Management of Plant Beneficial Fungal Endophytes to Improve the Performance of Agroecological Practices
Abstract
:1. Introduction
- -
- Access to resources: Among crop plant species, domestication and genetic selection often lasted for thousands of years, with more or less long distances of transportation from their native areas to the current zones of production [5]. Native areas should be regarded as a major source of information about the natural microbiota of the considered crop with a set of functions presumably essential to the plant holobiont life cycle naturally developed during evolution and early domestication. Of course, native areas of the considered crop plants must be known, the corresponding countries or zones must be accessible, the ancient ecosystems preserved, etc. [5]. The plant original diversities have to be explored across domestication steps, in several countries, with regard to the more or less progressive (e.g., intra- vs. intercontinental) dissemination. As early as possible in the development of microbial exploration projects, the terms and purposes of use must be established with the partner country or countries and adjusted as the databases evolve and their potential for use is assessed, both for plants and microbial strains (in accordance with ABS rules).
- -
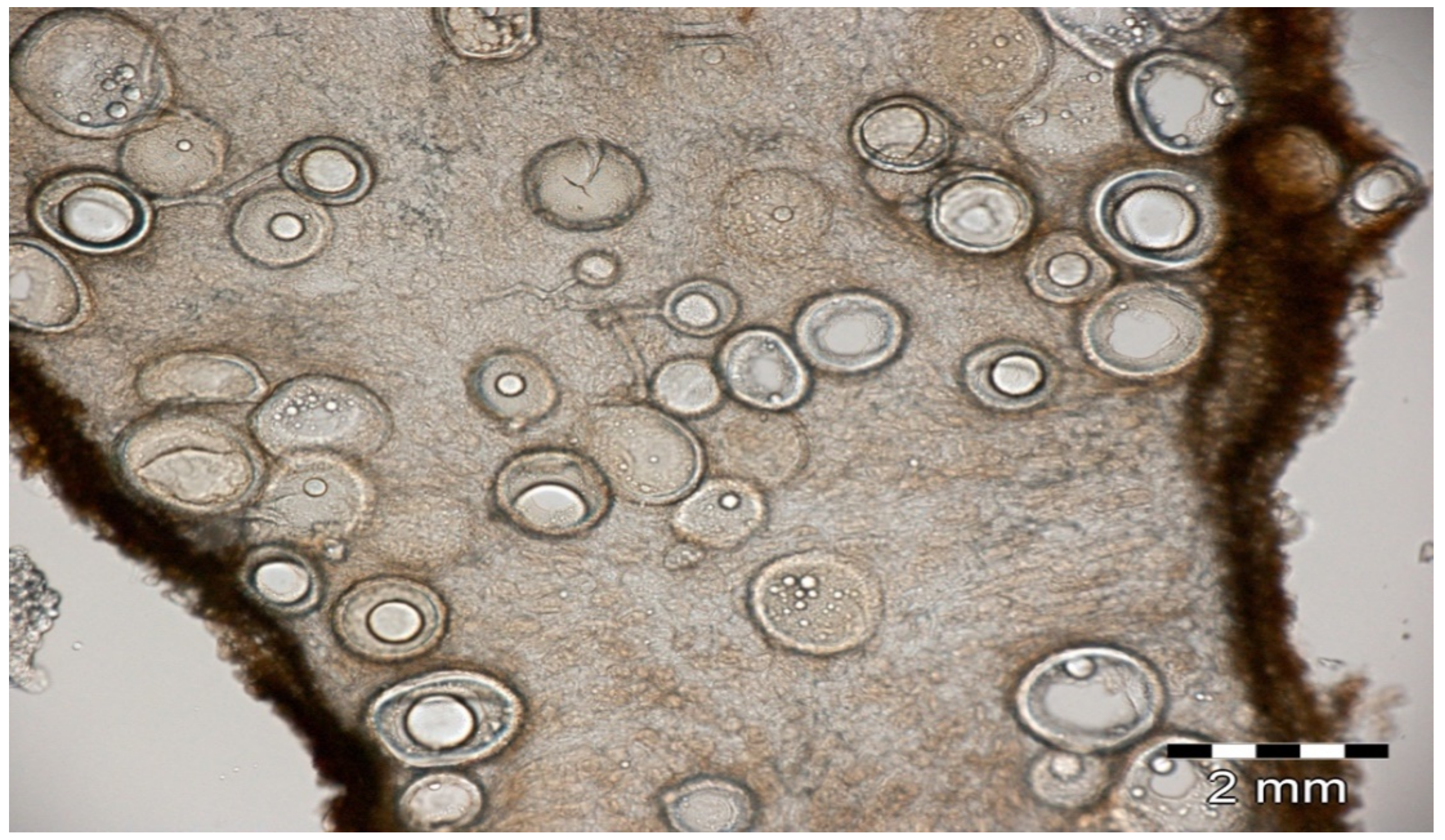
- Cultivability or the survival of microbial strains: Having the microbial strain available as a pure culture is a typical objective of an agro-microbiologist; however, non-cultivability is not an indicator of non-viability as evidenced by Xu et al. (1982) on different bacterial taxa [6]. Non-cultivability is considered a general fate of AM fungi (although Glomeromycota are still partially explored in terms of cultivability), and ECM are also not always easy to cultivate or to maintain over time in pure cultures. Plate cultures of the ECM ascomycete Tuber spp. are possible [7,8], but the presence of bacteria of the genus Rhodopseudomonas sp. (Figure 2) seems obligate [9]. Cultivability can thus constitute a real obstacle to agronomic use. Molecular methods of global analyses of microbial communities (metagenomic) can be used in parallel with microbial isolation trials to evaluate the relative rates of non-culturable microbial taxa and thus evaluate the representativity of the isolates.
- -
- Inoculation method: Depending on the plant and the cultivation methods (the need for a nursery stage, direct sowing, mechanized or not, cuttings, grafting, etc.), and also depending on the microbial strain and the form of inoculum, supply must be adapted.
2. Mycorrhizal Symbioses
2.1. Arbuscular Mycorrhizal Symbioses
2.2. Other Plant-Growth-Promoting Endophytic Fungi Outside the Glomeromycota
2.3. Dark Septate Endophytic Fungi (DSE)
2.4. Ectomycorrhizal Symbioses
2.5. Mycorrhization Helper Bacteria (MHB)
3. Agroecological Applications
3.1. Reductionist Approaches—ECM, AM or MHB Field Trials
3.1.1. ECM Inoculation of Forest Trees
3.1.2. The Effect of Soil AM Spores Inoculation on Plant Species
- The controlled mycorrhization of an exotic tree leguminous, Acacia holosericea, in a Sahelian ecosystem (Burkina Faso)
- The impact of mycorrhiza-based ecological engineering strategies on Ziziphus mauritiana Lam. and its native mycorrhizal communities on the route of the Great Green Wall (Senegal)
3.1.3. MHB (Mycorrhiza Helper Bacteria)
- Holistic approaches, acting on agricultural or forestry practices: associating grain legumes and cereals or using Lavandula as a soil stimulator in association with Cupressus
3.1.4. Mixed Legume/Cereals Crop Practices
3.1.5. Nurse Plants in Tree Planting
- Lavandula/Cupressus atlantica association
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Arbuscular mycorrhizae |
| ECM | Ecto-mycorrhizae |
| MHB | Mycorrhizae Helper Bacteria |
References
- EBIC—The European Biostimulants Industry Council. Available online: https://biostimulants.eu/ (accessed on 17 August 2022).
- v2_nese_40_internet.pdf. Available online: https://agriculture.gouv.fr/sites/minagri/files/v2_nese_40_internet.pdf#page=7 (accessed on 17 June 2022).
- Altieri, M.A. Agroecology: A new research and development paradigm for world agriculture. Agric. Ecosyst. Environ. 1989, 27, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Duponnois, R.; Ramanankierana, H.; Hafidi, M.; Baohanta, R.; Baudoin, E.; Thioulouse, J.; Sanguin, H.; Ba, A.; Galiana, A.; Bally, R.; et al. Native plant resources to optimize the performances of forest rehabilitation in Mediterranean and tropical environment: Some examples of nursing plant species that improve the soil mycorrhizal potential. C. R. Biol. 2013, 336, 265–272. [Google Scholar] [CrossRef]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.-S.; Roberts, N.; Singleton, F.L.; Attwell, R.W.; Grimes, D.J.; Colwell, R.R. Survival and viability of nonculturableEscherichia coli andVibrio cholerae in the estuarine and marine environment. Microb. Ecol. 1982, 8, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, G.; Comandini, O. Tuber. In Ectomycorrhizal Fungi Key Genera in Profile; Cairney, J.W.G., Chambers, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 163–186. [Google Scholar] [CrossRef]
- Iotti, M.; Amicucci, A.; Stocchi, V.; Zambonelli, A. Morphological and molecular characterization of mycelia of some Tuber species in pure culture. New Phytol. 2002, 155, 499–505. [Google Scholar] [CrossRef]
- Le Roux, C.; Tournier, E.; Lies, A.; Sanguin, H.; Chevalier, G.; Duponnois, R.; Mousain, D.; Prin, Y. Bacteria of the genus Rhodopseudomonas (Bradyrhizobiaceae): Obligate symbionts in mycelial cultures of the black truffles Tuber melanosporum and Tuber brumale. SpringerPlus 2016, 5, 1085. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Abdelhameid, N.M. Effect of Mycorrhizal Inoculation and Potassium Fertilization on Grain Yield and Nutrient Uptake of Sweet Sorghum Cultivated under Water Stress in Calcareous Soil. Egypt. J. Soil Sci. 2020, 60, 17–29. [Google Scholar] [CrossRef]
- Chalot, M.; Javelle, A.; Blaudez, D.; Lambilliote, R.; Cooke, R.; Sentenac, H.; Wipf, D.; Botton, B. An update on nutrient transport processes in ectomycorrhizas. In Diversity and Integration in Mycorrhizas; Springer: Dordrecht, The Netherlands, 2002; pp. 165–175. [Google Scholar] [CrossRef]
- Linderman, R.G. Mycorrhizal interactions in the rhizosphere. In The Rhizosphere and Plant Growth; Keister, D.L., Cregan, P.B., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 343–348. [Google Scholar] [CrossRef]
- Cassán, F.; Perrig, D.; Sgroy, V.; Luna, V. Basic and Technological Aspects of Phytohormone Production by Microorganisms: Azospirillum sp. as a Model of Plant Growth Promoting Rhizobacteria. In Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 141–182. [Google Scholar] [CrossRef]
- Basu, S.; Rabara, R.C.; Negi, S. AMF: The future prospect for sustainable agriculture. Physiol. Mol. Plant Pathol. 2018, 102, 36–45. [Google Scholar] [CrossRef]
- Tahat, M.M.; Alananbeh, K.M.; Othman, Y.A.; Leskovar, D.I. Soil Health and Sustainable Agriculture. Sustainability 2020, 12, 4859. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Caeiro, M.F.; Mahdhi, M.; Tenreiro, R.; Ulm, F.; Mars, M.; Cruz, C.; Dias, T. Arbuscular mycorrhizal traits are good indicators of soil multifunctionality in drylands. Geoderma 2021, 397, 115099. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with Mycorrhizas in Forestry and Agriculture; Australian Centre for International Agricultural Research: Canberra, Australia, 1996; Volume 32, p. 374. [Google Scholar]
- Öpik, M.; Zobel, M.; Cantero, J.J.; Davison, J.; Facelli, J.M.; Hiiesalu, I.; Jairus, T.; Kalwij, J.M.; Koorem, K.; Leal, M.E.; et al. Global sampling of plant roots expands the described molecular diversity of arbuscular mycorrhizal fungi. Mycorrhiza 2013, 23, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Corradi, N.; Bonfante, P. The Arbuscular Mycorrhizal Symbiosis: Origin and Evolution of a Beneficial Plant Infection. PLOS Pathog. 2012, 8, e1002600. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Maiti, D.; Singh, R.K.; Variar, M. Rice-based crop rotation for enhancing native arbuscular mycorrhizal (AM) activity to improve phosphorus nutrition of upland rice (Oryza sativa L.). Biol. Fertil. Soils 2012, 48, 67–73. [Google Scholar] [CrossRef]
- Paszkowski, U.; Jakovleva, L.; Boller, T. Maize mutants affected at distinct stages of the arbuscular mycorrhizal symbiosis. Plant J. 2006, 47, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Khalafallah, A.; Abo-Ghalia, H.H. Effect of arbuscular mycorrhizal fungi on the metabolic products and activity of antioxidant system in wheat plants subjected to short-term water stress, followed by recovery at different growth stages. J. Appl. Sci. Res. 2008, 4, 559–569. [Google Scholar]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Ashwath, N.; Jasper, D.A. Mycorrhizas in the Kakadu region of tropical Australia. Plant Soil 1996, 184, 159–171. [Google Scholar] [CrossRef]
- Agriculture et Agroalimentaire Canada. 2014. Available online: https://agriculture.canada.ca/fr/agriculture-et-agroalimentaire-canada (accessed on 17 August 2022).
- Declerck, S.; de Boulois, H.D.; de Stage, M.; Chave, M. Evaluation In Vitro du Potentiel Bioprotecteur des Champignons Mycorhiziens à Arbuscules contre le Flétrissement Bactérien de la Tomate; Unité de Microbiologie: Montpellier, France, 2012; p. 69. [Google Scholar]
- Micro-organismes: Santé et la Nutrition des Plantes. Available online: https://www.lallemandplantcare.com/fr/belgique/ (accessed on 18 August 2022).
- Colpaert, J.V.; Janos, D.P. On publishing in Mycorrhiza. Mycorrhiza 2018, 28, 209–211. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Varma, A.; Rexer, K.H.; Hassel, A.; Kost, G.; Sarbhoy, A.; Bisren, P.; Franken, P. Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 1998, 90, 896–903. [Google Scholar] [CrossRef]
- Varma, A.; Singh, A.N.; Sudha; Sahay, N.S.; Sharma, J.B.; Roy, A.; Kumari, M.; Rana, D.S.; Thakran, S.; Deka, D.; et al. Piriformospora indica: An Axenically Culturable Mycorrhiza-Like Endosymbiotic Fungus. In Fungal Associations; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 125–150. [Google Scholar] [CrossRef]
- Weiss, M.; Selosse, M.-A.; Rexer, K.-H.; Urban, A.; Oberwinkler, F. Sebacinales: A hitherto overlooked cosm of heterobasidiomycetes with a broad mycorrhizal potential. Mycol. Res. 2004, 108, 1003–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jisha, S.; Kk, S.; Manjula, S. Multifunctional aspects of Piriformospora indica in plant endosymbiosis. Mycology 2019, 10, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Weiß, M.; Waller, F.; Zuccaro, A.; Selosse, M.-A. Sebacinales—One thousand and one interactions with land plants. New Phytol. 2016, 211, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Mensah, R.A.; Li, D.; Liu, F.; Tian, N.; Sun, X.; Hao, X.; Lai, Z.; Cheng, C. Versatile Piriformospora indica and Its Potential Applications in Horticultural Crops. Hortic. Plant J. 2020, 6, 111–121. [Google Scholar] [CrossRef]
- Jafari, M.; Yari, M.; Ghabooli, M.; Sepehri, M.; Ghasemi, E.; Jonker, A. Inoculation and co-inoculation of alfalfa seedlings with root growth promoting microorganisms (Piriformospora indica, Glomus intraradices and Sinorhizobium meliloti) affect molecular structures, nutrient profiles and availability of hay for ruminants. Anim. Nutr. 2018, 4, 90–99. [Google Scholar] [CrossRef]
- Thürich, J.; Meichsner, D.; Furch, A.C.; Pfalz, J.; Krüger, T.; Kniemeyer, O.; Brakhage, A.; Oelmüller, R. Arabidopsis thaliana responds to colonisation of Piriformospora indica by secretion of symbiosis-specific proteins. PLoS ONE 2018, 13, e0209658. [Google Scholar] [CrossRef]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Hodkinson, T.R.; Doohan, F.M.; Saunders, M.J.; Murphy, B.R. Endophytes for a Growing World; Cambridge University Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Khan, A.L.; Hamayun, M.; Khan, S.A.; Kang, S.-M.; Shinwari, Z.K.; Kamran, M. Pure culture of Metarhizium anisopliae LHL07 reprograms soybean to higher growth and mitigates salt stress. World J Microbiol Biotechnol 2012, 28, 1483–1494. [Google Scholar] [CrossRef]
- Santos, M.; Cesanelli, I.; Diánez, F.; Sánchez-Montesinos, B.; Moreno-Gavíra, A. Advances in the Role of Dark Septate Endophytes in the Plant Resistance to Abiotic and Biotic Stresses. J. Fungi 2021, 7, 939. [Google Scholar] [CrossRef]
- 33-1.pdf. Available online: https://www.fungaldiversity.org/fdp/sfdp/33-1.pdf (accessed on 27 June 2022).
- Morte, A.; Lovisolo, C.; Schubert, A. Effect of drought stress on growth and water relations of the mycorrhizal association Helianthemum almeriense-Terfezia claveryi. Mycorrhiza 2000, 10, 115–119. [Google Scholar] [CrossRef]
- Calvaruso, C.; Turpault, M.P.; Leclerc, E.; Ranger, J.; Garbaye, J.; Uroz, S.; Frey-Klett, P. Influence of Forest Trees on the Distribution of Mineral Weathering-Associated Bacterial Communities of the Scleroderma citrinum Mycorrhizosphere. Appl. Environ. Microbiol. 2010, 76, 4780–4787. [Google Scholar] [CrossRef] [Green Version]
- Plassard, C.; Scheromm, P.; Mousain, D.; Bousquet, N.; Salsac, L. Ectomycorrhizal symbiosis and mineral nutrition. Source-sink relationships. Bull. Soc. Bot. Fr. Actual. Bot. 1988. Available online: https://scholar.google.com/scholar_lookup?title=Ectomycorrhizal+symbiosis+and+mineral+nutrition.+Source-sink+relationships.&author=Plassard+C.&publication_year=1988 (accessed on 27 June 2022).
- Lies, A.; Delteil, A.; Prin, Y.; Duponnois, R. Using Mycorrhiza Helper Microorganisms (MHM) to Improve the Mycorrhizal Efficiency on Plant Growth. In Role of Rhizospheric Microbes in Soil; Meena, V.S., Ed.; Springer: Singapore, 2018; Volume 1, pp. 277–298. [Google Scholar] [CrossRef]
- Garbaye, J. Tansley Review No. 76 Helper bacteria: A new dimension to the mycorrhizal symbiosis. New Phytol. 1994, 128, 197–210. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Garbaye, J.; Tarkka, M. The mycorrhiza helper bacteria revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef]
- Barea, J.-M.; Pozo, M.J.; Azcón, R.; Azcón-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [Green Version]
- Bournaud, C.; James, E.K.; De Faria, S.M.; Lebrun, M.; Melkonian, R.; Duponnois, R.; Tisseyre, P.; Moulin, L.; Prin, Y. Interdependency of efficient nodulation and arbuscular mycorrhization in Piptadenia gonoacantha, a Brazilian legume tree. Plant Cell Environ. 2018, 41, 2008–2020. [Google Scholar] [CrossRef]
- Bonfante, P.; Balestrini, R.; Mend, K. Gen Storage and secretion processes in the spore of Gigaspora margarita Becker &Hall as revealed by high-pressure freezing and freeze substitution. New Phytol. 1994, 128, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Kratz, R.F. Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesisl; MIT Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Desirò, A.; Salvioli, A.; Ngonkeu, E.L.; Mondo, S.; Epis, S.; Faccio, A.; Kaech, A.; Pawlowska, T.; Bonfante, P. Detection of a novel intracellular microbiome hosted in arbuscular mycorrhizal fungi. ISME J. 2014, 8, 257–270. [Google Scholar] [CrossRef] [Green Version]
- Emmett, B.D.; Lévesque-Tremblay, V.; Harrison, M.J. Conserved and reproducible bacterial communities associate with extraradical hyphae of arbuscular mycorrhizal fungi. ISME J. 2021, 15, 2276–2288. [Google Scholar] [CrossRef]
- Mugnier, J.; Mosse, B. Spore germination and viability of a vesicular arbuscular mycorrhizal fungus, Glomus mosseae. Trans. Br. Mycol. Soc. 1987, 88, 411–413. [Google Scholar] [CrossRef]
- Carpenter-Boggs, L.; Loynachan, T.E.; Stahl, P.D. Spore germination of Gigaspora margarita stimulated by volatiles of soil-isolated actinomycetes. Soil Biol. Biochem. 1995, 27, 1445–1451. [Google Scholar] [CrossRef]
- Abdellatif, L.; Lokuruge, P.; Hamel, C. Axenic growth of the arbuscular mycorrhizal fungus Rhizophagus irregularis and growth stimulation by coculture with plant growth-promoting rhizobacteria. Mycorrhiza 2019, 29, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Mayo, K.; Davis, R.E.; Motta, J. Stimulation of Germination of Spores of Glomus Versiforme by Spore-Associated Bacteria. Mycologia 1986, 78, 426–431. [Google Scholar] [CrossRef]
- Kiddee, S.; Yuttavanichakul, W.; Boonkerd, N.; Teaumroong, N.; Saito, K.; Tittabutr, P. Secretion compounds from Brevibacillus sp. SUT47 promote spore propagation of Acaulospora tuberculata colonizing maize roots (Zea mays L. cultivar Suwan 5). ScienceAsia 2020, 46, 634. [Google Scholar] [CrossRef]
- Bourles, A.; Guentas, L.; Charvis, C.; Gensous, S.; Majorel, C.; Crossay, T.; Amir, H. Co-inoculation with a bacterium and arbuscular mycorrhizal fungi improves root colonization, plant mineral nutrition, and plant growth of a Cyperaceae plant in an ultramafic soil. Mycorrhiza 2020, 30, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, U.; Ouziad, F.; Marner, F.-J.; Bothe, H. The bacterium Paenibacillus validus stimulates growth of the arbuscular mycorrhizal fungus Glomus intraradices up to the formation of fertile spores. FEMS Microbiol. Lett. 2006, 254, 258–267. [Google Scholar] [CrossRef]
- Pinto, D.; Santos, M.A.; Chambel, L. Thirty years of viable but nonculturable state research: Unsolved molecular mechanisms. Crit. Rev. Microbiol. 2015, 41, 61–76. [Google Scholar] [CrossRef]
- Jdo, M.I.; Cranenbrouck, S.; Declerck, S. Methods for large-scale production of AM fungi: Past, present, and future. Mycorrhiza 2011, 21, 1–16. [Google Scholar] [CrossRef]
- Ducousso, M.; Galiana, A.; Chaix, G.; Prin, Y. Relative infectivity of two Pisolithus spp. strains inoculated to the nitrogen-fixing legume tree Acacia crassicarpa A. Cunn. ex Benth. in a field experiment in Madagascar. Eur. J. Soil Biol. 2004, 40, 105–111. [Google Scholar] [CrossRef]
- Prin, Y.; Galiana, A.; le Roux, C.; Meleard, B.; Razafimaharo, V.; Ducousso, M.; Chaix, G. Molecular tracing of Brady-rhizobium strains helps to correctly interpret Acacia mangium response to inoculation in a reforestation experiment in Madagascar. Biol. Fertil. Soils 2003, 37, 64–69. [Google Scholar] [CrossRef]
- Bilgo, A.; Sangare, S.K.; Thioulouse, J.; Prin, Y.; Hien, V.; Galiana, A.; Baudoin, E.; Hafidi, M.; Ba, A.; Duponnois, R. Response of native soil microbial functions to the controlled mycorrhization of an exotic tree legume, Acacia holosericea in a Sahelian ecosystem. Mycorrhiza 2012, 22, 175–187. [Google Scholar] [CrossRef]
- Degens, B.P.; Harris, J.A. Development of a physiological approach to measuring the catabolic diversity of soil microbial communities. Soil Biol. Biochem. 1997, 29, 1309–1320. [Google Scholar] [CrossRef]
- Okafor, J.C. Improving Edible Species of Forest Products. 1991. Available online: https://scholar.google.com/scholar_lookup?title=%5BImproving+edible+species+of+forest+products%5D&author=Okafor%2C+J.C.&publication_year=1991 (accessed on 18 August 2022).
- Dia, A.; Duponnois, R. Le projet majeur africain de la Grande muraille verte: Concepts et mise en œuvre; IRD, Institut de Recherche pour le Réveloppement: Paris, France, 2010. [Google Scholar]
- Bâ, A.; Guissou, T.; Duponnois, R.; Plenchette, C.; Sacko, O.; Sidibé, D.; Windou, B. Mycorhization contrôlée et fertilisation phosphatée : Applications à la domestication du jujubier. Fruits 2001, 56, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Thioye, B.; Sanguin, H.; Kane, A.; de Faria, S.M.; Fall, D.; Prin, Y.; Sanogo, D.; N’Diaye, C.; Duponnois, R.; Sylla, S.N.; et al. Impact of mycorrhiza-based inoculation strategies on Ziziphus mauritiana Lam. and its native mycorrhizal communities on the route of the Great Green Wall (Senegal). Ecol. Eng. 2019, 128, 66–76. [Google Scholar] [CrossRef]
- Bâ, A.M.; Dalpé, Y.; Guissou, T. Les glomales d’Acacia holosericea et d’Acacia mangium. BOIS For. Trop. 1996, 250, 5–18. [Google Scholar] [CrossRef]
- Garofalo, P.; Paolo, E.D.; Rinaldi, M.; Garofalo, P.; Paolo, E.D.; Rinaldi, M. Durum wheat (Triticum durum Desf.) in rotation with faba bean (Vicia faba var. minor L.): Long-term simulation case study. Crop Pasture Sci. 2009, 60, 240–250. [Google Scholar] [CrossRef]
- Köpke, U.; Nemecek, T. Ecological services of faba bean. Field Crops Res. 2010, 115, 217–233. [Google Scholar] [CrossRef]
- Wahbi, S.; Prin, Y.; Thioulouse, J.; Sanguin, H.; Baudoin, E.; Maghraoui, T.; Oufdou, K.; Le Roux, C.; Galiana, A.; Hafidi, M.; et al. Impact of Wheat/Faba Bean Mixed Cropping or Rotation Systems on Soil Microbial Functionalities. Front. Plant Sci. 2016, 7, 1364. [Google Scholar] [CrossRef] [Green Version]
- Mitter, B.; Pfaffenbichler, N.; Flavell, R.; Compant, S.; Antonielli, L.; Petric, A.; Berninger, T.; Naveed, M.; Sheibani-Tezerji, R.; von Maltzahn, G.; et al. A New Approach to Modify Plant Microbiomes and Traits by Introducing Beneficial Bacteria at Flowering into Progeny Seeds. Front. Microbiol. 2017, 8, 11. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2017.00011 (accessed on 27 June 2022). [CrossRef] [Green Version]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The plant endosphere world—Bacterial life within plants. Environ. Microbiol. 2021, 23, 1812–1829. [Google Scholar] [CrossRef]
- Duponnois, R.; Ouahmane, L.; Kane, A.; Thioulouse, J.; Hafidi, M.; Boumezzough, A.; Prin, Y.; Baudoin, E.; Galiana, A.; Dreyfus, B. Nurse shrubs increased the early growth of Cupressus seedlings by enhancing belowground mutualism and soil microbial activity. Soil Biol. Biochem. 2011, 43, 2160–2168. [Google Scholar] [CrossRef]
- Ouahmane, L.; Thioulouse, J.; Hafidi, M.; Prin, Y.; Ducousso, M.; Galiana, A.; Plenchette, C.; Kisa, M.; Duponnois, R. Soil functional diversity and P solubilization from rock phosphate after inoculation with native or allochtonous arbuscular mycorrhizal fungi. For. Ecol. Manag. 2007, 241, 200–208. [Google Scholar] [CrossRef]
- Ouahmane, L.; Hafidi, M.; Thioulouse, J.; Ducousso, M.; Kisa, M.; Prin, Y.; Galiana, A.; Boumezzough, A.; Duponnois, R. Improvement of Cupressus atlantica Gaussen growth by inoculation with native arbuscular mycorrhizal fungi. J. Appl. Microbiol. 2007, 103, 683–690. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasslahsen, B.; Prin, Y.; Ferhout, H.; Smouni, A.; Duponnois, R. Management of Plant Beneficial Fungal Endophytes to Improve the Performance of Agroecological Practices. J. Fungi 2022, 8, 1087. https://doi.org/10.3390/jof8101087
Nasslahsen B, Prin Y, Ferhout H, Smouni A, Duponnois R. Management of Plant Beneficial Fungal Endophytes to Improve the Performance of Agroecological Practices. Journal of Fungi. 2022; 8(10):1087. https://doi.org/10.3390/jof8101087
Chicago/Turabian StyleNasslahsen, Bouchra, Yves Prin, Hicham Ferhout, Abdelaziz Smouni, and Robin Duponnois. 2022. "Management of Plant Beneficial Fungal Endophytes to Improve the Performance of Agroecological Practices" Journal of Fungi 8, no. 10: 1087. https://doi.org/10.3390/jof8101087
APA StyleNasslahsen, B., Prin, Y., Ferhout, H., Smouni, A., & Duponnois, R. (2022). Management of Plant Beneficial Fungal Endophytes to Improve the Performance of Agroecological Practices. Journal of Fungi, 8(10), 1087. https://doi.org/10.3390/jof8101087







