Antifungal Combinations against Candida Species: From Bench to Bedside
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. In Vitro Studies and Experimental Models of Infection
3.1.1. Azoles plus Echinocandins
3.1.2. Polyenes plus Echinocandins
3.1.3. Polyenes plus Azoles
3.1.4. 5-FC Combination Therapies
3.1.5. Other Combinations
3.2. Clinical Cases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2016, 374, 794–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, K.; Schorr, C.; Reboli, A.C.; Zanotti, S.; Tsigrelis, C. Incidence and mortality of sepsis, severe sepsis, and septic shock in intensive care unit patients with candidemia. Infect. Dis. 2015, 47, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Brescini, L.; Mazzanti, S.; Morroni, G.; Pallotta, F.; Masucci, A.; Orsetti, E.; Montalti, R.; Barchiesi, F. Candidemia in Internal Medicine: Facing the New Challenge. Mycopathologia 2022, 187, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers. 2018, 4, 18026. [Google Scholar] [CrossRef]
- Brescini, L.; Mazzanti, S.; Orsetti, E.; Morroni, G.; Masucci, A.; Pocognoli, A.; Barchiesi, F. Species distribution and antifungal susceptibilities of bloodstream Candida isolates: A nine-years single center survey. J. Chemother. 2020, 32, 244–250. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Mota Fernandes, C.; Dasilva, D.; Haranahalli, K.; McCarthy, J.B.; Mallamo, J.; Ojima, I.; Del Poeta, M. The Future of Antifungal Drug Therapy: Novel Compounds and Targets. Antimicrob. Agents Chemother. 2021, 65, e01719-20. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [Green Version]
- Ullmann, A.J.; Cornely, O.A.; Donnelly, J.P.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Developing European guidelines in clinical microbiology and infectious diseases. Clin. Microbiol. Infect. 2012, 18 (Suppl. S7), 1–8. [Google Scholar] [CrossRef] [Green Version]
- CLSI. M27, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2017. [Google Scholar]
- EUCAST. E Def 7.3.2. Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts. Available online: https://www.eucast.org/astoffungi/methodsinantifungalsusceptibilitytesting/susceptibility_testing_of_yeasts (accessed on 22 November 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Heyn, K.; Tredup, A.; Salvenmoser, S.; Müller, F.M. Effect of voriconazole combined with micafungin against Candida, Aspergillus, and Scedosporium spp. and Fusarium solani. Antimicrob. Agents Chemother. 2005, 49, 5157–5159. [Google Scholar] [CrossRef] [Green Version]
- Karlowsky, J.A.; Hoban, D.J.; Zhanel, G.G.; Goldstein, B.P. In vitro interactions of anidulafungin with azole antifungals, amphotericin B and 5-fluorocytosine against Candida species. Int. J. Antimicrob. Agents 2006, 27, 174–177. [Google Scholar] [CrossRef]
- Siopi, M.; Siafakas, N.; Zerva, L.; Meletiadis, J. Evaluation of paper gradient concentration strips for antifungal combination testing of Candida spp. Mycoses 2015, 58, 679–687. [Google Scholar] [CrossRef]
- Caballero, U.; Kim, S.; Eraso, E.; Quindós, G.; Vozmediano, V.; Schmidt, S.; Jauregizar, N. In vitro Synergistic Interactions of Isavuconazole and Echinocandins against Candida auris. Antibiotics 2021, 10, 355. [Google Scholar] [CrossRef]
- Katragkou, A.; McCarthy, M.; Meletiadis, J.; Hussain, K.; Moradi, P.W.; Strauss, G.E.; Myint, K.L.; Zaw, M.H.; Kovanda, L.L.; Petraitiene, R.; et al. In vitro combination therapy with isavuconazole against Candida spp. Med. Mycol. 2017, 55, 859–868. [Google Scholar] [CrossRef]
- Graybill, J.R.; Bocanegra, R.; Najvar, L.K.; Hernandez, S.; Larsen, R.A. Addition of caspofungin to fluconazole does not improve outcome in murine candidiasis. Antimicrob. Agents Chemother. 2003, 47, 2373–2375. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.L.; Lehman, V.N.; Averette, A.F.; Perfect, J.R.; Heitman, J. Posaconazole exhibits in vitro and in vivo synergistic antifungal activity with caspofungin or FK506 against Candida albicans. PLoS ONE 2013, 8, e57672. [Google Scholar] [CrossRef] [Green Version]
- Barchiesi, F.; Spreghini, E.; Fothergill, A.W.; Arzeni, D.; Greganti, G.; Giannini, D.; Rinaldi, M.G.; Scalise, G. Caspofungin in combination with amphotericin B against Candida glabrata. Antimicrob. Agents Chemother. 2005, 49, 2546–2549. [Google Scholar] [CrossRef] [Green Version]
- Denardi, L.B.; Keller, J.T.; Oliveira, V.; Mario, D.A.N.; Santurio, J.M.; Alves, S.H. Activity of Combined Antifungal Agents Against Multidrug-Resistant Candida glabrata Strains. Mycopathologia 2017, 182, 819–828. [Google Scholar] [CrossRef]
- Alves, I.A.; Bandeira, L.A.; Mario, D.A.; Denardi, L.B.; Neves, L.V.; Santurio, J.M.; Alves, S.H. Effects of antifungal agents alone and in combination against Candida glabrata strains susceptible or resistant to fluconazole. Mycopathologia 2012, 174, 215–221. [Google Scholar] [CrossRef]
- Olson, J.A.; Adler-Moore, J.P.; Smith, P.J.; Proffitt, R.T. Treatment of Candida glabrata infection in immunosuppressed mice by using a combination of liposomal amphotericin B with caspofungin or micafungin. Antimicrob. Agents Chemother. 2005, 49, 4895–4902. [Google Scholar] [CrossRef] [Green Version]
- Tobudic, S.; Kratzer, C.; Lassnigg, A.; Graninger, W.; Presterl, E. In vitro activity of antifungal combinations against Candida albicans biofilms. J. Antimicrob. Chemother. 2010, 65, 271–274. [Google Scholar] [CrossRef] [Green Version]
- Serena, C.; Mariné, M.; Quindós, G.; Carrillo, A.J.; Cano, J.F.; Pastor, F.J.; Guarro, J. In vitro interactions of micafungin with amphotericin B against clinical isolates of Candida spp. Antimicrob. Agents Chemother. 2008, 52, 1529–1532. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Reyes, G.H.; Long, L.A.; Mukherjee, P.K.; Ghannoum, M.A. Efficacy of caspofungin combined with amphotericin B against azole-resistant Candida albicans. J. Antimicrob. Chemother. 2003, 51, 1427–1429. [Google Scholar] [CrossRef] [Green Version]
- Reginatto, P.; Bergamo, V.Z.; Berlitz, S.J.; Guerreiro, I.C.K.; De Andrade, S.F.; Fuentefria, A.M. Rational selection of antifungal drugs to propose a new formulation strategy to control Candida biofilm formation on venous catheters. Braz. J. Microbiol. 2020, 51, 1037–1049. [Google Scholar] [CrossRef]
- Barchiesi, F.; Spreghini, E.; Tomassetti, S.; Giannini, D.; Scalise, G. Caspofungin in combination with amphotericin B against Candida parapsilosis. Antimicrob. Agents Chemother. 2007, 51, 941–945. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.E.; Diekema, D.J.; Messer, S.A.; Pfaller, M.A.; Klepser, M.E. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemother. 2002, 49, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Louie, A.; Kaw, P.; Banerjee, P.; Liu, W.; Chen, G.; Miller, M.H. Impact of the order of initiation of fluconazole and amphotericin B in sequential or combination therapy on killing of Candida albicans in vitro and in a rabbit model of endocarditis and pyelonephritis. Antimicrob. Agents Chemother. 2001, 45, 485–494. [Google Scholar] [CrossRef] [Green Version]
- Chassot, F.; Venturini, T.P.; Piasentin, F.B.; Santurio, J.M.; Svidzinski, T.I.E.; Alves, S.H. Activity of antifungal agents alone and in combination against echinocandin-susceptible and -resistant Candida parapsilosis strains. Rev. Iberoam. Micol. 2019, 36, 44–47. [Google Scholar] [CrossRef]
- Siopi, M.; Neroutsos, E.; Zisaki, K.; Gamaletsou, M.; Pirounaki, M.; Tsirigotis, P.; Sipsas, N.; Dokoumetzidis, A.; Goussetis, E.; Zerva, L.; et al. Bioassay for Determining Voriconazole Serum Levels in Patients Receiving Combination Therapy with Echinocandins. Antimicrob. Agents Chemother. 2015, 60, 632–636. [Google Scholar] [CrossRef]
- O’Brien, B.; Chaturvedi, S.; Chaturvedi, V. In vitro Evaluation of Antifungal Drug Combinations against Multidrug-Resistant Candida auris Isolates from New York Outbreak. Antimicrob. Agents Chemother. 2020, 64, e02195-19. [Google Scholar] [CrossRef] [PubMed]
- Te Dorsthorst, D.T.; Verweij, P.E.; Meletiadis, J.; Bergervoet, M.; Punt, N.C.; Meis, J.F.; Mouton, J.W. In vitro interaction of flucytosine combined with amphotericin B or fluconazole against thirty-five yeast isolates determined by both the fractional inhibitory concentration index and the response surface approach. Antimicrob. Agents Chemother. 2002, 46, 2982–2989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larwood, D.J. Nikkomycin Z-Ready to Meet the Promise? J. Fungi 2020, 6, 261. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.Y.; Hui, M. Effects of Echinocandins in Combination with Nikkomycin Z against Invasive Candida albicans Bloodstream Isolates and the fks Mutants. Antimicrob. Agents Chemother. 2017, 61, e00619-17. [Google Scholar] [CrossRef] [Green Version]
- Kovács, R.; Nagy, F.; Tóth, Z.; Bozó, A.; Balázs, B.; Majoros, L. Synergistic effect of nikkomycin Z with caspofungin and micafungin against Candida albicans and Candida parapsilosis biofilms. Lett. Appl. Microbiol. 2019, 69, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Gil-Lamaignere, C.; Müller, F.M. Differential effects of the combination of caspofungin and terbinafine against Candida albicans, Candida dubliniensis and Candida kefyr. Int. J. Antimicrob. Agents 2004, 23, 520–523. [Google Scholar] [CrossRef]
- Kaneko, Y.; Fukazawa, H.; Ohno, H.; Miyazaki, Y. Combinatory effect of fluconazole and FDA-approved drugs against Candida albicans. J. Infect. Chemother. 2013, 19, 1141–1145. [Google Scholar] [CrossRef]
- Khodavandi, A.; Alizadeh, F.; Vanda, N.A.; Karimi, G.; Chong, P.P. Possible mechanisms of the antifungal activity of fluconazole in combination with terbinafine against Candida albicans. Pharm. Biol. 2014, 52, 1505–1509. [Google Scholar] [CrossRef]
- Pai, M.P.; Samples, M.L.; Mercier, R.C.; Spilde, M.N. Activities and ultrastructural effects of antifungal combinations against simulated Candida endocardial vegetations. Antimicrob. Agents Chemother. 2008, 52, 2367–2376. [Google Scholar] [CrossRef] [Green Version]
- Pai, M.P. Antifungal combinations against simulated Candida albicans endocardial vegetations. Antimicrob. Agents Chemother. 2009, 53, 2629–2631. [Google Scholar] [CrossRef]
- Natarajan, G.; Lulic-Botica, M.; Rongkavilit, C.; Pappas, A.; Bedard, M. Experience with caspofungin in the treatment of persistent fungemia in neonates. J. Perinatol. 2005, 25, 770–777. [Google Scholar] [CrossRef] [Green Version]
- Ostrosky-Zeichner, L.; Kontoyiannis, D.; Raffalli, J.; Mullane, K.M.; Vazquez, J.; Anaissie, E.J.; Lipton, J.; Jacobs, P.; Van Rensburg, J.H.; Rex, J.H.; et al. International, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 654–661. [Google Scholar] [CrossRef]
- Lefort, A.; Chartier, L.; Sendid, B.; Wolff, M.; Mainardi, J.L.; Podglajen, I.; Desnos-Ollivier, M.; Fontanet, A.; Bretagne, S.; Lortholary, O.; et al. Diagnosis, management and outcome of Candida endocarditis. Clin. Microbiol. Infect. 2012, 18, E99–E109. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.N.; Lo, K.Y.; Tong, G.M.; Chan, S.F.; Lo, M.W.; Mak, S.K.; Wong, A.K. Treatment of fungal peritonitis with a combination of intravenous amphotericin B and oral flucytosine, and delayed catheter replacement in continuous ambulatory peritoneal dialysis. Perit. Dial. Int. 2008, 28, 155–162. [Google Scholar] [CrossRef]
- Rex, J.H.; Pappas, P.G.; Karchmer, A.W.; Sobel, J.; Edwards, J.E.; Hadley, S.; Brass, C.; Vazquez, J.A.; Chapman, S.W.; Horowitz, H.W.; et al. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin. Infect. Dis. 2003, 36, 1221–1228. [Google Scholar] [CrossRef]
- Alvarez, C.; Andes, D.R.; Kang, J.Y.; Krug, C.; Kwon, G.S. Antifungal Efficacy of an Intravenous Formulation Containing Monomeric Amphotericin B, 5-Fluorocytosine, and Saline for Sodium Supplementation. Pharm. Res. 2017, 34, 1115–1124. [Google Scholar] [CrossRef] [Green Version]
- Steier, Z.; Vermitsky, J.P.; Toner, G.; Gygax, S.E.; Edlind, T.; Katiyar, S. Flucytosine antagonism of azole activity versus Candida glabrata: Role of transcription factor Pdr1 and multidrug transporter Cdr1. Antimicrob. Agents Chemother. 2013, 57, 5543–5547. [Google Scholar] [CrossRef] [Green Version]
- Baltch, A.L.; Bopp, L.H.; Smith, R.P.; Ritz, W.J.; Michelsen, P.B. Anticandidal effects of voriconazole and caspofungin, singly and in combination, against Candida glabrata, extracellularly and intracellularly in granulocyte-macrophage colony stimulating factor (GM-CSF)-activated human monocytes. J. Antimicrob. Chemother. 2008, 62, 1285–1290. [Google Scholar] [CrossRef]
- Vakil, R.; Knilans, K.; Andes, D.; Kwon, G.S. Combination antifungal therapy involving amphotericin B, rapamycin and 5-fluorocytosine using PEG-phospholipid micelles. Pharm. Res. 2008, 25, 2056–2064. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Ramani, R.; Ghannoum, M.A.; Killian, S.B.; Holliday, N.; Knapp, C.; Ostrosky-Zeichner, L.; Messer, S.A.; Pfaller, M.A.; Iqbal, N.J.; et al. Multilaboratory testing of antifungal combinations against a quality control isolate of Candida krusei. Antimicrob. Agents Chemother. 2008, 52, 1500–1502. [Google Scholar] [CrossRef]
- Shuford, J.A.; Piper, K.E.; Steckelberg, J.M.; Patel, R. In vitro biofilm characterization and activity of antifungal agents alone and in combination against sessile and planktonic clinical Candida albicans isolates. Diagn. Microbiol. Infect. Dis. 2007, 57, 277–281. [Google Scholar] [CrossRef]
- Girmenia, C.; Venditti, M.; Martino, P. Fluconazole in combination with flucytosine in the treatment of fluconazole-resistant Candida infections. Diagn. Microbiol. Infect. Dis. 2003, 46, 227–231. [Google Scholar] [CrossRef]
- Roling, E.E.; Klepser, M.E.; Wasson, A.; Lewis, R.E.; Ernst, E.J.; Pfaller, M.A. Antifungal activities of fluconazole, caspofungin (MK0991), and anidulafungin (LY 303366) alone and in combination against Candida spp. and Crytococcus neoformans via time-kill methods. Diagn. Microbiol. Infect. Dis. 2002, 43, 13–17. [Google Scholar] [CrossRef]
- Guo, P.; He, Y.; Fan, R.; Wu, Z.; Chen, Y.; Huang, Y.; Liao, K.; Chen, P. A case series of medically managed Candida parapsilosis complex prosthetic valve endocarditis. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 1. [Google Scholar] [CrossRef]
- Noguchi, H.; Matsumoto, T.; Kimura, U.; Hiruma, M.; Kano, R.; Yaguchi, T.; Fukushima, S.; Ihn, H. Fungal melanonychia caused by Candida parapsilosis successfully treated with oral fosravuconazole. J. Dermatol. 2019, 46, 911–913. [Google Scholar] [CrossRef]
- Kalkanci, A.; Yesilirmak, N.; Ozdemir, H.B.; Unal, E.A.; Erdoğan, M.; Seker, T.; Tum, A.E.; Karakus, A.K.; Hizel, K.; Bilgihan, K. Impact of Iontophoresis and PACK-CXL Corneal Concentrations of Antifungals in an In vivo Model. Cornea 2018, 37, 1463–1467. [Google Scholar] [CrossRef]
- Kubota, K.; Soma, K.; Uehara, M.; Inaba, T.; Saito, A.; Takeda, N.; Hatano, M.; Morita, H.; Inuzuka, R.; Hirata, Y.; et al. Combined Surgical and Medical Therapy for Candida Prosthetic Endocarditis in a Patient with Repaired Tetralogy of Fallot. Int. Heart J. 2018, 59, 877–880. [Google Scholar] [CrossRef] [Green Version]
- Tu, E.Y.; Majmudar, P.A. Adjuvant Stromal Amphotericin B Injection for Late-Onset DMEK Infection. Cornea 2017, 36, 1556–1558. [Google Scholar] [CrossRef]
- Al-Sweih, N.; Ahmad, S.; Khan, S.; Khan, Z.; Joseph, L.; Vayalil, S.; Chandy, R. Persistent Candida conglobata bloodstream infection in a preterm neonate successfully treated by combination therapy with amphotericin B and caspofungin. J. Mycol. Med. 2017, 27, 271–276. [Google Scholar] [CrossRef]
- Carrega, G.; Cavagnaro, L.; Basso, M.; Riccio, G.; Ronca, A.; Salomone, C.; Burastero, G. Azole-resistant Candida albicans prosthetic joint infection treated with prolonged administration of anidulafungin and two-stage exchange with implant of a mega-prosthesis. J. Chemother. 2017, 29, 386–388. [Google Scholar] [CrossRef]
- Scemla, A.; Charlier, C.; Noel, L.H.; Amazzough, K.; Von Rosen, F.T.; Lesavre, P.; Lortholary, O. Pauci-immune crescentic glomerulonephritis without ANCA in a patient presenting with Candida parapsilosis endocarditis. Med. Mal. Infect. 2016, 46, 163–165. [Google Scholar] [CrossRef]
- Charlier, C.; El Sissy, C.; Bachelier-Bassi, S.; Scemla, A.; Quesne, G.; Sitterlé, E.; Legendre, C.; Lortholary, O.; Bougnoux, M.E. Acquired Flucytosine Resistance during Combination Therapy with Caspofungin and Flucytosine for Candida glabrata Cystitis. Antimicrob. Agents Chemother. 2015, 60, 662–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbst, M.; Gazendam, R.; Reimnitz, D.; Sawalle-Belohradsky, J.; Groll, A.; Schlegel, P.G.; Belohradsky, B.; Renner, E.; Klepper, J.; Grimbacher, B.; et al. Chronic Candida albicans Meningitis in a 4-Year-Old Girl with a Homozygous Mutation in the CARD9 Gene (Q295X). Pediatr. Infect. Dis. J 2015, 34, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.; Guitard, J.; Peltier, J.; Tligui, M.; Benbouzid, S.; Elhaj, S.A.; Rondeau, E.; Hennequin, C. Caspofungin irrigation through percutaneous calicostomy catheter combined with oral flucytosine to treat fluconazole-resistant symptomatic candiduria. J. Mycol. Med. 2015, 25, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Valentine, G.; Thomas, T.A.; Nguyen, T.; Lai, Y.C. Chronic granulomatous disease presenting as hemophagocytic lymphohistiocytosis: A case report. Pediatrics 2014, 134, e1727–e1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiMondi, V.P.; Townsend, M.L.; Johnson, M.; Durkin, M. Antifungal catheter lock therapy for the management of a persistent Candida albicans bloodstream infection in an adult receiving hemodialysis. Pharmacotherapy 2014, 34, e120–e127. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ramos, J.; Salavert-Lleti, M.; Monte-Boquet, E.; Lorente-Fernández, L.; Gil-Gómez, I.; Poveda-Andrés, J.L. Anidulafungin-induced alopecia. Ann. Pharmacother. 2014, 48, 660–662. [Google Scholar] [CrossRef]
- Hagiya, H.; Kajioka, H. Successful treatment of recurrent candidemia due to candidal thrombophlebitis associated with a central venous catheter using a combination of fosfluconazole and micafungin. Intern. Med. 2013, 52, 2139–2143. [Google Scholar] [CrossRef] [Green Version]
- Jarque, I.; Tormo, M.; Bello, J.L.; Rovira, M.; Batlle, M.; Julià, A.; Tabares, S.; Rivas, C.; Fernández-Sevilla, A.; García-Boyero, R.; et al. Caspofungin for the treatment of invasive fungal disease in hematological patients (ProCAS Study). Med. Mycol. 2013, 51, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.C.; Holland, E.J. Infectious endophthalmitis after Boston type 1 keratoprosthesis implantation. Cornea 2012, 31, 346–349. [Google Scholar] [CrossRef]
- Cheng, I.; Chen, Y.L.; Lin, C.H.; Jow, G.M.; Mu, S.C. Complicated Candida parapsilosis peritonitis on peritoneal dialysis in a neonate with renal failure because of bilateral adrenal abscesses. Kaohsiung J. Med. Sci. 2011, 27, 466–468. [Google Scholar] [CrossRef] [Green Version]
- Radike, K.; Kunzmann, S.; Abele-Horn, M.; Beer, M.; Hebestreit, H. Osteoarticular infection by Candida albicans in an infant with cystic fibrosis. J. Med. Microbiol. 2011, 60 Pt 10, 1542–1545. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Fish, D.; Burger, H.; Weiser, B.; Ross, J.S.; Jones, D.; Robstad, K.; Li, X.; Chaturvedi, V. Successful surgical intervention for the management of endocarditis due to multidrug resistant Candida parapsilosis: Case report and literature review. Mycopathologia 2011, 172, 287–292. [Google Scholar] [CrossRef]
- Mahdy, R.A.; Nada, W.M.; Wageh, M.M. Topical amphotericin B and subconjunctival injection of fluconazole (combination therapy) versus topical amphotericin B (monotherapy) in treatment of keratomycosis. J. Ocul. Pharmacol. Ther. 2010, 26, 281–285. [Google Scholar] [CrossRef]
- Mahdy, R.A.; Nada, W.M.; Wageh, M.M.; Kader, M.A.; Saleh, M.M.; Alswad, M.M. Assessment safety and efficacy of a combination therapy of topical amphotericin B and subconjunctival fluconazole for the treatment of fungal keratitis. Cutan. Ocul. Toxicol. 2010, 29, 193–197. [Google Scholar] [CrossRef]
- Okamoto, T.; Koh, K.; Takita, J.; Furuya, A.; Kato, M.; Ida, K. Voriconazole-micafungin combination therapy for acute lymphoblastic leukemia. Pediatr. Int. 2010, 52, 137–141. [Google Scholar] [CrossRef]
- Chew, A.C.; Mehta, J.S.; Li, L.; Busmanis, I.; Tan, D.T. Fungal endophthalmitis after descemet stripping automated endothelial keratoplasty--a case report. Cornea 2010, 29, 346–349. [Google Scholar] [CrossRef]
- Glick, J.A.; Graham, R.S.; Voils, S.A. Candida meningitis post Gliadel wafer placement successfully treated with intrathecal and intravenous amphotericin B. Ann. Pharmacother. 2010, 44, 215–218. [Google Scholar] [CrossRef]
- Bernbeck, B.; Janssen, G.; Winterhalter, S.; Schneider, D.T.; Wessalowski, R. Long time survival after reduced chemotherapy ina 15-year-old patient with AML and Candida krusei sepsis and eye involvement. Klin. Padiatr. 2009, 221, 384–385. [Google Scholar] [CrossRef]
- Haase, R.; Kreft, B.; Foell, J.; Kekulé, A.S.; Merkel, N. Successful treatment of Candida albicans septicemia in a preterm infant with severe congenital ichthyosis (Harlequin baby). Pediatr. Dermatol. 2009, 26, 575–578. [Google Scholar] [CrossRef]
- Varisco, B.M.; Benner, K.W.; Prabhakaran, P. Neonatal peritoneal candidiasis successfully treated with anidulafungin add-on therapy. Ann. Pharmacother. 2009, 43, 1907–1910. [Google Scholar] [CrossRef]
- Falcone, M.; Barzaghi, N.; Carosi, G.; Grossi, P.; Minoli, L.; Ravasio, V.; Rizzi, M.; Suter, F.; Utili, R.; Viscoli, C.; et al. Candida infective endocarditis: Report of 15 cases from a prospective multicenter study. Medicine 2009, 88, 160–168. [Google Scholar] [CrossRef]
- Bland, C.M.; Thomas, S. Micafungin plus fluconazole in an infected knee with retained hardware due to Candida albicans. Ann. Pharmacother. 2009, 43, 528–531. [Google Scholar] [CrossRef]
- Wellinghausen, N.; Moericke, A.; Bundschuh, S.; Friedrich, W.; Schulz, A.S.; Gatz, S.A. Multifocal osteomyelitis caused by Candida dubliniensis. J. Med. Microbiol. 2009, 58 Pt 3, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Albano, L.; Bretagne, S.; Mamzer-Bruneel, M.F.; Kacso, I.; Desnos-Ollivier, M.; Guerrini, P.; Le Luong, T.; Cassuto, E.; Dromer, F.; Lortholary, O.; et al. Evidence that graft-site candidiasis after kidney transplantation is acquired during organ recovery: A multicenter study in France. Clin. Infect. Dis. 2009, 48, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Karatza, A.A.; Dimitriou, G.; Marangos, M.; Christofidou, M.; Pavlou, V.; Giannakopoulos, I.; Darzentas, A.; Mantagos, S.P. Successful resolution of cardiac mycetomas by combined liposomal Amphotericin B with Fluconazole treatment in premature neonates. Eur. J. Pediatr. 2008, 167, 1021–1023. [Google Scholar] [CrossRef]
- Gahn, B.; Schub, N.; Repp, R.; Gramatzki, M. Triple antifungal therapy for severe systemic candidiasis allowed performance of allogeneic stem cell transplantation. Eur. J. Med. Res. 2007, 12, 337–340. [Google Scholar]
- Kanavi, M.R.; Foroutan, A.R.; Kamel, M.R.; Afsar, N.; Javadi, M.A. Candida interface keratitis after deep anterior lamellar keratoplasty: Clinical, microbiologic, histopathologic, and confocal microscopic reports. Cornea 2007, 26, 913–916. [Google Scholar] [CrossRef]
- Olver, W.J.; Scott, F.; Shankland, G.S. Successful treatment of Candida krusei fungemia with amphotericin B and caspofungin. Med. Mycol. 2006, 44, 655–657. [Google Scholar] [CrossRef] [Green Version]
- Paula, C.R.; Krebs, V.L.; Auler, M.E.; Ruiz, L.S.; Matsumoto, F.E.; Silva, E.H.; Diniz, E.M.; Vaz, F.A. Nosocomial infection in newborns by Pichia anomala in a Brazilian intensive care unit. Med. Mycol. 2006, 44, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Al-Assiri, A.; Al-Jastaneiah, S.; Al-Khalaf, A.; Al-Fraikh, H.; Wagoner, M.D. Late-onset donor-to-host transmission of Candida glabrata following corneal transplantation. Cornea 2006, 25, 123–125. [Google Scholar] [CrossRef]
- Pelletier, R.; Alarie, I.; Lagacé, R.; Walsh, T.J. Emergence of disseminated candidiasis caused by Candida krusei during treatment with caspofungin: Case report and review of literature. Med. Mycol. 2005, 43, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Lye, D.C.; Hughes, A.; O’Brien, D.; Athan, E. Candida glabrata prosthetic valve endocarditis treated successfully with fluconazole plus caspofungin without surgery: A case report and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 753–755. [Google Scholar] [CrossRef]
- Fourtounas, C.; Marangos, M.; Kalliakmani, P.; Savidaki, E.; Goumenos, D.S.; Vlachojannis, J.G. Treatment of peritoneal dialysis related fungal peritonitis with caspofungin plus amphotericin B combination therapy. Nephrol. Dial. Transplant. 2006, 21, 236–237. [Google Scholar] [CrossRef] [Green Version]
- Wagner, D.; Sander, A.; Bertz, H.; Finke, J.; Kern, W.V. Breakthrough invasive infection due to Debaryomyces hansenii (teleomorph Candida famata) and Scopulariopsis brevicaulis in a stem cell transplant patient receiving liposomal amphotericin B and caspofungin for suspected aspergillosis. Infection 2005, 33, 397–400. [Google Scholar] [CrossRef]
- Breit, S.M.; Hariprasad, S.M.; Mieler, W.F.; Shah, G.K.; Mills, M.D.; Grand, M.G. Management of endogenous fungal endophthalmitis with voriconazole and caspofungin. Am. J. Ophthalmol. 2005, 139, 135–140. [Google Scholar] [CrossRef]
- Solomon, R.; Biser, S.A.; Donnenfeld, E.D.; Perry, H.D.; Doshi, S.J.; Lee, C.C. Candida parapsilosis keratitis following treatment of epithelial ingrowth after laser in situ keratomileusis. Eye Contact Lens 2004, 30, 85–86. [Google Scholar] [CrossRef]
- Muallem, M.S.; Alfonso, E.C.; Romano, A.C.; Miller, D.; Kurstin, J.; Marangon, F.B.; Culbertson, W.W.; Yoo, S.H. Bilateral Candida parapsilosis interface keratitis after laser in situ keratomileusis. J. Cataract. Refract. Surg. 2003, 29, 2022–2025. [Google Scholar] [CrossRef]
- Mikamo, H.; Ninomiya, M.; Tamaya, T. Tuboovarian abscess caused by Candida glabrata in a febrile neutropenic patient. J. Infect. Chemother. 2003, 9, 257–259. [Google Scholar] [CrossRef]
- Shann, S.; Wilson, J. Treatment of Candida glabrata using topical amphotericin B and flucytosine. Sex. Transm. Infect. 2003, 79, 265–266. [Google Scholar] [CrossRef] [Green Version]
- Sutphin, J.E.; Pfaller, M.A.; Hollis, R.J.; Wagoner, M.D. Donor-to-host transmission of Candida albicans after corneal transplantation. Am. J. Ophthalmol. 2002, 134, 120–121. [Google Scholar] [CrossRef]
- Ramamohan, N.; Zeineh, N.; Grigoris, P.; Butcher, I. Candida glabrata infection after total hip arthroplasty. J. Infect. 2001, 42, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Soto-Hernández, J.L.; Ramírez-Crescencio, M.A.; Moreno Estrada, V.M.; Del Valle Robles, R. Candida albicans cerebral granulomas associated with a nonfunctional cerebrospinal fluid shunt: Case report. Neurosurgery 2000, 47, 973–976; discussion 976–977. [Google Scholar] [CrossRef] [PubMed]
- Tortorano, A.M.; Prigitano, A.; Morroni, G.; Brescini, L.; Barchiesi, F. Candidemia: Evolution of Drug Resistance and Novel Therapeutic Approaches. Infect. Drug Resist. 2021, 14, 5543–5553. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Campione, E.; Gaziano, R.; Doldo, E.; Marino, D.; Falconi, M.; Iacovelli, F.; Tagliaferri, D.; Pacello, L.; Bianchi, L.; Lanna, C.; et al. Antifungal Effect of All-trans Retinoic Acid against Aspergillus fumigatus In Vitro and in a Pulmonary Aspergillosis In Vivo Model. Antimicrob. Agents Chemother. 2021, 65, e01874-20. [Google Scholar] [CrossRef]
- Cosio, T.; Gaziano, R.; Zuccari, G.; Costanza, G.; Grelli, S.; Di Francesco, P.; Bianchi, L.; Campione, E. Retinoids in Fungal Infections: From Bench to Bedside. Pharmaceuticals 2021, 14, 962. [Google Scholar] [CrossRef]

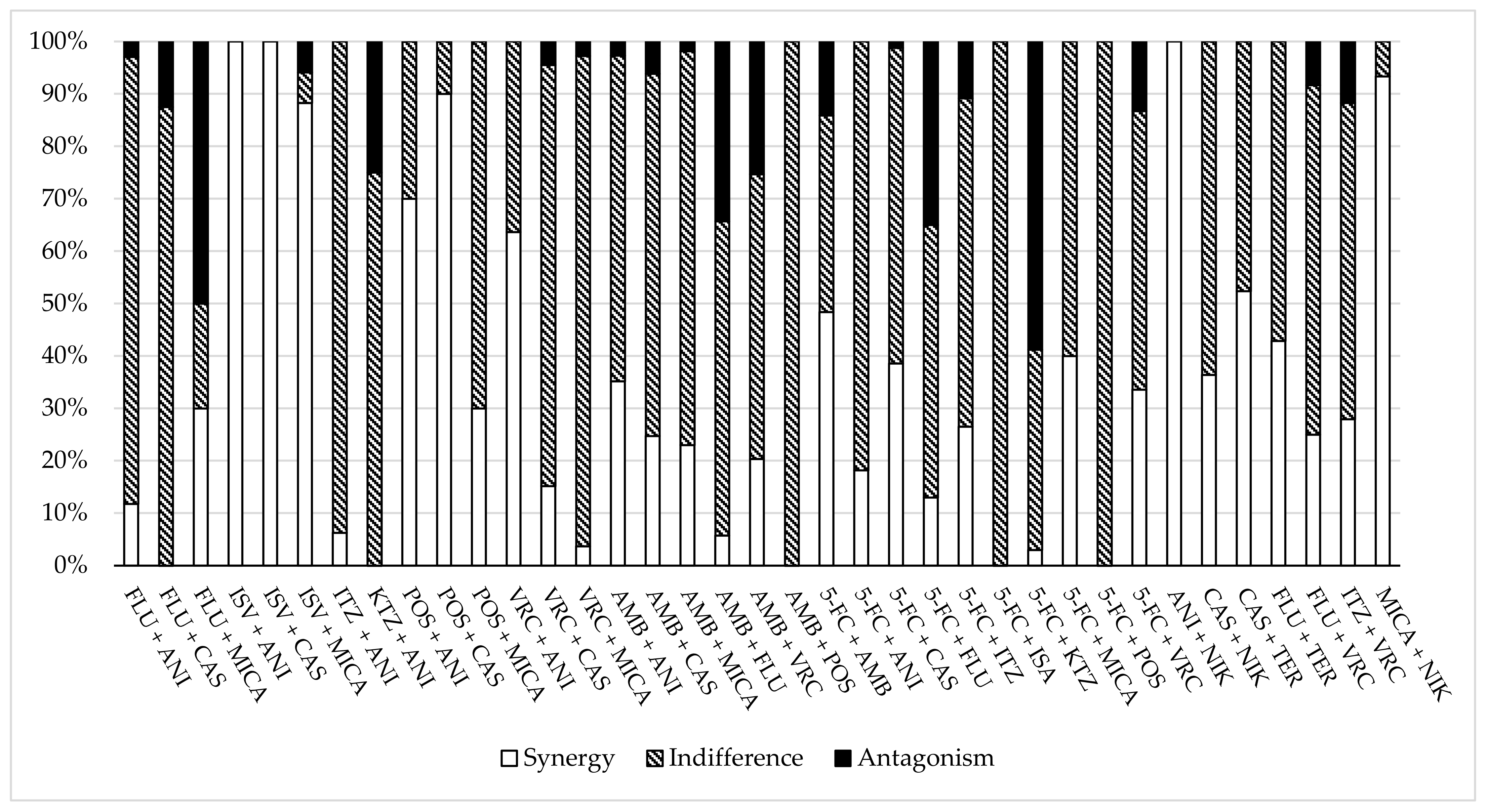
| C. alb | C. par | C. gla | C. tro | C. aur | C. kru | C. dub | C. lus | C. kef | All | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | I | A | S | I | A | S | I | A | S | I | A | S | I | A | S | I | A | S | I | A | S | I | A | S | I | A | S | I | A | |
| Azoles + Echinocandins | ||||||||||||||||||||||||||||||
| FLU + ANI | - | 6 | - | - | 4 | - | 4 | 9 | 1 | - | 6 | - | - | - | - | - | 4 | - | - | - | - | - | - | - | - | - | - | 4 | 29 | 1 |
| FLU + CAS | - | 2 | - | - | - | - | - | 8 | 2 | - | 2 | - | - | - | - | - | 2 | - | - | - | - | - | - | - | - | - | - | 0 | 14 | 2 |
| FLU + MICA | - | - | - | - | - | - | 3 | 2 | 5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3 | 2 | 5 |
| ISV + ANI | - | - | - | - | - | - | - | - | - | - | - | - | 12 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 12 | 0 | 0 |
| ISV + CAS | - | - | - | - | - | - | - | - | - | - | - | - | 12 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 12 | 0 | 0 |
| ISV + MICA | 1 | - | - | 1 | - | - | - | - | 1 | - | 1 | - | 12 | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | 15 | 1 | 1 |
| ITZ + ANI | - | 4 | - | - | 2 | - | 1 | 3 | - | - | 4 | - | - | - | - | - | 2 | - | - | - | - | - | - | - | - | - | - | 1 | 15 | 0 |
| KTZ + ANI | - | 4 | - | - | 2 | - | - | 4 | - | - | - | 4 | - | - | - | - | 2 | - | - | - | - | - | - | - | - | - | - | 0 | 12 | 4 |
| POS + ANI | - | - | - | - | - | - | 7 | 3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 7 | 3 | 0 |
| POS + CAS | 10 | - | - | - | - | - | 8 | 2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 18 | 2 | 0 |
| POS + MICA | - | - | - | - | - | - | 3 | 7 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3 | 7 | 0 |
| VRC + ANI | - | - | - | - | 1 | - | 7 | 3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 7 | 4 | 0 |
| VRC + CAS | - | 30 | - | - | 1 | - | 17 | 59 | 5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 17 | 90 | 5 |
| VRC + MICA | 1 | 54 | - | - | 13 | - | 3 | 16 | 3 | - | - | - | - | - | - | - | - | - | - | 19 | - | - | - | - | - | - | - | 4 | 102 | 3 |
| Polyenes + Echinocandins | ||||||||||||||||||||||||||||||
| AMB + ANI | 3 | 4 | - | 3 | 4 | - | 4 | 9 | 1 | 3 | 4 | - | - | - | - | - | 2 | - | - | - | - | - | - | - | - | - | - | 13 | 23 | 1 |
| AMB + CAS | 4 | 7 | - | - | 6 | - | 20 | 54 | 6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 24 | 67 | 6 |
| AMB + MICA | 4 | 33 | - | 7 | 10 | - | 3 | 21 | 3 | 7 | 8 | - | - | - | - | 9 | 26 | - | 7 | 13 | - | - | 10 | - | - | - | - | 37 | 121 | 3 |
| Polyenes + Azoles | ||||||||||||||||||||||||||||||
| AMB + FLU | - | 3 | 4 | 3 | 37 | 20 | 1 | - | - | - | 1 | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | 4 | 42 | 24 |
| AMB + VRC | - | - | - | - | 35 | 25 | 28 | 40 | 10 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 28 | 75 | 35 |
| AMB + POS | - | 10 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 | 10 | 0 |
| 5-FC combination | ||||||||||||||||||||||||||||||
| 5-FC + AMB | 5 | 4 | 5 | 12 | 35 | 13 | 55 | 23 | 6 | 1 | - | - | 16 | 6 | 2 | - | 1 | - | - | - | - | - | - | - | - | - | - | 89 | 69 | 26 |
| 5-FC + ANI | - | 4 | - | - | 4 | - | - | 4 | - | - | 4 | - | 6 | 9 | - | - | 2 | - | - | - | - | - | - | - | - | - | - | 6 | 27 | 0 |
| 5-FC + CAS | - | - | - | - | - | - | 26 | 41 | 1 | - | - | - | 6 | 9 | - | - | - | - | - | - | - | - | - | - | - | - | - | 32 | 50 | 1 |
| 5-FC + FLU | 6 | 1 | 5 | 4 | 40 | 16 | 1 | 1 | 14 | 1 | - | - | - | - | - | 1 | 10 | - | - | - | - | - | - | - | - | - | - | 13 | 52 | 35 |
| 5-FC + ITZ | - | - | - | - | - | - | 22 | 37 | 9 | - | - | - | - | 15 | - | - | - | - | - | - | - | - | - | - | - | - | - | 22 | 52 | 9 |
| 5-FC + ISA | - | - | - | - | - | - | - | - | - | - | - | - | - | 15 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 | 15 | 0 |
| 5-FC + KTZ | - | - | - | - | - | - | 2 | 26 | 40 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 26 | 40 |
| 5-FC + MICA | - | - | - | - | - | - | - | - | - | - | - | - | 6 | 9 | - | - | - | - | - | - | - | - | - | - | - | - | - | 6 | 9 | 0 |
| 5-FC + POS | - | - | - | - | - | - | - | - | - | - | - | - | - | 15 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0 | 15 | 0 |
| 5-FC + VRC | - | - | - | 10 | 37 | 13 | 25 | 37 | 6 | - | - | - | 13 | 2 | - | - | - | - | - | - | - | - | - | - | - | - | - | 48 | 76 | 19 |
| Other combinations | ||||||||||||||||||||||||||||||
| ANI + NIK | 4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4 | 0 | 0 |
| CAS + NIK | 4 | 2 | - | - | 5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4 | 7 | 0 |
| CAS + TER | 40 | 19 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 41 | - | - | - | - | 26 | - | - | 66 | 60 | 0 |
| FLU + TER | 3 | 4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3 | 4 | 0 |
| FLU + VRC | - | - | - | 15 | 40 | 5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 15 | 40 | 5 |
| ITZ + VRC | - | - | - | - | - | - | 19 | 41 | 8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 19 | 41 | 8 |
| MICA + NIK | 10 | - | - | 4 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 14 | 1 | 0 |
| Reference | Isolates and Species | Combinations | Methods | Results |
|---|---|---|---|---|
| Kalkanci et al., 2018 [58] | 12 corneas were inoculated with C. albicans | VRC + AMB | Corneal Infection Rabbit model | Two Log reduction in colony numbers compared to single treatment |
| Alvarez et al., 2017 [48] | C. albicans * | AMB + 5-FC | Systemic Infection Neutropenic Mouse model | No differences compared to monotherapy |
| Chen et al., 2013 [19] | Three C. albicans * | POS + CAS | Systemic Infection Mouse model | SYN in 1 isolate, NO SYN in drug resistant isolates |
| Olson et al., 2005 [23] | C. glabrata | AMB + CAS or AMB + MICA | Systemic Infection Neutropenic Mouse model | Improved activity of combination therapy |
| Barchiesi et al., 2005 [20] | C. glabrata * | CAS + AMB | Systemic Infection Neutropenic Mouse model | >100 fold CFU difference |
| Graybill et al., 2003 [18] | C. albicans | CAS + FLU | Systemic Infection Mouse model | No differences compared to monotherapy |
| Hossain et al., 2003 [26] | C. albicans * | CAS + AMB | Systemic Infection Mouse model | CAS + AMB prolonged survival compared with untreated control. Treatment of MICA with AMB + CAS, even at low dosage also tended to prolong survival |
| Louie et al., 2001 [30] | C. albicans | FLU + AMB | Rabbit model of endocarditis and pyelonephritis | No differences compared to monotherapy |
| Combinations | Number of Cases | Success n (%) | Failure n (%) |
|---|---|---|---|
| AMB + FLU | 142 | 100 (70.4%) | 42 (29.6%) |
| AMB + 5-FC | 24 | 15 (62.5%) | 9 (37.5%) |
| AMB + CAS | 15 | 8 (53.3%) | 7 (46.7%) |
| CAS + 5-FC | 13 | 4 (30.8%) | 9 (69.2%) |
| AMB + CAS + FLU | 11 | 5 (45.5%) | 6 (54.5%) |
| CAS + VRC | 9 | 7 (77.8%) | 2 (22.2%) |
| FLU + 5-FC | 8 | 6 (75.0%) | 2 (25.0%) |
| CAS + FLU | 6 | 4 (66.7%) | 2 (33.3%) |
| FLU + MICA | 4 | 3 (75.0%) | 1 (25.0%) |
| AMB + CAS + VRC | 2 | 2 (100%) | - |
| AMB + 5-FC + FLU | 1 | 1 (100%) | - |
| AMB + 5-FC + VRC | 1 | 1 (100%) | - |
| AMB + ANI | 1 | 1 (100%) | - |
| AMB + FLU + 5-FC + CAS | 1 | 1 (100%) | - |
| AMB + FLU + MICA | 1 | 1 (100%) | - |
| AMB + KTZ | 1 | 1 (100%) | - |
| AMB + KTZ | 1 | 1 (100%) | - |
| AMB + VRC | 1 | 1 (100%) | - |
| CAS + FLU + POS | 1 | 1 (100%) | - |
| CAS + ITZ | 1 | - | 1 (100%) |
| FLU + VRC | 1 | - | 1 (100%) |
| ITZ + EFI | 1 | - | 1 (100%) |
| MICA + VRC | 1 | 1 (100%) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fioriti, S.; Brescini, L.; Pallotta, F.; Canovari, B.; Morroni, G.; Barchiesi, F. Antifungal Combinations against Candida Species: From Bench to Bedside. J. Fungi 2022, 8, 1077. https://doi.org/10.3390/jof8101077
Fioriti S, Brescini L, Pallotta F, Canovari B, Morroni G, Barchiesi F. Antifungal Combinations against Candida Species: From Bench to Bedside. Journal of Fungi. 2022; 8(10):1077. https://doi.org/10.3390/jof8101077
Chicago/Turabian StyleFioriti, Simona, Lucia Brescini, Francesco Pallotta, Benedetta Canovari, Gianluca Morroni, and Francesco Barchiesi. 2022. "Antifungal Combinations against Candida Species: From Bench to Bedside" Journal of Fungi 8, no. 10: 1077. https://doi.org/10.3390/jof8101077






