Oral Chronic Hyperplastic Candidiasis and Its Potential Risk of Malignant Transformation: A Systematic Review and Prevalence Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Critical Appraisal
2.6. Statistical Analysis
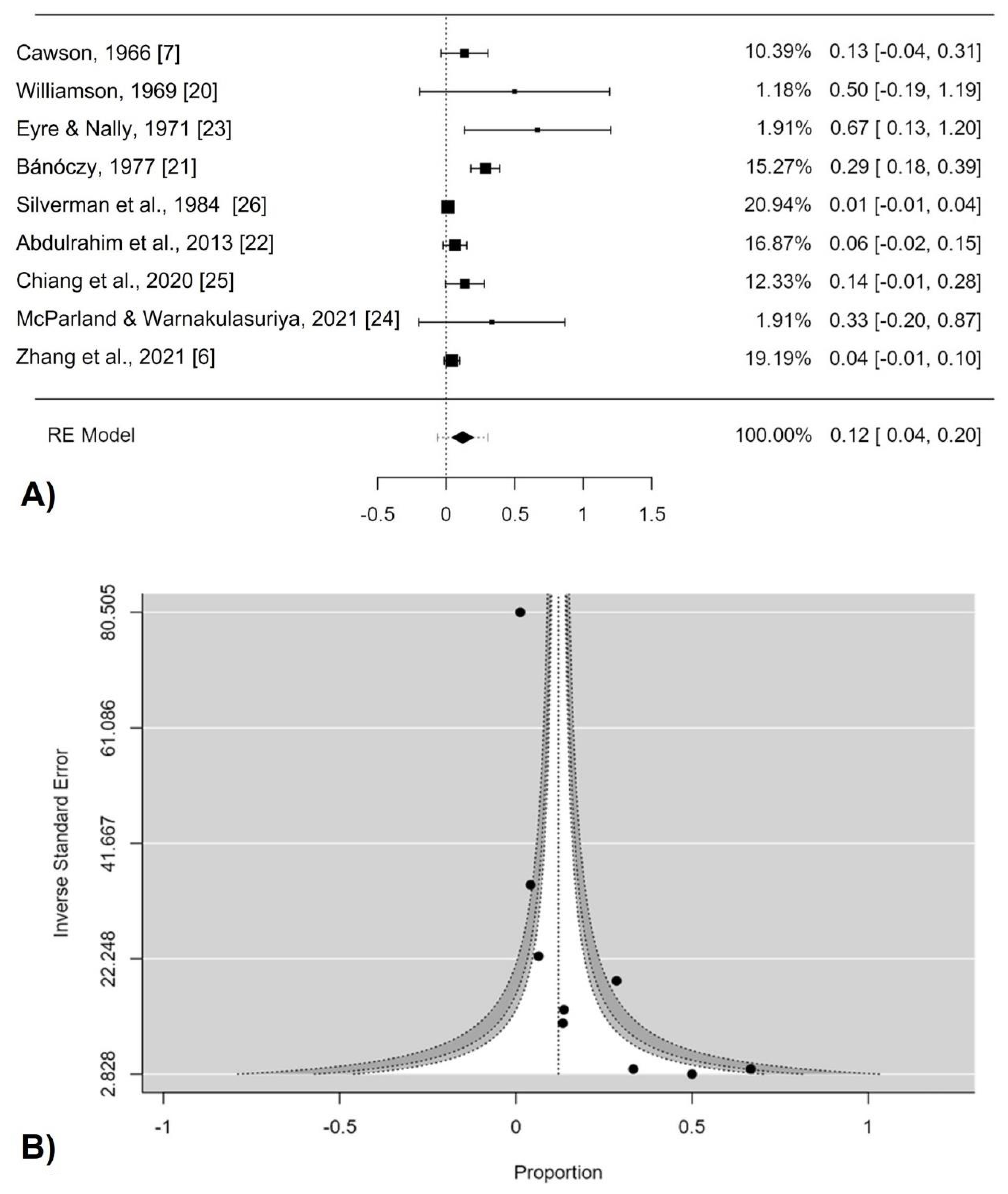
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Scores and Data Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Database | Search |
|---|---|
| PubMed | (“Candidiasis, Chronic Mucocutaneous” [Mesh] or “Chronic Hyperplastic Candidiasis” [All Fields] or “candidal leukoplakia” [All Fields] or “Candida leukoplakia” [All Fields]) and (malign* or premalign* or “potentially malignant disorder” or “precancer” or “cancer” [All Fields] or “Carcinoma, Squamous Cell” [Mesh] or “squamous cell carcinoma” [All Fields] or “oscc” [All Fields] or “transformation” [All Fields] or “risk” [All Fields] or “progression” [All Fields]). |
| Scopus | TITLE-ABS (“Chronic Hyperplastic Candidiasis” OR “Chronic Mucocutaneous Candidiasis” OR “candidal leukoplakia” OR “candida leukoplakia”) AND TITLE-ABS (“malignant” OR “malignant” OR “evolution” OR “evolves” OR “evolve” OR “progress” OR “progresses” OR “progression” OR “transformation” OR “prognosis” OR “prognostic” OR “prognoses”) |
| Web of Science | TS = (“Chronic hyperplastic candidiasis” OR “CHC”) AND TS = (“malignant” OR “malignant” OR “evolution” OR “evolves” OR “evolve” OR “progress” OR “progresses” OR “progression” OR “transformation” OR “prognosis” OR “prognostic” OR “prognoses”) AND article type: articles |
| LILACS | tw:(“Chronic hyperplastic candidiasis” OR “candidiasis hiperplasica cronica“ OR “candidiase hiperplasica“) AND tw:(“malignant” OR “malignent” OR “maligna” OR “malignas” OR “maligno” OR “malignos” OR “evolution” OR “evolução” OR “evolución” OR “progression” OR “progressão” OR “progressión” OR “avance” OR “avanço” OR “transformation” OR “transformação” OR “transformación” OR “prognosis” OR “prognostic” OR “prognoses” OR “prognóstico” OR “pronóstico” OR “prognósticos” OR “pronósticos”) |
| EMBASE | (‘Chronic hyperplastic candidiasis’) AND (‘malignant’ OR ‘malignent’ OR ‘evolution’ OR ‘evolves’ OR ‘evolve’ OR ‘progress’ OR ‘progresses’ OR ‘progression’ OR ‘transformation’ OR ‘prognosis’ OR ‘prognostic’ OR ‘prognoses’) |
Appendix B

References
- Scully, C.; Ei-Kabir, M.; Samaranayake, L.P. Candida and Oral Candidosis: A Review. Crit. Rev. Oral Biol. Med. 1994, 5, 125–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfredi, M.; Polonelli, L.; Aguirre-Urizar, J.M.; Carrozzo, M.; McCullough, M. Urban Legends Series: Oral Candidosis. Oral Dis. 2013, 19, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Lynch, N.; McCullough, M. Oral Fungal Infections: An Update for the General Practitioner. Aust. Dent. J. 2010, 55, 48–54. [Google Scholar] [CrossRef]
- Sitheeque, M.; Samaranayake, L. Chronic Hyperplastic Candidosis/Candidiasis (Candidal Leukoplakia). Crit. Rev. Oral Biol. Med. 2003, 14, 253–267. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, P.; Zhao, W.; Hua, H.; Yan, Z. Fluorescence Staining Vs. Routine Koh Smear for Rapid Diagnosis of Oral Candidiasis-a Diagnostic Test. Oral Dis. 2020, 26, 941–947. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, S.; Wang, X.; Gao, Y.; Yan, Z. Malignant Transformation and Treatment Recommendations of Chronic Hyperplastic Candidiasis-a Six-Year Retrospective Cohort Study. Mycoses 2021, 64, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Cawson, R.A.; Lehner, T. Chronic Hyperplastic Candidiasis--Candidal Leukoplakia. Br. J. Dermatol. 1968, 80, 9–16. [Google Scholar] [CrossRef]
- Pina, P.S.S.; Custódio, M.; Sugaya, N.N.; De Sousa, S.C.O.M. Histopathologic Aspects of the So-Called Chronic Hyperplastic Candidiasis: An Analysis of 36 Cases. J. Cutan. Pathol. 2021, 48, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Bartie, K.L.; Williams, D.W.; Wilson, M.J.; Potts, A.J.C.; Lewis, M.A.O. Pcr Fingerprinting of Candida Albicans Associated with Chronic Hyperplastic Candidosis and Other Oral Conditions. J. Clin. Microbiol. 2001, 39, 4066–4075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez-Garcia, A.; Rementeria, A.; Aguirre-Urizar, J.M.; Moragues, M.D.; Antoran, A.; Pellon, A.; Abad-Diaz-de-Cerio, A.; Hernando, F.L. Candida Albicans and Cancer: Can This Yeast Induce Cancer Development or Progression? Crit. Rev. Microbiol. 2016, 42, 181–193. [Google Scholar] [PubMed]
- Odell, E.; Kujan, O.; Warnakulasuriya, S.; Sloan, P. Oral Epithelial Dysplasia: Recognition, Grading and Clinical Significance. Oral Dis. 2021, 27, 1947–1976. [Google Scholar] [CrossRef]
- Brennan, M.; Migliorati, C.A.; Lockhart, P.B.; Wray, D.; Al-Hashimi, I.; Axéll, T.; Bruce, A.J.; Carpenter, W.; Eisenberg, E.; Epstein, J.B.; et al. Management of Oral Epithelial Dysplasia: A Review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, S19.e1–S19.e12. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral Potentially Malignant Disorders: A Consensus Report from an International Seminar on Nomenclature and Classification, Convened by the Who Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The Prisma 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Moola, S.; Riitano, D.; Lisy, K. The Development of a Critical Appraisal Tool for Use in Systematic Reviews Addressing Questions of Prevalence. Int. J. Health Policy Manag. 2014, 3, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newcombe, R.G. Two-Sided Confidence Intervals for the Single Proportion: Comparison of Seven Methods. Stat. Med. 1998, 17, 857–872. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W.-L. Outlier and Influence Diagnostics for Meta-Analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Thompson. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Williamson, D.M. Chronic Hyperplastic Candidiasis and Squamous Carcinoma. Br. J. Dermatol. 1969, 81, 125–127. [Google Scholar] [CrossRef]
- Banoczy, J. Follow-up Studies in Oral Leukoplakia. J. Maxillofac. Surg. 1977, 5, 69–75. [Google Scholar] [CrossRef]
- Abdulrahim, M.H.; McManus, B.A.; Flint, S.R.; Coleman, D.C. Genotyping Candida Albicans from Candida Leukoplakia and Non-Candida Leukoplakia Shows No Enrichment of Multilocus Sequence Typing Clades but Enrichment of Abc Genotype C in Candida Leukoplakia. PLoS ONE 2013, 8, e73738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyre, J.; Nally, F.F. Oral Candidosis and Carcinoma. Br. J. Dermatol. 1971, 85, 73–75. [Google Scholar] [CrossRef] [PubMed]
- McParland, H.; Warnakulasuriya, S. Lichenoid Morphology Could Be an Early Feature of Oral Proliferative Verrucous Leukoplakia. J. Oral Pathol. Med. 2021, 50, 229–235. [Google Scholar] [CrossRef]
- Chiang, W.; Liu, S.; Lin, J.; Chiu, S.; Gou, S.; Chiou, C.; Chang, C. Malignant Development in Patients with Oral Potentially Malignant Disorders Detected through Nationwide Screening: Outcomes of 5-Year Follow-up at a Single Hospital. Head Neck 2020, 42, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S., Jr.; Gorsky, M.; Lozada, F. Oral Leukoplakia and Malignant Transformation. A Follow-up Study of 257 Patients. Cancer 1984, 53, 563–568. [Google Scholar]
- El-Naggar, A.K.; Chan, J.K.; Takata, T.; Grandis, J.R.; Slootweg, P.J. The Fourth Edition of the Head and Neck World Health Organization Blue Book: Editors’ Perspectives. Hum. Pathol. 2017, 66, 10–12. [Google Scholar] [CrossRef]
- McCullough, M.; Jaber, M.; Barrett, A.W.; Bain, L.; Speight, P.M.; Porter, S.R. Oral yeast carriage correlates with presence of oral epithelial dysplasia. Oral Oncol. 2002, 38, 391–393. [Google Scholar] [CrossRef]
- Dilhari, A.; Weerasekera, M.M.; Siriwardhana, A.; Maheshika, O.; Gunasekara, C.; Karunathilaka, S.; Nagahawatte, A.; Fernando, N. Candida Infection in Oral Leukoplakia: An Unperceived Public Health Problem. Acta Odontol. Scand. 2016, 74, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A.O.; Williams, D.W. Diagnosis and Management of Oral Candidosis. Br. Dent. J. 2017, 223, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Shukla, K.; Vun, I.; Lov, I.; Laparidis, G.; McCamley, C.; Ariyawardana, A. Role of Candida Infection in the Malignant Transformation of Oral Leukoplakia: A Systematic Review of Observational Studies. Transl. Res. Oral Oncol. 2019, 4, 2057178X19828229. [Google Scholar] [CrossRef]
- Yang, S.-W.; Lee, Y.-C.; Lee, Y.-S.; Chang, L.-C.; Lai, Y.-R. Risk Assessment of Malignant Transformation of Oral Leukoplakia in Patients with Previous Oral Squamous Cell Carcinoma. Int. J. Oral Maxillofac. Surg. 2022, in press. [CrossRef] [PubMed]
- Aguirre-Urizar, J.M.; Lafuente-Ibanez de Mendoza, I.; Warnakulasuriya, S. Malignant Transformation of Oral Leukoplakia: Systematic Review and Meta-Analysis of the Last 5 Years. Oral Dis. 2021, 27, 1881–1895. [Google Scholar] [CrossRef] [PubMed]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; López, S.P.; Shanti, R.M. Potentially Malignant Disorders of the Oral Cavity and Oral Dysplasia: A Systematic Review and Meta-Analysis of Malignant Transformation Rate by Subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef]
- Axéll, T.; Pindborg, J.J.; Smith, C.J.; Van der Waal, I.; an International Collaborative Group on Oral White Lesions. Oral White Lesions with Special Reference to Precancerous and Tobacco-Related Lesions: Conclusions of an International Symposium Held in Uppsala, Sweden, May 18–21 1994. International Collaborative Group on Oral White Lesions. J. Oral Pathol. Med. 1996, 25, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Krogh, P.; Hald, B.; Holmstrup, P. Possible Mycological Etiology of Oral Mucosal Cancer: Catalytic Potential of Infecting Candida Albicans and Other Yeasts in Production of N-Nitrosobenzylmethylamine. Carcinogenesis 1987, 8, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Alnuaimi, A.D.; Ramdzan, A.N.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Kolev, S.D.; Reynolds, E.C.; McCullough, M.J. Candida Virulence and Ethanol-Derived Acetaldehyde Production in Oral Cancer and Non-Cancer Subjects. Oral Dis. 2016, 22, 805–814. [Google Scholar] [CrossRef]
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. Oral Microbiome: A New Biomarker Reservoir for Oral and Oropharyngeal Cancers. Theranostics 2017, 7, 4313–4321. [Google Scholar] [CrossRef]
- Delaloye, J.; Calandra, T. Invasive Candidiasis as a Cause of Sepsis in the Critically Ill Patient. Virulence 2014, 5, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Jayachandran, A.L.; Katragadda, R.; Thyagarajan, R.; Vajravelu, L.; Manikesi, S.; Kaliappan, S.; Jayachandran, B. Oral Candidiasis among Cancer Patients Attending a Tertiary Care Hospital in Chennai, South India: An Evaluation of Clinicomycological Association and Antifungal Susceptibility Pattern. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 8758461. [Google Scholar] [CrossRef] [Green Version]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Höfs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin Is a Fungal Peptide Toxin Critical for Mucosal Infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y. Oral Candidosis: Pathophysiology and Best Practice for Diagnosis, Classification, and Successful Management. J. Fungi 2021, 7, 555. [Google Scholar] [CrossRef] [PubMed]
- Theofilou, V.I.; Alfaifi, A.; Montelongo-Jauregui, D.; Pettas, E.; Georgaki, M.; Nikitakis, N.G.; Jabra-Rizk, M.A.; Sultan, A.S. The Oral Mycobiome: Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2022, 51, 413–420. [Google Scholar] [CrossRef]
- Lamey, P.J.; Lewis, M.A.; Macdonald, D.G. Treatment of Candidal Leukoplakia with Fluconazole. Br. Dent. J. 1989, 166, 296–298. [Google Scholar] [CrossRef]
- Alba, A.C.; Alexander, P.E.; Chang, J.; MacIsaac, J.; DeFry, S.; Guyatt, G.H. High Statistical Heterogeneity Is More Frequent in Meta-Analysis of Continuous Than Binary Outcomes. J. Clin. Epidemiol. 2016, 70, 129–135. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Patsopoulos, N.; Evangelou, E. Uncertainty in Heterogeneity Estimates in Meta-Analyses. BMJ 2007, 335, 914–916. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Gavaghan, D.; Egger, M. Publication and Related Bias in Meta-Analysis: Power of Statistical Tests and Prevalence in the Literature. J. Clin. Epidemiol. 2000, 53, 1119–1129. [Google Scholar] [CrossRef]
- Lane, P.W. Meta-Analysis of Incidence of Rare Events. Stat. Methods Med. Res. 2013, 22, 117–132. [Google Scholar] [CrossRef]
- Delgado-Rodriguez, M.; Llorca, J. Bias. J. Epidemiol. Community Health 2004, 58, 635–641. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, R.I.; Feinstein, A.R. The Problem of “Protopathic Bias” in Case-Control Studies. Am. J. Med. 1980, 68, 255–258. [Google Scholar] [CrossRef]
- Zambon, J.J.; Haraszthy, V.I. The Laboratory Diagnosis of Periodontal Infections. Periodontology 2000 1995, 7, 69–82. [Google Scholar] [CrossRef] [PubMed]


| Author and Year | Country | Study Type | Patients | Follow-Up (Years) | Method of Assessment | Malignant Development (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| n | Sex | Mean Age (Years) | |||||||
| F | M | ||||||||
| Cawson, 1966 [7] | UK | Case series | 15 | 4 | 11 | 50.5 | 10 | Biopsy (P.A.S) | 13.3 |
| Williamson, 1969 [20] | UK | Case series | 2 | 0 | 2 | 59.5 | - | Biopsy (P.A.S), culture | 50 |
| Eyre & Nally, 1971 [23] | UK | Case report | 3 | 0 | 3 | 55.7 | - | Scraping and culture | 66.6 |
| Bánóczy, 1977 [21] | Hungary | Cohort | 70 | - | - | - | 9.8 | Biopsy (P.A.S) | 28.7 |
| Silverman et al., 1984 [26] | USA | Cohort | 80 | - | - | 54 | 7.2 | Biopsy (P.A.S), culture | 4.4 |
| Abdulrahim et al., 2013 [22] | Ireland | Case-control | 31 | 14 | 17 | 57.8 | 2 | Biopsy (P.A.S) | 6.5 |
| Chiang et al., 2020 [25] | Taiwan | Cohort | 22 | - | - | - | 5 | Biopsy (P.A.S), | 13.6 |
| McParland & Warnakulasuriya, 2021 [24] | UK | Descriptive | 3 | - | - | - | 16 | Biopsy (P.A.S), | 33.3 |
| Zhang et al., 2021 [6] | China | Cohort | 48 | 13 | 35 | 54.9 | 6 | Biopsy (P.A.S), | 4.2 |
| Total | 274 | 31 | 68 | 55.4 | 8 | - | 12.4 | ||
| Author and Year | Location | Histopathology | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FOM | Retromolar | Gingiva | Palate | Tongue | Buccal | Lip | SCC | High-ED | Low-ED | No ED | |
| Cawson, 1966 [7] | 0 | 0 | 0 | 4 | 4 | 6 | 1 | - | - | - | - |
| Williamson, 1969 [20] | - | - | - | - | - | - | - | 1 | - | - | 1 |
| Eyre & Nally, 1971 [23] | - | - | - | - | - | 2 | 3 | 2 | - | - | - |
| Bánóczy, 1977 [21] | - | - | - | - | - | - | - | - | - | - | - |
| Silverman et al., 1984 [26] | - | - | - | - | - | - | - | 2 | - | - | - |
| Abdulrahim et al., 2013 [22] | 0 | 0 | 2 | 3 | 4 | 22 | - | 2 | 8 | 18 | 3 |
| Chiang et al., 2020 [25] | - | - | - | - | - | - | 3 | - | - | - | |
| McParland & Warnakulasuriya, 2021 [24] | - | - | - | - | - | - | - | - | - | - | - |
| Zhang et al., 2021 [6] | - | - | - | - | - | - | - | 0 | 1 | 9 | 38 |
| Total | - | - | 2 | 7 | 8 | 30 | 4 | 10 | 9 | 27 | 42 |
| Sample Size (n) | Pooled Data | Heterogeneity | ||||
|---|---|---|---|---|---|---|
| Studies | Patients | PP (95% CI) | p-Value | phet | I2 (%) | |
| Malignant development | ||||||
| 9 | 274 | PP = 12.1% (4.3–19.8) | 0.002 | 0.001 | 78.27 | |
| Subgroup analysis | ||||||
| Low and moderate risk of bias | 6 | 247 | PP = 9.8% (2.0–17.8) | 0.014 | 0.001 | 81.98 |
| High risk of bias | 3 | 27 | PP = 34.6% (−0.2–71.5) | 0.06 | 0.113 | 54.21 |
| Asian | 2 | 70 | PP = 6.5% (−1.5–14.5) | 0.111 | 0.229 | 31.03 |
| Non-Asian | 7 | 204 | PP = 16.3% (3.9–28.9) | 0.010 | 0.001 | 82.77 |
| Studies | Pooled Proportion | 95% CI |
|---|---|---|
| Overall | 12.1 | 4.3–19.8 |
| Omitting Cawson [7] | 12.0 | 3.7–20.4 |
| Omitting Williamson [20] | 11.6 | 3.9–19.3 |
| Omitting Eyre & Nally [23] | 10.8 | 3.4–18.1 |
| Omitting Bánóczy [21] | 6.6 | 1.1–12.1 |
| Omitting Silverman et al. [26] | 15.6 | 5.9–25.3 |
| Omitting Abdulrahim et al. [22] | 13.9 | 4.6–23.1 |
| Omitting Chiang et al. [25] | 12.0 | 3.6–20.4 |
| Omitting McParland & Warnakulasuriya [24] | 11.7 | 3.9–19.5 |
| Omitting Zhang et al. [6] | 15.6 | 4.7–26.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-Pouso, A.I.; Pérez-Jardón, A.; Caponio, V.C.A.; Spirito, F.; Chamorro-Petronacci, C.M.; Álvarez-Calderón-Iglesias, Ó.; Gándara-Vila, P.; Lo Muzio, L.; Pérez-Sayáns, M. Oral Chronic Hyperplastic Candidiasis and Its Potential Risk of Malignant Transformation: A Systematic Review and Prevalence Meta-Analysis. J. Fungi 2022, 8, 1093. https://doi.org/10.3390/jof8101093
Lorenzo-Pouso AI, Pérez-Jardón A, Caponio VCA, Spirito F, Chamorro-Petronacci CM, Álvarez-Calderón-Iglesias Ó, Gándara-Vila P, Lo Muzio L, Pérez-Sayáns M. Oral Chronic Hyperplastic Candidiasis and Its Potential Risk of Malignant Transformation: A Systematic Review and Prevalence Meta-Analysis. Journal of Fungi. 2022; 8(10):1093. https://doi.org/10.3390/jof8101093
Chicago/Turabian StyleLorenzo-Pouso, Alejandro I., Alba Pérez-Jardón, Vito Carlo Alberto Caponio, Francesca Spirito, Cintia M. Chamorro-Petronacci, Óscar Álvarez-Calderón-Iglesias, Pilar Gándara-Vila, Lorenzo Lo Muzio, and Mario Pérez-Sayáns. 2022. "Oral Chronic Hyperplastic Candidiasis and Its Potential Risk of Malignant Transformation: A Systematic Review and Prevalence Meta-Analysis" Journal of Fungi 8, no. 10: 1093. https://doi.org/10.3390/jof8101093
APA StyleLorenzo-Pouso, A. I., Pérez-Jardón, A., Caponio, V. C. A., Spirito, F., Chamorro-Petronacci, C. M., Álvarez-Calderón-Iglesias, Ó., Gándara-Vila, P., Lo Muzio, L., & Pérez-Sayáns, M. (2022). Oral Chronic Hyperplastic Candidiasis and Its Potential Risk of Malignant Transformation: A Systematic Review and Prevalence Meta-Analysis. Journal of Fungi, 8(10), 1093. https://doi.org/10.3390/jof8101093







