Characterization and Evaluation of Metarhizium spp. (Metsch.) Sorokin Isolates for Their Temperature Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates and Culture Conditions
2.2. Insect Culture
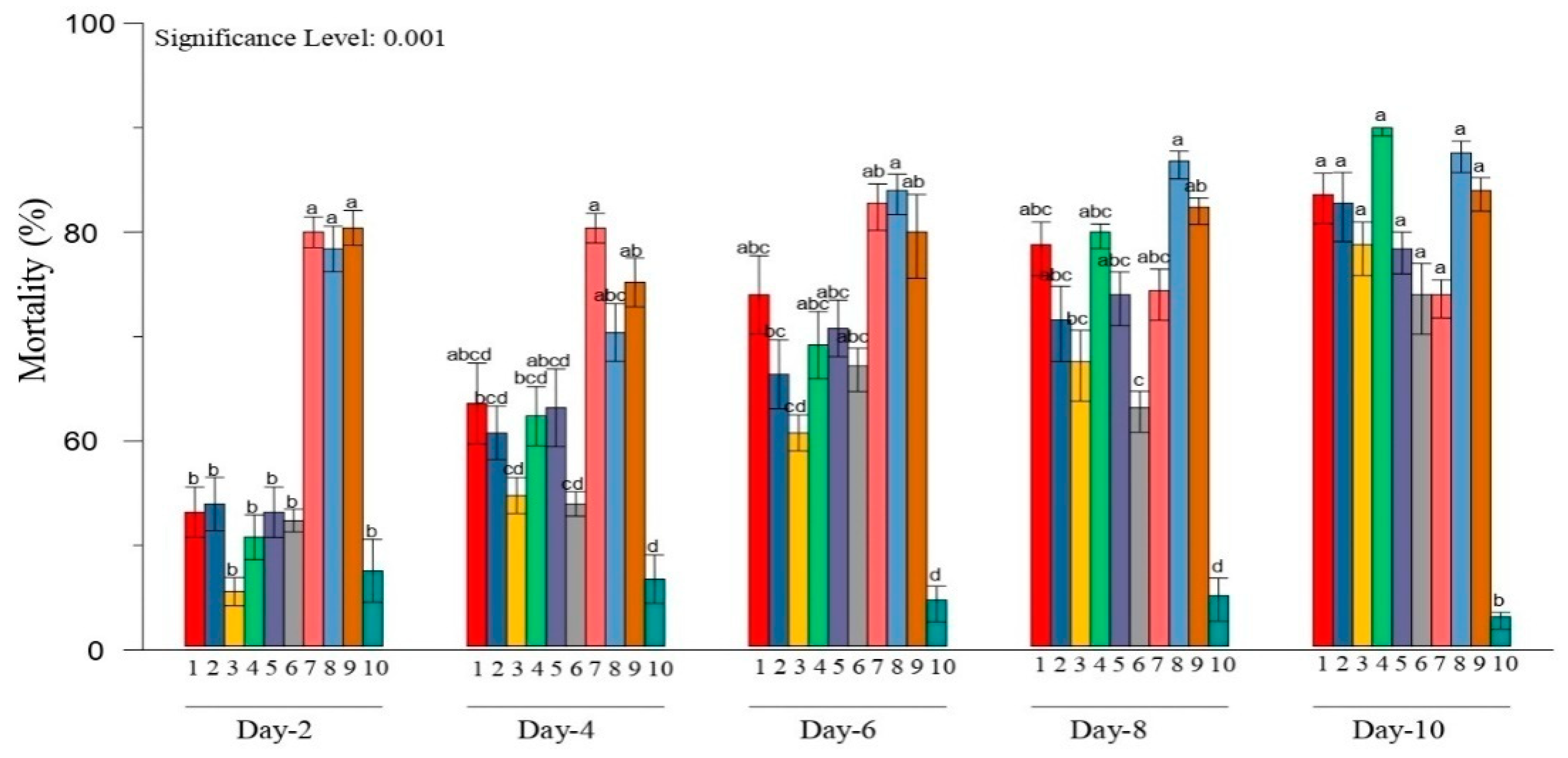
2.3. Bioassay of Metarhizium spp. against Hyblaea puera
2.4. Production of Cuticle-Degradation Enzymes
2.5. Estimation of Extracellular Enzyme Activity on H. puera
2.6. Temperature Optimization of Fungal Isolates Growth
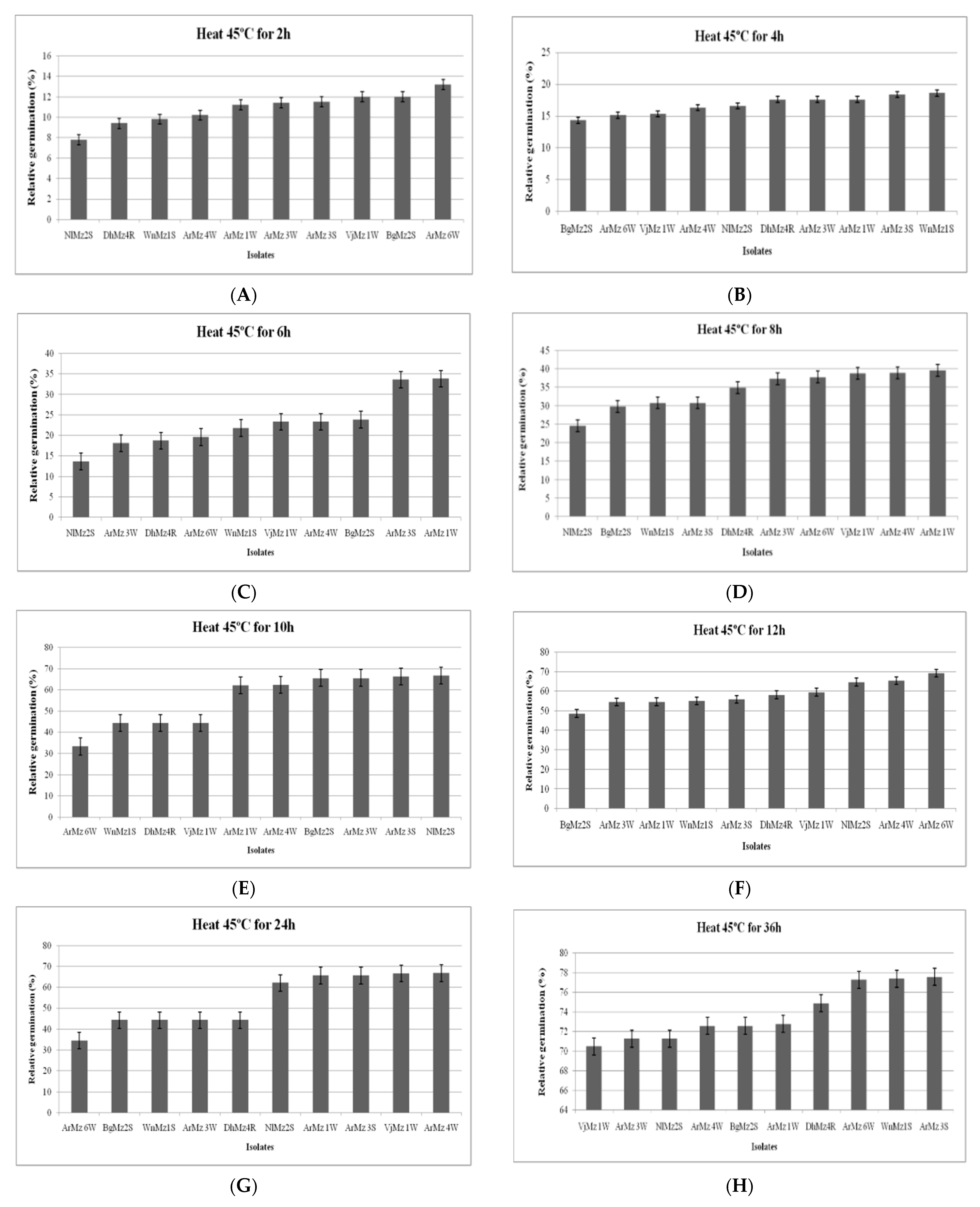
2.6.1. Effect of Heat Temperature on Relative Germination (RG)
2.6.2. Effect of Cold Temperature on Relative Germination (RG)
2.7. Analysis of Results
3. Results
3.1. Isolation and Identification of Fungi
3.2. Bioassay Tested Metarhiziumsp. Isolates against H. puera
3.3. Evaluation of Metarhizium sp. Isolates Effected Non-Extracellur Enzymes
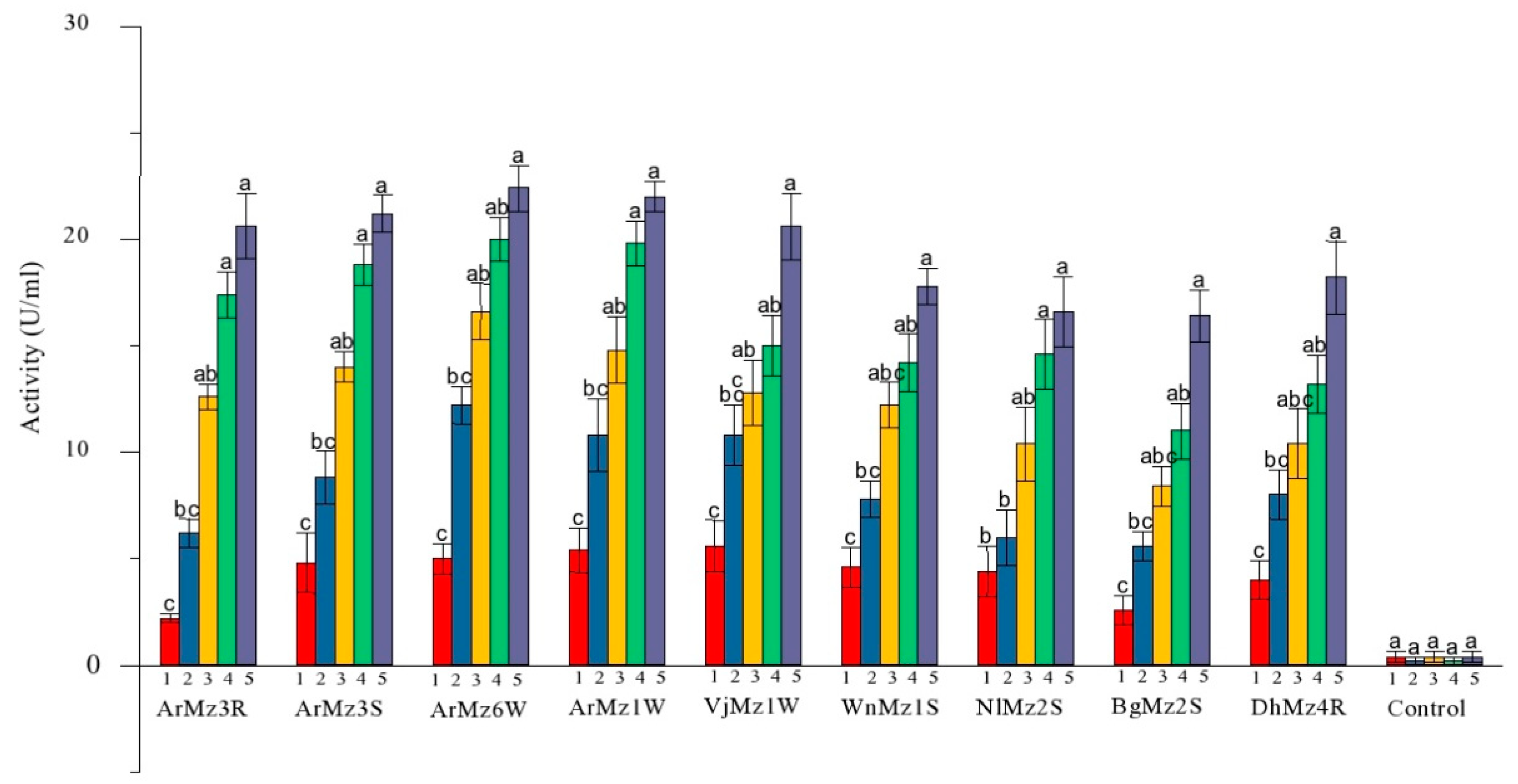
3.4. Extracellur Protease Enzymes Activity
3.5. Extracellur-Chitinases Enzymes Activity
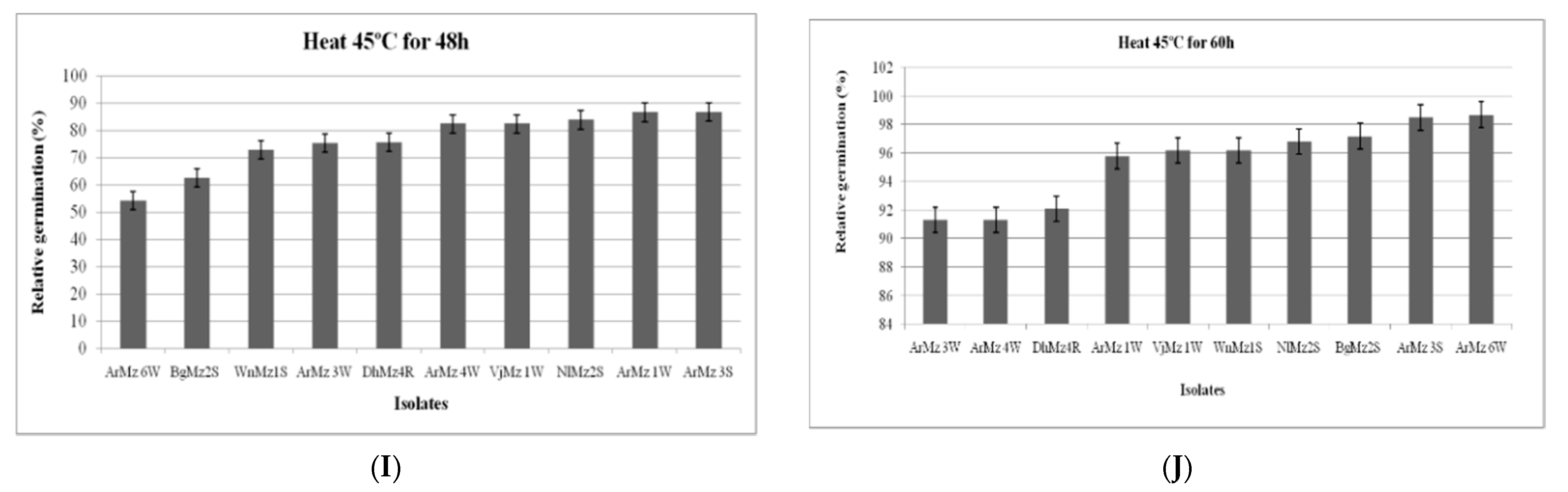
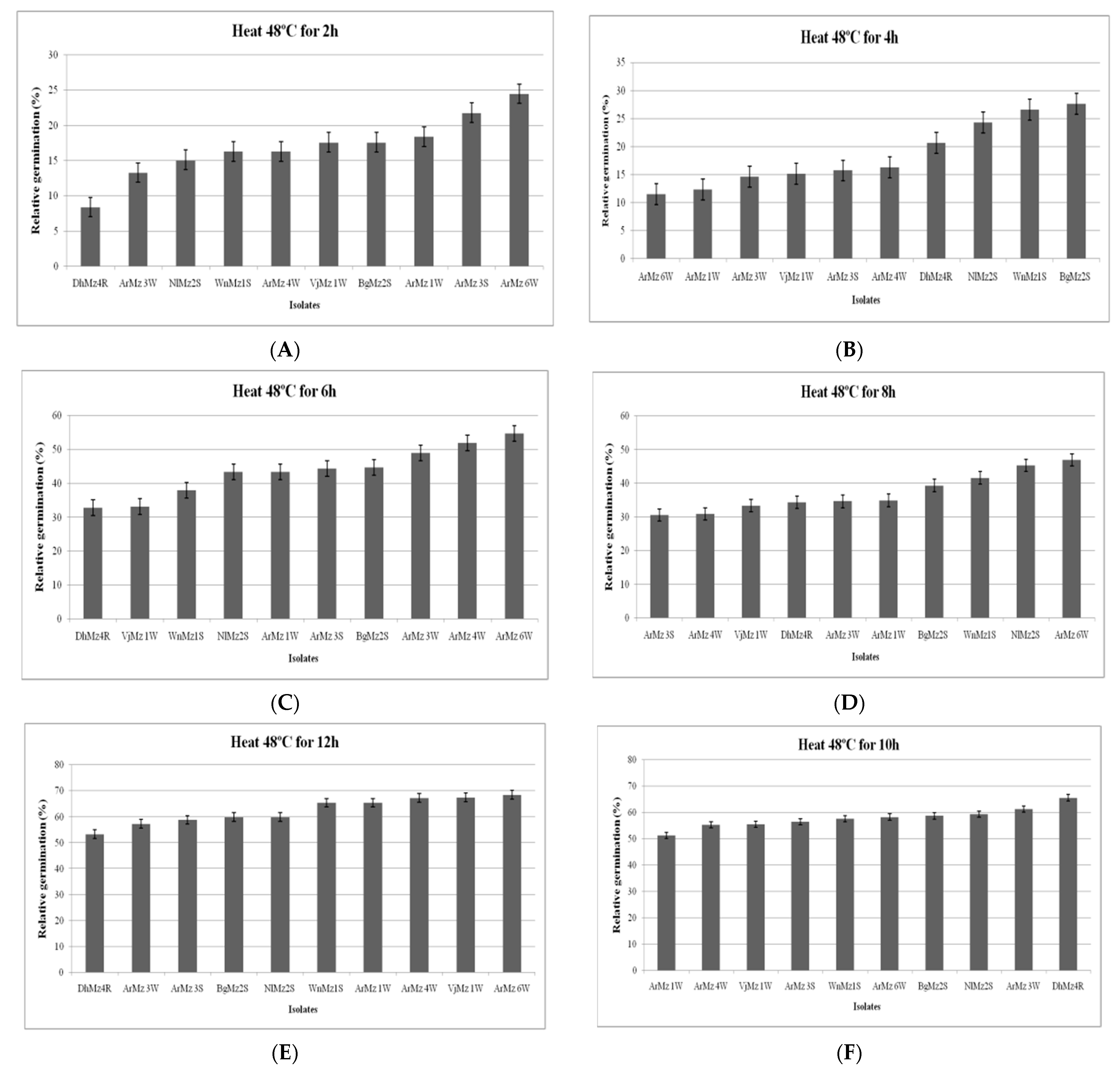
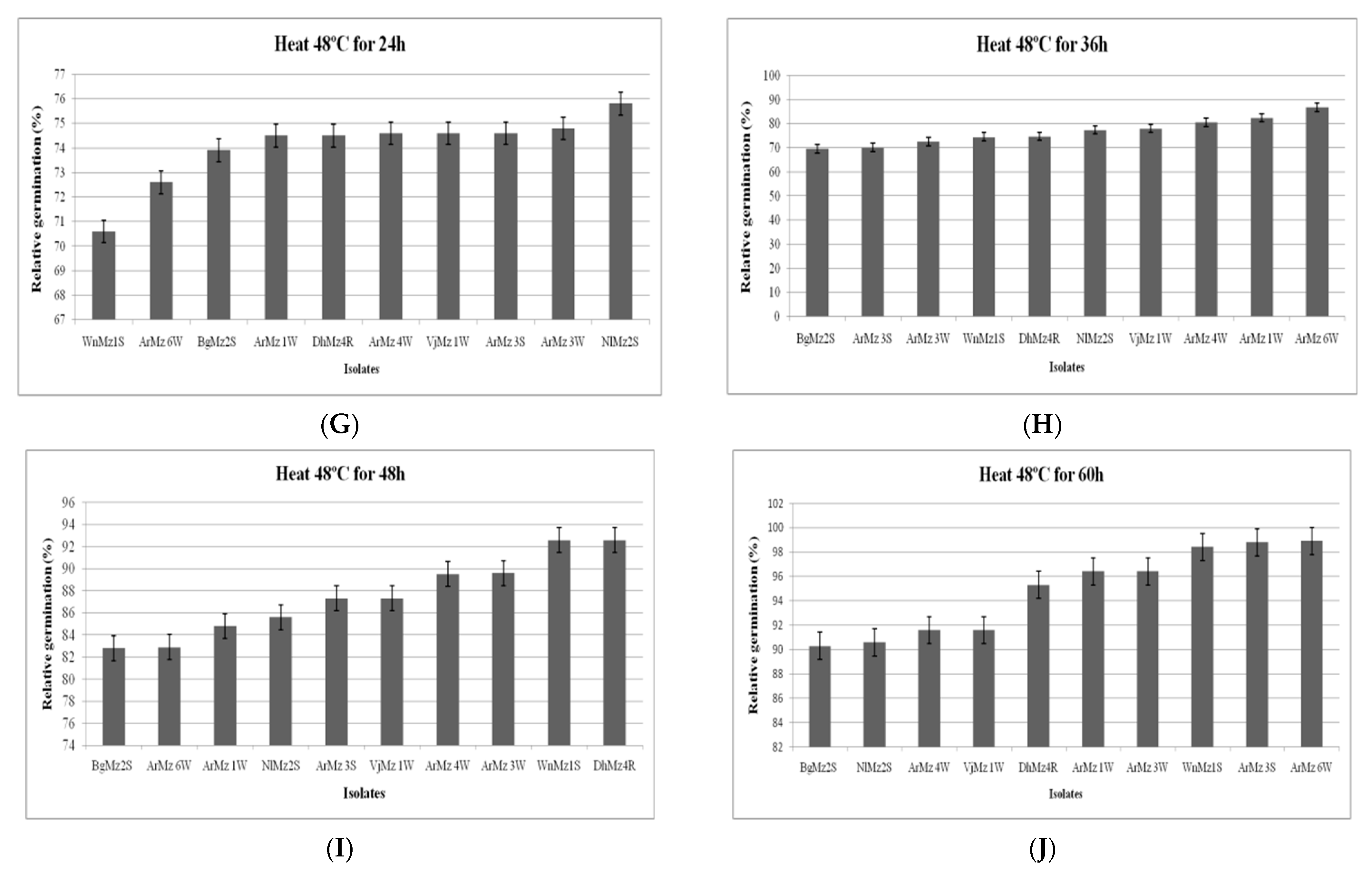
3.6. Heat Tolerance and Cold Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPF | Entomopathogenic Fungi |
| PDAY | Potato Dextrose Agar with Yeast extract |
| SDAY | Sabouraud Dextrose Agar with Yeast extract |
| ITS | ribosomal internal transcribed spacer |
| RPB1 | DNA-directed RNA polymerase II subunit |
References
- Metchnikoff, E. Diseases of the larva of the grain weevil. In Insects Harmful to Agriculture; Odessa Zemstvo Office: Odessa, Ukraine, 1879. (In Russian) [Google Scholar]
- Bischoff, J.F.; Rehner, S.A.; Humber, R.A. A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 2009, 101, 512–530. [Google Scholar] [CrossRef] [Green Version]
- Kepler, R.M.; Humber, R.A.; Bischoff, J.F.; Rehner, S.A. Clarification of generic and species boundaries for Metarhizium and related fungi through multigenephylogenetics. Mycologia 2014, 106, 811–829. [Google Scholar] [CrossRef]
- Chandra Teja, K.N.; Pand Rahman, S.J. Characterisation and evaluation of Metarhizium anisopliae (Metsch.) Sorokin strains for their temperature tolerance. Mycology 2016, 7, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Keyser, C.A.; Fernandes, É.K.K.; Rangel, D.E.N.; Roberts, D.W. Heat-induced post-stress growth delay: A biological trait of many Metarhizium isolates reducing biocontrol efficacy. J. Invertebr. Pathol. 2014, 120, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rangel, D.E.N.; Fernandes, É.K.K.; Dettenmaier, S.J.; Roberts, D.W. Thermotolerance of germlings and mycelium of the insect-pathogenic fungus Metarhizium spp. and mycelial recovery after heat stress. J. Basic. Microbiol. 2010, 50, 344–350. [Google Scholar] [CrossRef]
- Fernandes, E.K.K.; Keyser, C.A.; Chong, J.P.; Rangel, D.E.N.; Miller, M.P.; Roberts, D.W. Characterization of Metarhizium species and varieties based on molecular analysis, heat tolerance and cold activity. J. App. Microbiol. 2010, 108, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Hunt, V.L.; Zhong, W.; McClure, C.D.; Mlynski, D.T.; Duxbury, E.M.L.; Keith Charnley, A.; Priest, N.K. Cold-seeking behaviour mitigates reproductive losses from fungal infection in Drosophila. J. Anim. Ecol. 2016, 85, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, L.; Butt, T.M.; Jenkinson, P. Effect of Artificial Culture Media on Germination, Growth, Virulence and Surface Properties of the Entomopathogenic Hyphomycete (Metarhizium anisopliae). Myco. Res. 2002, 106, 705715. [Google Scholar] [CrossRef]
- Inglis, G.D.; Duke, G.M.; Kawchuk, L.M.; Goettel, M.S. Influence of oscillating temperatures on the competitive infection and colonization of the migratory grasshopper by Beauveria bassiana and Metarhizium flavoviride. Biol. Control. 1999, 14, 111–120. [Google Scholar] [CrossRef]
- Nishi, O.; Hasegawa, K.; Iiyama, K.; Yasunaga-Aoki, C.; Shimizu, S. Phylogenetic analysis of Metarhizium spp. isolated from soil in Japan. Appl. Entomol. Zool. 2011, 46, 301–309. [Google Scholar] [CrossRef]
- Lekime, M.; Focant, C.; Farnir, F.; Mignon, B.; Losson, B. Pathogenicity and Theromotolerance of Entomopathogenic Fungi for the Control of the Scab Mite, Psoroptesovis. Exp. Appl. Acarol. 2008, 46, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bidochka, M.J.; Kamp, A.M.; Lavender, T.M.; Dekoning, J.; De Croos, J.N. Habitat association in two genetic groups of the insect-pathogenic fungus Metarhizium anisopliae: Uncovering cryptic species. Appl. Environ. Microbiol. 2001, 67, 1335–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braga, G.U.L.; Flint, S.D.; Messias, C.L.; Anderson, A.J.; Roberts, D.W. Effect of UV-B on conidia and germlings of the entomopathogenic hyphomycete Metarhizium anisopliae. Mycol. Res. 2001, 105, 874–882. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Anderson, A.J.; Roberts, D.W. Evaluating physical and nutritional stress during mycelial growth as inducers of tolerance to heat and UV-B radiation in Metarhizium anisopliae conidia. Mycol. Res. 2008, 112, 1362–1372. [Google Scholar] [CrossRef]
- Liu, H.; Skinner, M.; Brownbridge, M.; Parker, B.L. Characterization of Beauveria bassiana and Metarhizium anisopliae isolates for management of tarnished plant bug, Lygus lineolaris (Hemiptera: Miridae). J. Invertebr. Pathol. 2003, 82, 139–147. [Google Scholar] [CrossRef]
- Hegedus, D.D.; Khachatourians, G.G. The impact of biotechnology on hyphomycetous fungal insect biocontrol agents. Biotechnol. Adv. 1995, 13, 455–490. [Google Scholar] [CrossRef]
- Krieger de Moraes, C.; Schrank, A.; Vainstein, M.H. Regulation of extracellular chitinases and proteases in the entomopathogen and acaricides Metarhizium anisopliae. Curr. Microbiol. 2003, 46, 205–210. [Google Scholar] [CrossRef]
- St. Leger, R.J.; Bidochka, M.J.; Roberts, D.W. Construction of an improved mycoinsecticide over expressing a toxic protease. Proc. Natl. Acad. Sci. USA 1996, 93, 6349–6354. [Google Scholar] [CrossRef] [Green Version]
- Nahar, P.; Ghormade, V.; Deshpande, M.V. The extracellular constitutive production of chitin deacetylase in Metarhizium anisopliae: Possible edge to entomopathogenic fungi in the biological control of insect pests. J. Invertebr. Pathol. 2004, 85, 80–88. [Google Scholar] [CrossRef]
- Revathi, N.; Ravikumar, G.; Kalaiselvi, M.; Gomathi, D.; Uma, C. Pathogenicity of three entomopathogenic fungi against Helicoverpa armigera. J. Plant Pathol. Microbiol. 2011, 2, 114. [Google Scholar] [CrossRef] [Green Version]
- Sapna Bai, N.; Remadevi, O.K.; Sasidharan, T.O.; Balachander, M.; Priyadarsanan, D.R. Cuticle degrading enzyme production by some isolates of the Entomopathogenic fungus, Metarhizium anisopliae (Metsch.). J. Bio-Sci. 2012, 20, 25–32. [Google Scholar]
- Ramanujam, B.; Balachander, M.; Roopa, G.; Rangeshwaran, R.; Karmakar, P. Chitinase activity and virulence of different isolates of Beauveria bassiana, Metarhizium anisopliae and Lecanicillium spp. J. Biol. Control 2011, 25, 223–228. [Google Scholar]
- Beeson, C.F.C. The Ecology and Control of the Forest Insects of India and the Neighboring Countries; Government of India: New Delhi, India, 1941; p. 767. [Google Scholar]
- Sudheendrakumar, V.V.; Mohammed Ali, I.M.; Varma, R.V. Nuclear Polyhedrosis Virus of the teak defoliator Hyblaeapuera. J. Invert. Pathol. 1988, 51, 307–308. [Google Scholar] [CrossRef]
- Senthil-Nathan, S.; Saehoon, K. Behavioral and physiological effects of Melia azedarach L. extract on the teak defoliator Hyblaeapuera Cramer (Lepidoptera: Hyblaeidae). Crop Prot. 2006, 25, 287–291. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. A Review of Bio Pesticides and Their Mode of Action against Insect Pests. In Environmental Sustainability-Role of Green Technologies; Springer: Dordrecht, The Netherlands, 2015; pp. 49–63. [Google Scholar]
- Velavan, V.; Sivakumar, G.; Rangeswaran, R.; Sasidharan, T.O.; Sundararaj, R. Metarhiziummajus and Metarhiziumrobertsii show enhanced activity against the coleopteran pests Holotricha serrata L. and Oryctes rhinoceros L. J. Biol. Control 2017, 31, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Veen, K.H.; Ferron, P. A selective medium for the isolation of Beauveria tenella and of Metarhiziumanisopliae. J. Invertebr. Pathol. 1966, 8, 268–269. [Google Scholar] [CrossRef]
- Riddell, R.W. Permanent stained mycological preparations obtained by slide culture. Mycologia 1950, 42, 265–270. [Google Scholar] [CrossRef]
- Senthil-Nathan, S.; Choi, M.Y.; Paik, C.H.; Seo, H.Y. Food consumption, utilization, and detoxification enzyme activity of the rice leaffolder larvae after treatment with Dysoxylum triterpenes. Pest. Biochem. Physiol. 2007, 88, 260–267. [Google Scholar] [CrossRef]
- Remadevi, O.K.; Sasidharan, T.O.; Balachander, M.; Sapna Bai, N. Prospects of developing Metarhizium based mycoinsecticide for pest management in forestry. J. Biopestic. 2010, 3, 38–41. [Google Scholar]
- Dhar, P.; Kaur, G. Cuticle-Degrading Proteases Produced by Metarhizium anisopliae and Their Induction in Different Media. Indian J. Microbiol. 2010, 50, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.A.; Ghormade, V.; Kulkarni, G.; Kapoor, M.; Chavan, S.B.; Rajendran, A.; Patil, S.K.; Shouche, Y.; Deshpande, M.V. Comparison of Metarhizium isolates for biocontrol of Helicoverpa armigera (Lepidoptera: Noctuidae) in chickpea. Biocontrol. Sci. Technol. 2008, 18, 809–828. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. Physiological and biochemical effect of Neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front. Physiol. 2013, 4, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, S.C. Modi Comparative Analysis of Chitinase Activity by Four Different Assays from Soil Born Actinomycetes. In Proceedings of the 4th International Conference on Multidisciplinary Research & Practice (4ICMRP) Gujarat University, Ahmedabad, India, 22 December 2017; pp. 185–190. [Google Scholar]
- Milner, R.J.; Huppatz, R.J.; Swaris, S.C. A new method for assessment of germination of Metarhizium conidia. J. Invertebr. Pathol. 1991, 57, 121–123. [Google Scholar] [CrossRef]
- Braga, G.U.L.; Flint, S.D.; Miller, C.D.; Anderson, A.J.; Roberts, D.W. Variability in response to UV-B among species and strains of Metarhizium isolated from sites at latitudes from 61N to 54 S. J. Invertebr. Pathol. 2001, 78, 98–108. [Google Scholar] [CrossRef]
- Velavan, V.; Rangeshwaran, R.; Sivakumar, G.; Sasidharan, T.O.; Sundararaj, R.; Kandan, A. Occurrence of Metarhizium spp. isolated from forest soils in South India and their potential in biological control of banana stem weevil Odoiporus longicollis Oliver. Egypt. J. Biol. Pest Control 2021, 31, 131–143. [Google Scholar] [CrossRef]
- Lee, W.W.; Shin, T.Y.; Bae, S.M.; Woo, S.D. Screening and evaluation of entomopathogenic fungi against the green peach aphid, Myzus persicae, using multiple tools. J. Asia Pac. Entomol. 2015, 18, 607–615. [Google Scholar] [CrossRef]
- Li, J.; Feng, M.G. Intra specific tolerance of Metarhizium anisopliae conidia to the upper thermal limits of summer with a description of a quantitative assay system. Mycol. Res. 2009, 113, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Rangel, D.E.N.; Anderson, A.J.; Roberts, D.W. Growth of Metarhizium anisopliae on non-preferred carbon sources yields conidia with increased UV-B tolerance. J. Invertebr. Pathol. 2006, 93, 127–134. [Google Scholar] [CrossRef]
- Vasantha-Srinivasan, P.; Karthi, S.; Chellappandian, M.; Ponsankar, A.; Thanigaivel, A.; Senthil-Nathan, S.; Chandramohan, D.; Ganesan, R. Aspergillus flavus (Link) toxins reduces the fitness of dengue vector Aedes aegypti (Linn.) and their non-target toxicity against aquatic predator. Microb. Pathog. 2019, 128, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Karthi, S.; Vasantha-Srinivasan, P.; Ganesan, R.; Ramasamy, V.; Senthil-Nathan, S.; Khater, H.F.; Radhakrishnan, N.; Amala, K.; Kim, T.J.; El-Sheikh, M.A.; et al. Target Activity of Isaria tenuipes (Hypocreales: Clavicipitaceae) Fungal Strains against Dengue Vector Aedes aegypti (Linn.) and Its Non-Target Activity Against Aquatic Predators. J. Fungi. 2020, 6, 196. [Google Scholar] [CrossRef]
- Nishi, O.; Iiyama, K.; Yasunaga-Aoki, C.; Shimizu, S. Comparison of the germination rates of Metarhizium spp. conidia from Japan at high and low temperatures. Lett. Appl. Microbiol. 2013, 57, 554–560. [Google Scholar] [CrossRef]
- Gurvinder, K.; Padmaja, V. Relationships among activities of extracellular enzyme production and virulence against Helicoverpa armigera in Beauveria bassiana. J. Basic Microbiol. 2009, 49, 264–274. [Google Scholar]
- Charnley, A.K.; St. Leger, R.J. The Role of Cuticle Degrading Enzymes in Fungal Pathogenesis of Insects. In The Fungal Spore and Disease Initiation in Plants and Animals; Plenum Publishing Co.: New York, NY, USA, 1991; pp. 267–286. [Google Scholar]
- St. Leger, R.J.; Cooper, R.M.; Charnley, A.K. Cuticle-degrading enzymes of entomopathogenic fungi: Cuticle degradationin vitro by enzymes from entomopathogens. J. Invertebr. Pathol. 1986, 47, 167–177. [Google Scholar] [CrossRef]
- Dhanapal, R.; Kumar, D.V.S.R.; Lakshmipathy, R.; Sandhya Rani, C.; Manoj Kumar, R. Pathogenicity testing of indigenous isolates of entomopathogenic fungus, Lecanicillium lecanii against tobacco caterpillar, Spodoptera litura. J. Experi. Zool. India 2019, 22, 753–756. [Google Scholar]
- Dhanapal, R.; Kumar, D.V.S.R.; Lakshmipathy, R.; Sandhya Rani, C.; Manoj Kumar, R. Exploration of indigenous strains of the green muscardine fungus from soils and their pathogenicity against the tobacco caterpillar, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2020, 30, 34. [Google Scholar] [CrossRef]
- Dhanapal, R.; Kumar, D.V.S.R.; Lakshmipathy, R.; Sandhya Rani, C.; Manoj Kumar, R. Isolation of indigenous strains of the white halo fungus as a biological control agent against 3rd instar larvae of tobacco caterpillar, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2020, 30, 89. [Google Scholar] [CrossRef]
- Karthi, S.; Vaideki, K.; Shivakumar, M.S.; Ponsankar, A.; Thanigaivel, A.; Chellappandian, M.; Vasantha-Srinivasan, P.; Chanthini, K.M.; Hunter, W.B.; Senthil-Nathan, S. Effect of on the mortality of Aspergillus flavus and activity of antioxidant enzymes of Spodoptera litura Fab. (Lepidoptera: Noctuidae) larvae. Pestic. Biochem. Physiol. 2018, 149, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Podder, D.; Mukherjee, A. An insight of anopheline larvicidal mechanism of Trichoderma asperellum (TaspSKGN2). Sci. Rep. 2021, 11, 16029. [Google Scholar] [CrossRef] [PubMed]
- Kirubakaran, S.A.; Senthil-Nathan, S.; Revathi, K.; Chandrasekaran, R.; Senthil-Nathan, S. Effect of oil-formulated Metarhiziumanisopliae and Beauveria bassiana against the rice leaffolder Cnaphalocrocis medinalis Guenée (Lepidoptera: Pyralidae). Arch. Phytopathol. Plant Prot. 2014, 47, 977–992. [Google Scholar] [CrossRef]
- Sangeetha, A.; Arivudainambi, S. Studies on the efficacy of bio—products in the management of teak skeletonizer Eutectonamachaeralis Walker (Pyralidae: Lepidoptera). J. Biopest. 2012, 5, 129–134. [Google Scholar]
- Loganathan, J.; David, P.M.M. Laboratory and field evaluation of Bacillus thuriengiensis Berliner products against the teak defoliator Hyblaea puera Cramer. Insect. Sci. Appl. 2000, 20, 61–65. [Google Scholar]
- Biji, C.P.; Sudheendrakumar, V.V.; Sajeev, T.V. Quantitative estimation of Hyblaea puera NPV production in three larval stages of the teak defoliator, Hyblaea puera (Cramer). J. Virol. Meth. 2006, 136, 78–82. [Google Scholar] [CrossRef]
- Narayanan, K. Insect defence: Its impact on microbial control of insect pests. Curr. Sci. 2004, 86, 800–814. [Google Scholar]
- Remadevi, O.K.; SapnaBai, N.; Sasidharan, T.O.; Balachander, M.; Dharmarajan, P. Attempts at controlling Teak Defoliator (Hyblaea puera Cramer, Lepidoptera, Hyblaeidae) with the entomopathogenic fungus, Metarhizium anisopliae (Metsch.): Laboratory, nursery and field trials. Int. J. Pest Manag. 2013, 59, 236–242. [Google Scholar] [CrossRef]
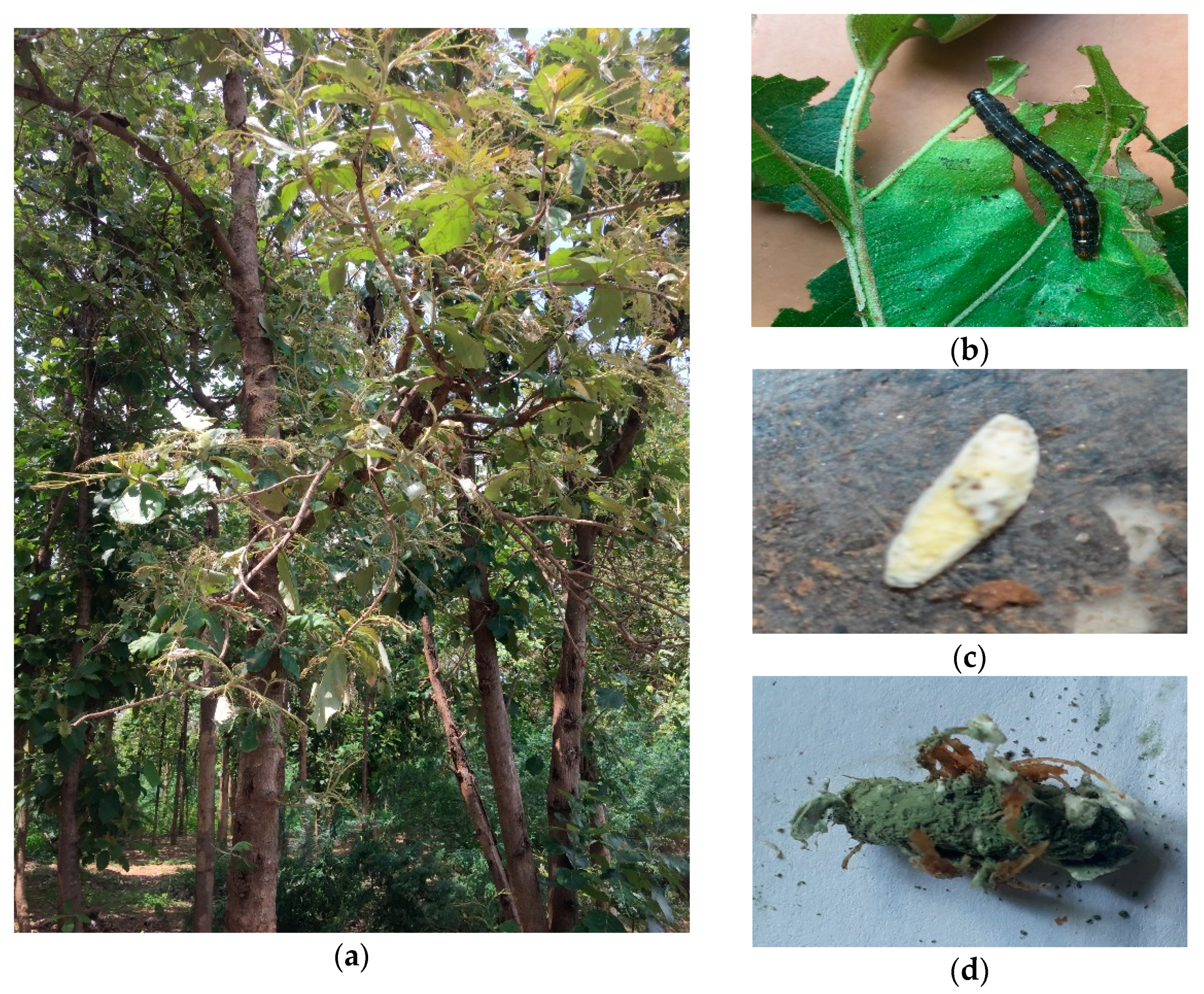


| Fungi | Type of Forest | Source | Region | Geographical Origin | Accession Number | |
|---|---|---|---|---|---|---|
| ITS | RPB1 | |||||
| M.robertsii ArMz3R | WEF | Insect(Protaetia aurichalcea) | Aralam | 11′99° N 75′76° E | KU983799 | KU680339 |
| M.robertsii ArMz3S | WEF | Insect(P. aurichalcea) | Aralam | 11′99° N 75′76° E | KU983775 | KU680335 |
| M.robertsii ArMz6W | WEF | Insect (P. aurichalcea) | Aralam | 11′99° N 75′76° E | KU983797 | KU680341 |
| M.quizhouense ArMz1W | WEF | Insect(Eutectona machaeralis) | Aralam | 11′99° N 75′76° E | KU870314 | Not yet |
| M.majus VjMz1W | WEF | Insect(Melolontha guttigera) | Coorg | 12′20° N 75′80° E | KU983771 | KU680342 |
| M.anisopliae WnMz1S | WEF | soil | Aralam | 11′60° N 76′08° E | KU983788 | KU680323 |
| M.anisopliae NlMz2S | MDF | soil | Bengaluru | 12′97° N 77′59° E | KU983785 | KU680325 |
| M.anisopliae BgMz2S | DDF | soil | Bengaluru | 12′80° N 77′57° E | KU983780 | KU680320 |
| M.anisopliae DhMz4R | MDF | soil | Palakkad | 10′77° N 76′65° E | KU983784 | KU680328 |
| Isolates Name | % Mortality of H. puera Due Protease | % Mortality of H. puera Due Chitinase | ||||||
|---|---|---|---|---|---|---|---|---|
| 103 * | 104 * | 105 * | 106 * | 103 * | 104 * | 105 * | 106 * | |
| M. robertsii ArMz3R | 8.499 ab | 13.400 ab | 24.121 a | 26.929 b | 9.862 b | 12.826 e | 19.355 de | 23.619 c |
| M. robertsii ArMz3S | 11.465 a | 14.771 a | 24.539 a | 27.807 a | 8.879 b | 19.594 a | 20.270 cd | 27.618 ab |
| M. robertsii ArMz6W | 8.702 ab | 13.285 ab | 22.481 b | 26.371 b | 8.350 b | 15.728 cd | 20.518 cd | 27.489 ab |
| M. quizhouense ArMz1W | 8.280 ab | 14.503 a | 24.850 a | 27.063 a | 13.241 a | 19.575 a | 23.607 ab | 28.214 a |
| M. majus VjMz1W | 5.476 bc | 13.067 ab | 22.160 bc | 27.489 a | 13.241 a | 19.234 ab | 25.673 a | 27.083 ab |
| M. anisopliae WnMz1S | 3.965 c | 13.341 ab | 21.524 cd | 27.943 a | 8.813 b | 12.109 ef | 24.057 ab | 26.648 b |
| M. anisopliae NlMz2S | 2.573 cd | 12.947 ab | 20.997 d | 27.618 a | 8.501 b | 14.019 de | 17.910 ef | 26.916 b |
| M. anisopliae BgMz2S | 2.639 cd | 12.419 abc | 19.333 e | 23.333 f | 8.879 b | 14.111 de | 19.965 de | 22.704 c |
| M. anisopliae DhMz4R | 4.151 c | 10.899 bc | 19.218 e | 25.284 e | 9.912 b | 17.039 bc | 22.265 bc | 26.772 b |
| Control | 0.573 d | 1.440 d | 6.957 f | 10.184 g | 1.606 c | 2.639 g | 4.151 g | 9.873 d |
| SE | 18.701 | 7.849 | 3.186 | 2.348 | 27.273 | 11.393 | 8.032 | 3.159 |
| CD(0.01) | 4.459 | 2.478 | 1.184 | 1.050 | 4.400 | 3.019 | 2.897 | 1.414 |
| CD(0.05) | 3.072 | 1.849 | 0.883 | 0.783 | 3.282 | 2.252 | 2.161 | 1.055 |
| Isolates | Proteases Enzyme Activity (U/mL) | Compatative Analysis | ||||
|---|---|---|---|---|---|---|
| Day-1 | Day-2 | Day-3 | Day-4 | Day-5 | ||
| ArMz3R | 2.2 ± 0.2 c | 6.2 ± 0.6 bc | 12.6 ± 0.6 ab | 17.4 ± 1.1 a | 20.6 ± 1.5 a | 20.6 ± 3.4 b |
| ArMz3S | 4.8 ± 1.3 c | 8.8 ± 1.2 bc | 14 ± 0.7 ab | 18.8 ± 0.9 a | 21.2 ± 0.8 a | 21.2 ± 1.7 ab |
| ArMz6W | 5 ± 0.7 c | 12.2 ± 0.8 bc | 16.6 ± 1.3 ab | 20 ± 1 ab | 22.4 ± 1.0 a | 22.4 ± 3.3 a |
| ArMz1W | 5.4 ± 1.0 c | 10.8 ± 1.7 bc | 14.8 ± 1.5 ab | 19.8 ± 1.0 a | 22 ± 0.7 a | 22.0 ± 2.4 a |
| VjMz1W | 5.6 ± 1.2 c | 10.8 ± 1.4 bc | 12.8 ± 1.5 abc | 15 ± 1.41 ab | 20.6 ± 1.5 a | 20.6 ± 3.1 b |
| WnMz1S | 4.6 ± 0.9 c | 7.8 ± 0.8 bc | 12.2 ± 1.0 abc | 14.2 ± 1.3 ab | 17.8 ± 0.8 a | 17.8 ± 3.1 cd |
| NlMz2S | 4.4 ± 1.2 b | 6 ± 1.3 b | 10.4 ± 1.7 ab | 14.6 ± 1.6 a | 16.6 ± 1.6 a | 16.6 ± 1.7 de |
| BgMz2S | 2.6 ± 0.6 c | 5.6 ± 0.6 bc | 8.4 ± 0.92 abc | 11 ± 1.30 ab | 16.4 ± 1.2 a | 16.4 ± 2.2 e |
| DhMz4R | 4 ± 0.8 c | 8 ± 1.1 bc | 10.4 ± 1.6 abc | 13.2 ± 1.3 ab | 18.2 ± 1.7 a | 18.2 ± 1.4 c |
| Control | 0.4 ± 0.2 a | 0.2 ± 0.2 a | 0.4 ± 0.24 a | 0.2 ± 0.2 a | 0.4 ± 0.2 a | 0.4 ± 0.9 f |
| CV | 5.714 | |||||
| CD(0.01) | 1.745 | |||||
| Isolates | Chitinases Enzyme Activity (U/mL) | Comparative Analysis | ||||
|---|---|---|---|---|---|---|
| Day-1 | Day-2 | Day-3 | Day-4 | Day-5 | ||
| ArMz3R | 3.2 ± 1.0 c | 6.6 ± 1.0 bc | 10.4 ± 1.2 abc | 15.2 ± 1.8 ab | 21.4 ± 1.0 a | 21.4 ± 2.4 a |
| ArMz3S | 5.6 ± 1.2 b | 8 ± 2.5 b | 12 ± 2.1 ab | 16.4 ± 2.1 ab | 21.4 ± 1.6 a | 21.4 ± 3.6 a |
| ArMz6W | 4.6 ± 1.0 b | 6.4 ± 1.3 b | 8.8 ± 1.0 b | 14 ± 1.4 ab | 21.2 ± 1.6 a | 21.2 ± 3.7 ab |
| VjMz1W | 5.6 ± 1.2 a | 9.6 ± 2.1 a | 11.8 ± 2.0 a | 14.2 ± 2.2 a | 16.4 ± 2.1 a | 16.4 ± 4.8 c |
| ArMz1W | 3.2 ± 0.8 c | 8.6 ± 2.1 bc | 14.6 ± 2.0 abc | 16.6 ± 2.2 ab | 21.4 ± 1.6 a | 21.4 ± 3.6 a |
| WnMz1S | 6.4 ± 1.2 b | 12 ± 2.1 ab | 16.8 ± 1.8 ab | 19.2 ± 1.2 a | 21.6 ± 1.2 a | 21.6 ± 2.7 a |
| NlMz2S | 6.8 ± 1.5 b | 8.6 ± 2.4 b | 12.6 ± 2.6 ab | 20.2 ± 2.5 a | 21.2 ± 1.4 a | 20.2 ± 3.3 ab |
| BgMz2S | 4.4 ± 1.0 b | 5.2 ± 1.1 b | 7 ± 1.3 b | 15.8 ± 1.8 ab | 20.2 ± 1.4 a | 20.2 ± 3.3 ab |
| DhMz4R | 4.4 ± 1.1 b | 7.4 ± 1.2 b | 9.6 ± 1.2 ab | 13.8 ± 1.8 ab | 19.2 ± 1.2 a | 19.2 ± 2.9 b |
| Control | 0.4 ± 0.2 a | 0.2 ± 0.2 a | 0.4 ± 0.2 a | 0.2 ± 0.2 a | 0.4 ± 0.2 a | 0.4 ± 0.8 d |
| CV | 8.429 | |||||
| CD(0.01) | 2.684 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velavan, V.; Dhanapal, R.; Ramkumar, G.; Karthi, S.; Senthil-Nathan, S.; Ndomba, O.A.; Kweka, E.J. Characterization and Evaluation of Metarhizium spp. (Metsch.) Sorokin Isolates for Their Temperature Tolerance. J. Fungi 2022, 8, 68. https://doi.org/10.3390/jof8010068
Velavan V, Dhanapal R, Ramkumar G, Karthi S, Senthil-Nathan S, Ndomba OA, Kweka EJ. Characterization and Evaluation of Metarhizium spp. (Metsch.) Sorokin Isolates for Their Temperature Tolerance. Journal of Fungi. 2022; 8(1):68. https://doi.org/10.3390/jof8010068
Chicago/Turabian StyleVelavan, Viswakethu, Rajendran Dhanapal, Govindaraju Ramkumar, Sengodan Karthi, Sengottayan Senthil-Nathan, Osmund A. Ndomba, and Eliningaya J. Kweka. 2022. "Characterization and Evaluation of Metarhizium spp. (Metsch.) Sorokin Isolates for Their Temperature Tolerance" Journal of Fungi 8, no. 1: 68. https://doi.org/10.3390/jof8010068
APA StyleVelavan, V., Dhanapal, R., Ramkumar, G., Karthi, S., Senthil-Nathan, S., Ndomba, O. A., & Kweka, E. J. (2022). Characterization and Evaluation of Metarhizium spp. (Metsch.) Sorokin Isolates for Their Temperature Tolerance. Journal of Fungi, 8(1), 68. https://doi.org/10.3390/jof8010068









