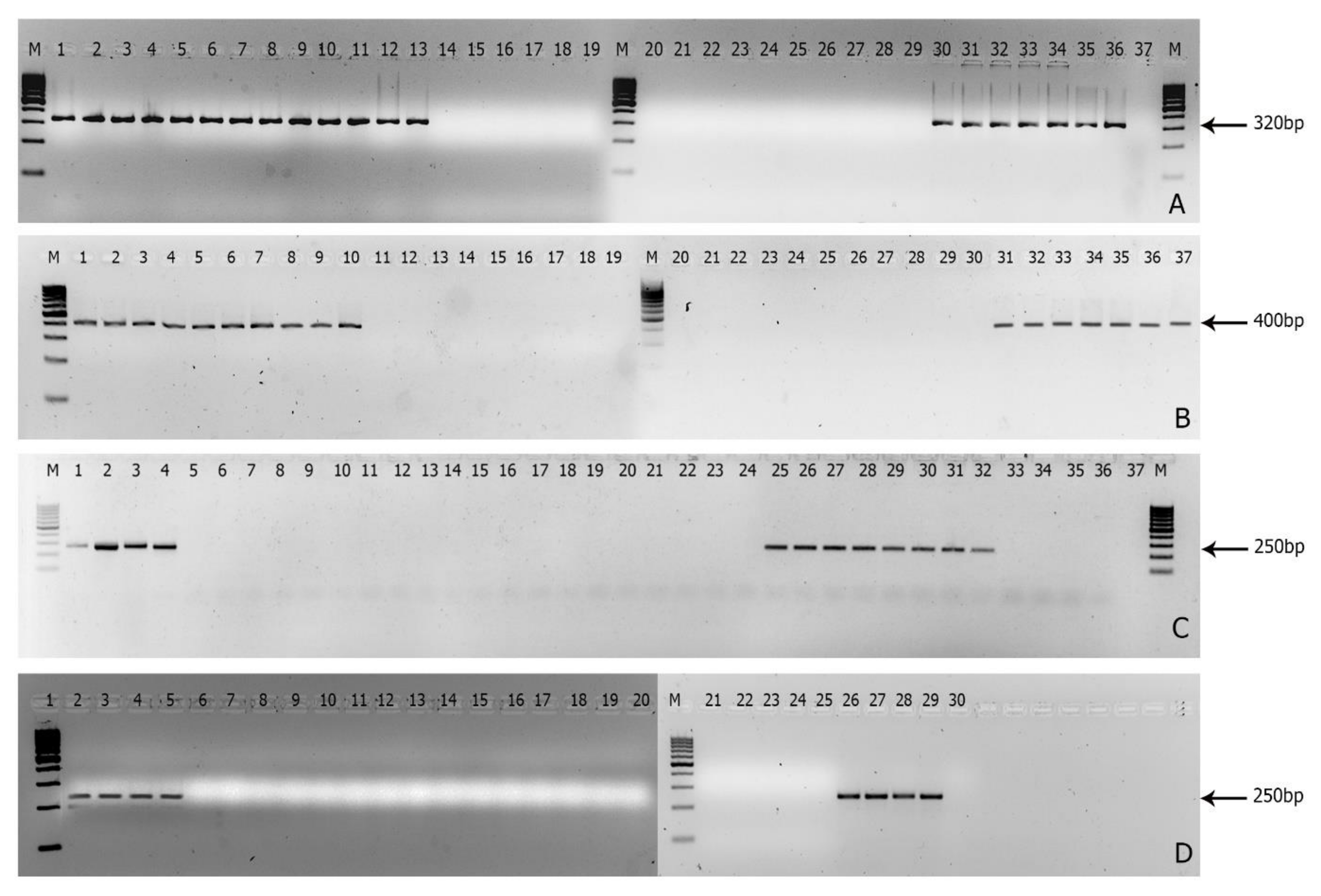
3.1. Screening of Race-Specific Markers and Its Specificity to Indian Foc
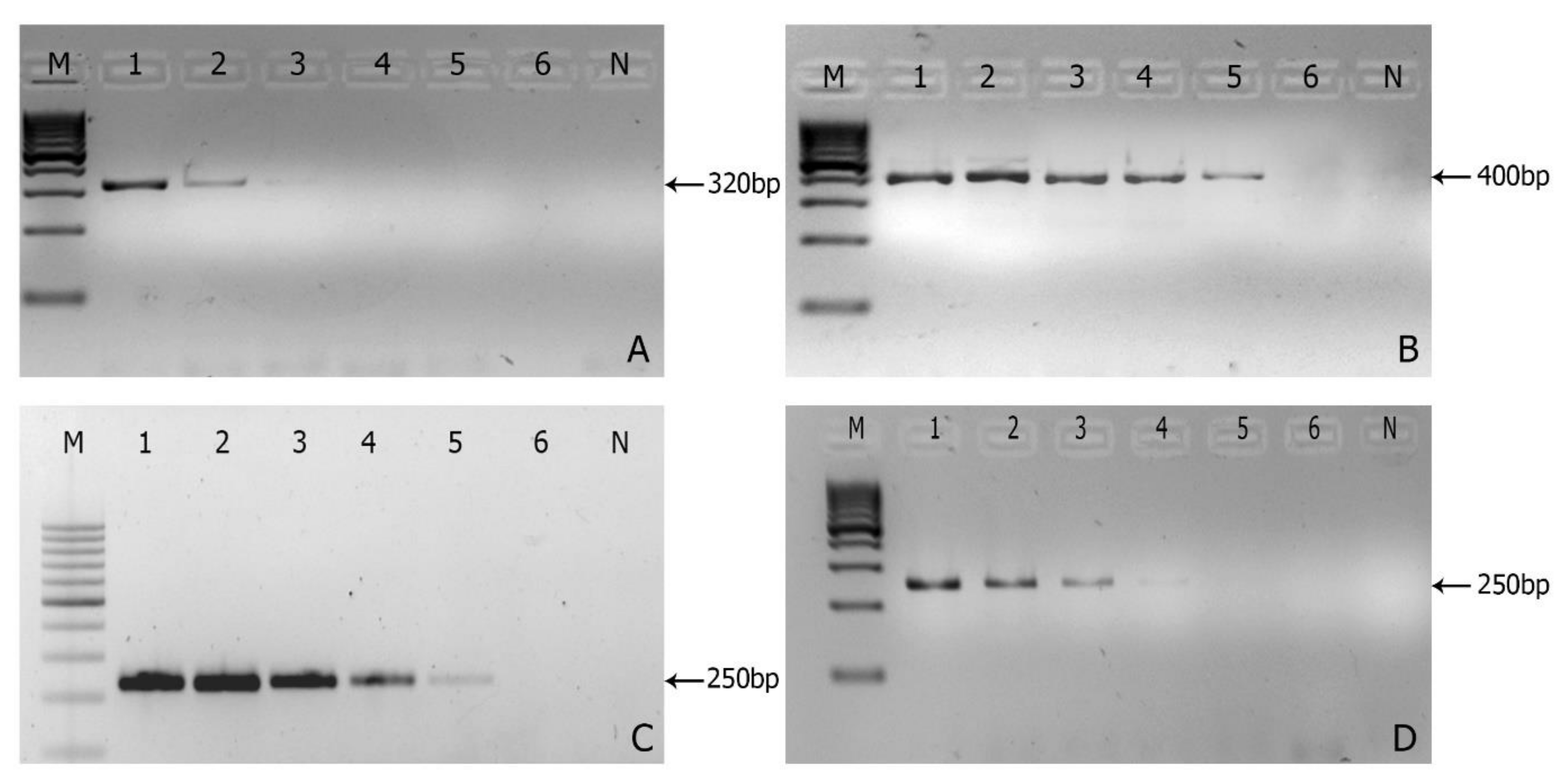
3.2. Sensitivity of the Markers
3.3. In Planta Detection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAOSTAT Database Collections. Available online: https://www.fao.org/faostat/en/#data/ (accessed on 27 September 2021).
- Stover, R.H. Fusarial Wilt (Panama Disease) of Bananas and Other Musa Species; Commonwealth Mycological Institute: Kew, UK, 1962. [Google Scholar]
- Stover, R.H. Fusarium wilt of banana: Some history and current status of the disease. In Fusarium Wilt of Banana; Ploetz, R.C., Ed.; American Phytopathology Society Press: St. Paul, MN, USA, 1990; Volume 1, p. 70. [Google Scholar]
- Thangavelu, R.; Loganathan, M.; Arthee, R.; Prabakaran, M.; Uma, S. Fusarium Wilt: A Threat to Banana Cultivation and Its Management; CABI: Wallingford, UK, 2020; Volume 15, pp. 1–24. [Google Scholar] [CrossRef]
- Altendorf, S. Banana Fusarium Wilt Tropical Race 4: A Mounting Threat to Global Banana Markets? FAO: Rome, Italy, 2019. [Google Scholar]
- Musapedia Banana-Producing Countries. Available online: https://www.promusa.org/Banana-producing+countries+portal#footnotefao (accessed on 27 September 2021).
- Mustaffa, M.M.; Thangavelu, R. Status of Fusarium wilt in India. In V International Symposium on Banana: ISHS-ProMusa Symposium on Global Perspectives on Asian Challenges 897; ISHS: Leuven, Belgium, 2011; Volume 897, pp. 323–330. [Google Scholar]
- Thangavelu, R.; Gopi, M.; Pushpakanth, P.; Loganathan, M.; Edwin Raj, E.; Marimuthu, N.; Prabakaran, M.; Uma, S. First Report of Fusarium oxysporum f. sp. cubense VCG 0125 and VCG 01220 of Race 1 Infecting Cavendish Bananas (Musa sp. AAA) in India. Plant Dis. 2021, 105, 1215. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, R.; Edwin Raj, E.; Muthukathan, G.; Murugan, L.; Uma, S. Draft Genome Resource of a Novel Virulent Fusarium oxysporum f. sp. cubense Race 1 Strain (VCG 0124) Infecting Cavendish (AAA) Group of Banana in India. Plant Dis. 2021, 105, 2708–2710. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, L.M.; Smith, L.J.; Aitken, E.A.B. Identification and characterization of nonpathogenic Fusarium oxysporum capable of increasing and decreasing Fusarium wilt severity. Mycol. Res. 2006, 110, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Diener, A.C.; Ausubel, F.M. Resistance to Fusarium oxysporum 1, a Dominant Arabidopsis Disease-Resistance Gene, Is Not Race Specific. Genetics 2005, 171, 305–321. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.; Toms, S. The contours of critical accounting. Crit. Perspect. Account. 2010, 21, 171–182. [Google Scholar] [CrossRef]
- Daniells, J.; Davis, D.; Peterson, R.; Pegg, K. Goldfinger: Not as resistant to Sigatoka/yellow Sigatoka as first thought. Infomusa 1995, 4, 6. [Google Scholar]
- Yergeau, E.; Filion, M.; Vujanovic, V.; St-Arnaud, M. A PCR-denaturing gradient gel electrophoresis approach to assess Fusarium diversity in asparagus. J. Microbiol. Methods 2005, 60, 143–154. [Google Scholar] [CrossRef]
- Jurado, M.; Vázquez, C.; Marín, S.; Sanchis, V.; Teresa González-Jaén, M. PCR-based strategy to detect contamination with mycotoxigenic Fusarium species in maize. Syst. Appl. Microbiol. 2006, 29, 681–689. [Google Scholar] [CrossRef]
- Kung’u, J.; Jefries, P. Races and virulence of Fusarium oxysporum f. sp. cubense on local banana cultivars in Kenya. Ann. Appl. Biol. 2001, 139, 343–349. [Google Scholar] [CrossRef]
- Thangavelu, R.; Mustaffa, M.M. First Report on the Occurrence of a Virulent Strain of Fusarium Wilt Pathogen (Race-1) Infecting Cavendish (AAA) Group of Bananas in India. Plant Dis. 2010, 94, 1379. [Google Scholar] [CrossRef]
- Li, B.; Du, J.; Lan, C.; Liu, P.; Weng, Q.; Chen, Q. Development of a loop-mediated isothermal amplification assay for rapid and sensitive detection of Fusarium oxysporum f. sp. cubense race 4. Eur. J. Plant Pathol. 2013, 135, 903–911. [Google Scholar] [CrossRef]
- Dita, M.A.; Waalwijk, C.; Buddenhagen, I.W.; Souza, J.T.; Kema, G.H.J. A molecular diagnostic for tropical race 4 of the banana fusarium wilt pathogen. Plant Pathol. 2010, 59, 348–357. [Google Scholar] [CrossRef]
- Aguayo, J.; Mostert, D.; Fourrier-Jeandel, C.; Cerf-Wendling, I.; Hostachy, B.; Viljoen, A.; Ioos, R. Development of a hydrolysis probe-based real-time assay for the detection of tropical strains of Fusarium oxysporum f. sp. cubense race 4. PLoS ONE 2017, 12, e0171767. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Chang, J.Y.; Liu, E.T.; Chao, C.P.; Huang, J.W.; Chang, P.F.L. Development of a molecular marker for specific detection of Fusarium oxysporum f. sp. cubense race 4. Eur. J. Plant Pathol. 2009, 123, 353–365. [Google Scholar] [CrossRef]
- García-Bastidas, F.; Ordóñez, N.; Konkol, J.; Al-Qasim, M.; Naser, Z.; Abdelwali, M.; Salem, N.; Waalwijk, C.; Ploetz, R.C.; Kema, G.H.J. First Report of Fusarium oxysporum f. sp. cubense Tropical Race 4 Associated with Panama Disease of Banana outside Southeast Asia. Plant Dis. 2014, 98, 694. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C. Fusarium wilt of banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordoñez, N.; García-Bastidas, F.; Laghari, H.B.; Akkary, M.Y.; Harfouche, E.N.; al Awar, B.N.; Kema, G.H.J. First Report of Fusarium oxysporum f. sp. cubense Tropical Race 4 Causing Panama Disease in Cavendish Bananas in Pakistan and Lebanon. Plant Dis. 2016, 100, 209. [Google Scholar] [CrossRef]
- Chittarath, K.; Mostert, D.; Crew, K.S.; Viljoen, A.; Kong, G.; Molina, A.B.; Thomas, J.E. First Report of Fusarium oxysporum f. sp. cubense Tropical Race 4 (VCG 01213/16) Associated with Cavendish Bananas in Laos. Plant Dis. 2018, 102, 449. [Google Scholar] [CrossRef]
- Hung, T.N.; Hung, N.Q.; Mostert, D.; Viljoen, A.; Chao, C.P.; Molina, A.B. First report of fusarium wilt on Cavendish bananas, caused by Fusarium oxysporum f. sp. cubense tropical race 4 (VCG 01213/16), in Vietnam. Plant Dis. 2018, 102, 448. [Google Scholar] [CrossRef]
- Zheng, S.-J.; García-Bastidas, F.A.; Li, X.; Zeng, L.; Bai, T.; Xu, S.; Yin, K.; Li, H.; Fu, G.; Yu, Y.; et al. New Geographical Insights of the Latest Expansion of Fusarium oxysporum f. sp. cubense Tropical Race 4 Into the Greater Mekong Subregion. Front. Plant Sci. 2018, 9, 457. [Google Scholar] [CrossRef] [Green Version]
- Thangavelu, R.; Mostert, D.; Gopi, M.; Devi, P.G.; Padmanaban, B.; Molina, A.B.; Viljoen, A. First detection of Fusarium oxysporum f. sp. cubense tropical race 4 (TR4) on Cavendish banana in India. Eur. J. Plant Pathol. 2019, 154, 777–786. [Google Scholar] [CrossRef]
- Lin, Y.H.; Su, C.C.; Chao, C.P.; Chen, C.Y.; Chang, C.J.; Huang, J.W.; Chang, P.F.L. A molecular diagnosis method using real-time PCR for quantification and detection of Fusarium oxysporum f. sp. cubense race 4. Eur. J. Plant Pathol. 2013, 135, 395–405. [Google Scholar] [CrossRef]
- Ordóñez, N.; Salacinas, M.; Mendes, O.; Seidl, M.F.; Meijer, H.J.G.; Schoen, C.D.; Kema, G.H.J. A loop-mediated isothermal amplification (LAMP) assay based on unique markers derived from genotyping by sequencing data for rapid in planta diagnosis of Panama disease caused by Tropical Race 4 in banana. Plant Pathol. 2019, 68, 1682–1693. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.L.; Sun, L.X.; Ruan, X.L.; Qiu, D.Y.; Chen, D.H.; Cai, X.Q.; Li, H.P. Development of a single-tube duplex real-time fluorescence method for the rapid quantitative detection of Fusarium oxysporum f. sp. cubense race 1 (FOC1) and race 4 (FOC4) using TaqMan probes. Crop Prot. 2015, 68, 27–35. [Google Scholar] [CrossRef]
- Li, M.; Shi, J.; Xie, X.; Leng, Y.; Wang, H.; Xi, P.; Zhou, J.; Zhong, S.; Jiang, Z. Identification and application of a unique genetic locus in diagnosis of Fusarium oxysporum f. sp. cubense tropical race 4. Can. J. Plant Pathol. 2013, 35, 482–493. [Google Scholar] [CrossRef]
- Mostert, D.; Molina, A.B.; Daniells, J.; Fourie, G.; Hermanto, C.; Chao, C.-P.; Fabregar, E.; Sinohin, V.G.; Masdek, N.; Thangavelu, R.; et al. The distribution and host range of the banana Fusarium wilt fungus, Fusarium oxysporum f. sp. cubense, in Asia. PLoS ONE 2017, 12, e0181630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czislowski, E.; Fraser-Smith, S.; Zander, M.; O’Neill, W.T.; Meldrum, R.A.; Tran-Nguyen, L.T.T.; Batley, J.; Aitken, E.A.B. Investigation of the diversity of effector genes in the banana pathogen, Fusarium oxysporum f. sp. cubense, reveals evidence of horizontal gene transfer. Mol. Plant Pathol. 2018, 19, 1155–1171. [Google Scholar] [CrossRef] [Green Version]
- Lievens, B.; van Baarlen, P.; Verreth, C.; van Kerckhove, S.; Rep, M.; Thomma, B.P.H.J. Evolutionary relationships between Fusarium oxysporum f. sp. lycopersici and F. oxysporum f. sp. radicis-lycopersici isolates inferred from mating type, elongation factor-1α and exopolygalacturonase sequences. Mycol. Res. 2009, 113, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- De Sain, M.; Rep, M. The role of pathogen-secreted proteins in fungal vascular wilt diseases. Int. J. Mol. Sci. 2015, 16, 23970–23993. [Google Scholar] [CrossRef]
- Van Dam, P.; Fokkens, L.; Schmidt, S.M.; Linmans, J.H.J.; Kistler, H.C.; Ma, L.-J.; Rep, M. Effector profiles distinguish formae speciales of Fusarium oxysporum. Environ. Microbiol. 2016, 18, 4087–4102. [Google Scholar] [CrossRef]
- Jangir, P.; Mehra, N.; Sharma, K.; Singh, N.; Rani, M.; Kapoor, R. Secreted in Xylem Genes: Drivers of Host Adaptation in Fusarium oxysporum. Front. Plant Sci. 2021, 12, 628611. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Ganapathi, T.R.; Mukherjee, P.K.; Bapat, V.A. MSI-99, a magainin analogue, imparts enhanced disease resistance in transgenic tobacco and banana. Planta 2003, 216, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Carvalhais, L.C.; Henderson, J.; Rincon-Florez, V.A.; O’Dwyer, C.; Czislowski, E.; Aitken, E.A.B.; Drenth, A. Molecular diagnostics of banana fusarium wilt targeting Secreted-in-xylem genes. Front. Plant Sci. 2019, 10, 547. [Google Scholar] [CrossRef]
- Li, C.; Mostert, G.; Zuo, C.; Beukes, I.; Yang, Q.; Sheng, O.; Kuang, R.; Wei, Y.; Hu, C.; Rose, L.; et al. Diversity and Distribution of the Banana Wilt Pathogen Fusarium oxysporum f. sp. cubense in China. Fungal Genom. Biol. 2013, 3, 6. [Google Scholar] [CrossRef]
- Raman, T.; Edwin Raj, E.; Muthukathan, G.; Loganathan, M.; Periyasamy, P.; Natesh, M.; Manivasakan, P.; Kotteeswaran, S.; Rajendran, S.; Subbaraya, U. Comparative Whole-Genome Sequence Analyses of Fusarium Wilt Pathogen (Foc R1, STR4 and TR4) Infecting Cavendish (AAA) Bananas in India, with a Special Emphasis on Pathogenicity Mechanisms. J. Fungi 2021, 7, 717. [Google Scholar] [CrossRef]
- Correll, J.C. Nitrate Nonutilizing Mutants of Fusarium oxysporum and Their Use in Vegetative Compatibility Tests. Phytopathology 1987, 77, 1640. [Google Scholar] [CrossRef]
- Li, C.; Shao, J.; Wang, Y.; Li, W.; Guo, D.; Yan, B.; Xia, Y.; Peng, M. Analysis of banana transcriptome and global gene expression profiles in banana roots in response to infection by race 1 and tropical race 4 of Fusarium oxysporum f. sp. cubense. BMC Genom. 2013, 14, 851. [Google Scholar] [CrossRef] [Green Version]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Biol. Report. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Winnenburg, R.; Baldwin, T.K.; Urban, M.; Rawlings, C.; Köhler, J.; Hammond-Kosack, K.E. PHI-base: A new database for pathogen-host interactions. Nucleic Acids Res. 2006, 34, D459–D464. [Google Scholar] [CrossRef]
- Winnenburg, R.; Urban, M.; Beacham, A.; Baldwin, T.K.; Holland, S.; Lindeberg, M.; Hansen, H.; Rawlings, C.; Hammond-Kosack, K.E.; Köhler, J. PHI-base update: Additions to the pathogen-host interaction database. Nucleic Acids Res. 2008, 36, D572–D576. [Google Scholar] [CrossRef]
- Schmidt, S.M.; Houterman, P.M.; Schreiver, I.; Ma, L.; Amyotte, S.; Chellappan, B.; Boeren, S.; Takken, F.L.W.; Rep, M. MITEs in the promoters of effector genes allow prediction of novel virulence genes in Fusarium oxysporum. BMC Genom. 2013, 14, 119. [Google Scholar] [CrossRef] [Green Version]
- Zuo, C.; Deng, G.; Li, B.; Huo, H.; Li, C.; Hu, C.; Kuang, R.; Yang, Q.; Dong, T.; Sheng, O.; et al. Germplasm screening of Musa spp. For resistance to Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4). Eur. J. Plant Pathol. 2018, 151, 723–734. [Google Scholar] [CrossRef]
- Fourie, G.; Steenkamp, E.T.; Gordon, T.R.; Viljoen, A. Evolutionary relationships among the Fusarium oxysporum f. sp. cubense vegetative compatibility groups. Appl. Environ. Microbiol. 2009, 75, 4770–4781. [Google Scholar] [CrossRef] [Green Version]
- Magdama, F.; Monserrate-Maggi, L.; Serrano, L.; Sosa, D.; Geiser, D.M.; Jiménez-Gasco, M.D. Comparative analysis uncovers the limitations of current molecular detection methods for Fusarium oxysporum f. sp. cubense Race 4 strains. PLoS ONE 2019, 14, e0222727. [Google Scholar] [CrossRef]
- Jelinski, N.A.; Broz, K.; Jonkers, W.; Ma, L.-J.; Kistler, H.C. Effector Gene Suites in Some Soil Isolates of Fusarium oxysporum Are Not Sufficient Predictors of Vascular Wilt in Tomato. Phytopathology 2017, 107, 842–851. [Google Scholar] [CrossRef]
- Rocha, L.O.; Laurence, M.H.; Ludowici, V.A.; Puno, V.I.; Lim, C.C.; Tesoriero, L.A.; Summerell, B.A.; Liew, E.C.Y. Putative effector genes detected in Fusarium oxysporum from natural ecosystems of Australia. Plant Pathol. 2016, 65, 914–929. [Google Scholar] [CrossRef] [Green Version]
- Fraser-Smith, S.; Czislowski, E.; Meldrum, R.A.; Zander, M.; O’Neill, W.; Balali, G.R.; Aitken, E.A.B. Sequence variation in the putative effector gene SIX8 facilitates molecular differentiation of Fusarium oxysporum f. sp. cubense. Plant Pathol. 2014, 63, 1044–1052. [Google Scholar] [CrossRef]
- Ayukawa, Y.; Hanyuda, S.; Fujita, N.; Komatsu, K.; Arie, T. Novel loop-mediated isothermal amplification (LAMP) assay with a universal QProbe can detect SNPs determining races in plant pathogenic fungi. Sci. Rep. 2017, 7, 4253. [Google Scholar] [CrossRef]
- Maymon, M.; Sela, N.; Shpatz, U.; Galpaz, N.; Freeman, S. The origin and current situation of Fusarium oxysporum f. sp. cubense tropical race 4 in Israel and the Middle East. Sci. Rep. 2020, 10, 1590. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, W.T.; Henderson, J.; Pattemore, J.A.; O’Dwyer, C.; Perry, S.; Beasley, D.R.; Tan, Y.P.; Smyth, A.L.; Goosem, C.H.; Thomson, K.M.; et al. Detection of Fusarium oxysporum f. sp. cubense tropical race 4 strain in northern Queensland. Australas. Plant Dis. Notes 2016, 11, 33. [Google Scholar] [CrossRef] [Green Version]
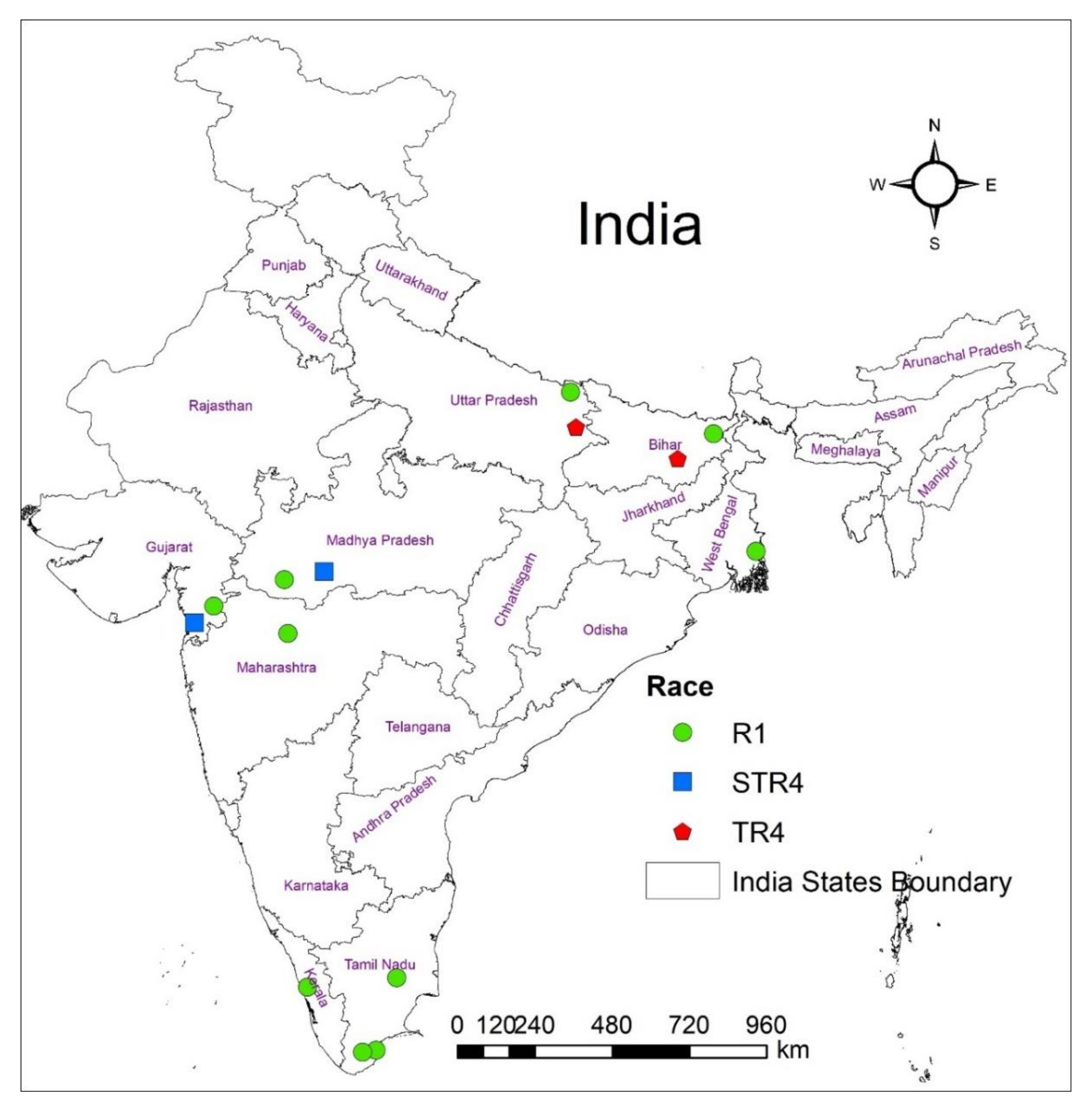

| S. No. | Isolate | Cultivar (Genome Status) | Geographical Locations | Race | VCG † | Pathogenicity on cv. Grande Naine (AAA) | PCR Diagnosis for | |||
|---|---|---|---|---|---|---|---|---|---|---|
| TR4 | STR4 | R4 | R1 | |||||||
| 1 | SU1 | Grande Naine (AAA) | Karjan, Gujarat | 1 | 01220 | + | - | - | - | + |
| 2 | SU2 | Grande Naine (AAA) | Karjan, Gujarat | 1 | 01220 | + | - | - | - | + |
| 3 | SU3 | Grande Naine (AAA) | Surat, Gujarat | 4 | 0120 | + | - | + | + | - |
| 4 | SU4 | Grande Naine (AAA) | Karjan, Gujarat | 1 | 01220 | + | - | - | - | + |
| 5 | SU5 | Grande Naine (AAA) | Karjan, Gujarat | 1 | 01220 | + | - | - | - | + |
| 6 | UP1 | Grande Naine (AAA) | Gurlirumkadava, UP | 1 | 0125 | + | - | - | - | + |
| 7 | UP2 | Grande Naine (AAA) | Pilavagam, UP | 1 | 01220 | + | - | - | - | + |
| 8 | UP3 | Grande Naine (AAA) | Kaleelnagar, UP | 1 | 0125 | + | - | - | - | + |
| 9 | UP4 | Grande Naine (AAA) | Puphyhee, UP | 4 | 01216 | + | + | - | + | - |
| 10 | UP5 | Grande Naine (AAA) | Sabeyadala, UP | 1 | 01220 | + | - | - | - | + |
| 11 | UP6 | Grande Naine (AAA) | Buchiyang, UP | 1 | 0125 | + | - | - | - | + |
| 12 | UP7 | Grande Naine (AAA) | Siswabazar,UP | 4 | 01216 | + | + | - | + | - |
| 13 | UP8 | Grande Naine (AAA) | Siswabazar,UP | 4 | 01216 | + | + | - | + | - |
| 14 | UP9 | Grande Naine (AAA) | Siswabazar,UP | 4 | 01216 | + | + | - | + | - |
| 15 | UP10 | Grande Naine (AAA) | Siswabazar,UP | 4 | 01216 | + | + | - | + | - |
| 16 | UP11 | Grande Naine (AAA) | Siswabazar,UP | 4 | 01216 | + | + | - | + | - |
| 17 | B1 | Robusta (AAA) | Kattihar, Bihar | 4 | 01213/16 | + | + | - | + | - |
| 18 | B2 | Robusta (AAA) | Kattihar, Bihar | 4 | 01213/16 | + | + | - | + | - |
| 19 | B3 | Robusta (AAA) | Kattihar, Bihar | 4 | 01213/16 | + | + | - | + | - |
| 20 | B4 | Grande Naine (AAA) | Kattihar, Bihar | 4 | 01213/16 | + | + | - | + | - |
| 21 | B5 | Grande Naine (AAA) | Katihar, Bihar | 4 | 01213/16 | + | + | - | + | - |
| 22 | B6 | Monthan (AAA) | Katihar, Bihar | 1 | 01220 | + | - | - | - | + |
| 23 | B7 | Monthan (AAA) | Katihar, Bihar | 1 | 01220 | + | - | - | - | + |
| 24 | B8 | Monthan (AAA) | Katihar, Bihar | 1 | 01220 | + | - | - | - | + |
| 25 | B9 | Malbhog (AAA) | Katihar Bihar | 1 | 0125 | + | - | - | - | + |
| 26 | B10 | Grande Naine (AAA) | Katihar Bihar | 4 | 01216 | + | + | - | + | - |
| 27 | B11 | Grande Naine (AAA) | Katihar, Bihar | 4 | 01216 | + | + | - | + | - |
| 28 | B12 | Grande Naine (AAA) | Purnia, Bihar | 4 | 01216 | + | + | - | + | - |
| 29 | B13 | Grande Naine (AAA) | Purnia, Bihar | 4 | 01216 | + | + | - | + | - |
| 30 | TN1 | Grande Naine (AAA) | Theni, TN | 1 | 0124 | + | - | - | - | + |
| 31 | TN2 | Grande Naine (AAA) | Theni, TN | 1 | 0125 | + | - | - | - | + |
| 32 | TN3 | Robuta (AAA) | Theni, TN | 1 | 01220 | + | - | - | - | + |
| 33 | TN4 | Grande Naine (AAA) | Theni, TN | 1 | 0125 | + | - | - | - | + |
| 34 | TN5 | Papoulou (AAB) | Theni, TN | 1 | 01220 | + | - | - | - | + |
| 35 | TN6 | Grande Naine (AAA) | Theni, TN | 1 | 0125 | + | - | - | - | + |
| 36 | TN7 | Grande Naine (AAA) | Theni, TN | 1 | 0125 | + | - | - | - | + |
| 37 | TN8 | Grande Naine (AAA) | Theni, TN | 1 | 0125 | + | - | - | - | + |
| 38 | TN9 | Grande Naine (AAA) | Theni, TN | 1 | 0124 | + | - | - | - | + |
| 39 | TN10 | Grande Naine (AAA) | Theni, TN | 1 | 0125 | + | - | - | - | + |
| 40 | TN11 | Grande Naine (AAA) | Theni, TN | 1 | 0125 | + | - | - | - | + |
| 41 | TN12 | Grande Naine (AAA) | Theni, TN | 1 | 0124 | + | - | - | - | + |
| 42 | TN13 | Grande Naine (AAA) | Theni. TN | 1 | 01220 | + | - | - | - | + |
| 43 | TN14 | Grande Naine (AAA) | Theni, TN | 1 | 0125 | + | - | - | - | + |
| 44 | TN15 | Karpuravalli (ABB) | Kattuputhur, TN | 1 | 0125 | + | - | - | - | + |
| 45 | TN16 | Rasthali (AAB) | Namakkal, TN | 1 | 0125 | + | - | - | - | + |
| 46 | TN17 | Rasthali (AAB) | Tirunelveli, TN | 1 | 0125 | + | - | - | - | + |
| 47 | TN18 | Monthan (ABB) | Tuticorin, TN | 1 | 01220 | + | - | - | - | + |
| 48 | TN19 | Ney Poovan (AB) | Tuticorin, TN | 1 | 01220 | + | - | - | - | + |
| 49 | TN20 | Grande Naine (AAA) | Coimbatore, TN | 1 | * | + | - | - | - | + |
| 50 | TN21 | Karpuravalli (ABB) | Coimbatore, TN | 1 | 01220 | + | - | - | - | + |
| 51 | TN22 | Rasthali (AAB) | Coimbatore, TN | 1 | * | + | - | - | - | + |
| 52 | TN23 | Ney Poovan (AB) | Coimbatore, TN | 1 | * | + | - | - | - | + |
| 53 | Ke1 | Rasthali (AAB) | Thrissur, Kerala | 1 | * | + | - | - | - | + |
| 54 | Ke2 | Rasthali (AAB) | Thrissur, Kerala | 1 | * | + | - | - | - | + |
| 55 | Ke3 | Rasthali (AAB) | Thrissur, Kerala | 1 | * | + | - | - | - | + |
| 56 | Ke4 | Big Ebanga (AAB) | Thrissur, Kerala | 1 | 0125 | + | - | - | - | + |
| 57 | Ke5 | Rasthali (AAB) | Thrissur, Kerala | 1 | * | + | - | - | - | + |
| 58 | MP1 | Grande Naine (AAA) | Burhanpur, MP | 4 | 0120 | + | - | + | + | - |
| 59 | MP2 | Grande Naine (AAA) | Burhanpur, MP | 4 | 0120 | + | - | + | + | - |
| 60 | MP3 | Grande Naine (AAA) | Burhanpur, MP | 4 | 0120 | + | - | + | + | - |
| 61 | MP4 | Grande Naine (AAA) | Burhanpur, MP | 1 | * | + | - | - | - | + |
| Primer Name | Gene ID | Primer sequence (5′ to 3′) | Length (bp) | Annealing Conditions |
|---|---|---|---|---|
| FocR1F FocR1R | XM_018394505.1 (Hypothetical protein) | TACCTCCTTGGTCGACAGGT CAGACTTCCAACGTCTCGGT | 320 bp | 62 °C for 30 s 30 cycles |
| FocR4F FocR4R | KF548063.1 (SIX8a) | CGCACTCTTACGTTGAGGAT TCCACGCAACACTAGCTACT | 400 bp | 66 °C for 30 s 30 cycles |
| FocTR4F FocTR4R | KX434998.1 (SIX1a) | TGATTTGCCGTGGAATGACA TGGTCTTGACACGACCCA | 250 bp | 65 °C for 30 s 30 cycles |
| FocSTR4F FocSTR4R | KM503196.1 (SIX7a) | GCGCAAGTAGTCTTGCTTCC ATTAAGCGGTTGGCGTATTG | 250 bp | 58 °C for 30 s 30 cycles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangavelu, R.; Edwinraj, E.; Gopi, M.; Pushpakanth, P.; Sharmila, K.; Prabaharan, M.; Loganathan, M.; Uma, S. Development of PCR-Based Race-Specific Markers for Differentiation of Indian Fusarium oxysporum f. sp. cubense, the Causal Agent of Fusarium Wilt in Banana. J. Fungi 2022, 8, 53. https://doi.org/10.3390/jof8010053
Thangavelu R, Edwinraj E, Gopi M, Pushpakanth P, Sharmila K, Prabaharan M, Loganathan M, Uma S. Development of PCR-Based Race-Specific Markers for Differentiation of Indian Fusarium oxysporum f. sp. cubense, the Causal Agent of Fusarium Wilt in Banana. Journal of Fungi. 2022; 8(1):53. https://doi.org/10.3390/jof8010053
Chicago/Turabian StyleThangavelu, Raman, Esack Edwinraj, Muthukathan Gopi, Periyasamy Pushpakanth, Kotteswaran Sharmila, Manivasakan Prabaharan, Murugan Loganathan, and Subbaraya Uma. 2022. "Development of PCR-Based Race-Specific Markers for Differentiation of Indian Fusarium oxysporum f. sp. cubense, the Causal Agent of Fusarium Wilt in Banana" Journal of Fungi 8, no. 1: 53. https://doi.org/10.3390/jof8010053
APA StyleThangavelu, R., Edwinraj, E., Gopi, M., Pushpakanth, P., Sharmila, K., Prabaharan, M., Loganathan, M., & Uma, S. (2022). Development of PCR-Based Race-Specific Markers for Differentiation of Indian Fusarium oxysporum f. sp. cubense, the Causal Agent of Fusarium Wilt in Banana. Journal of Fungi, 8(1), 53. https://doi.org/10.3390/jof8010053







