In Vitro Antifungal Activity of LL-37 Analogue Peptides against Candida spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Purification of Antimicrobial Peptides
2.2. Microorganisms
2.3. Susceptibility Assay of Candida Planktonic Cells
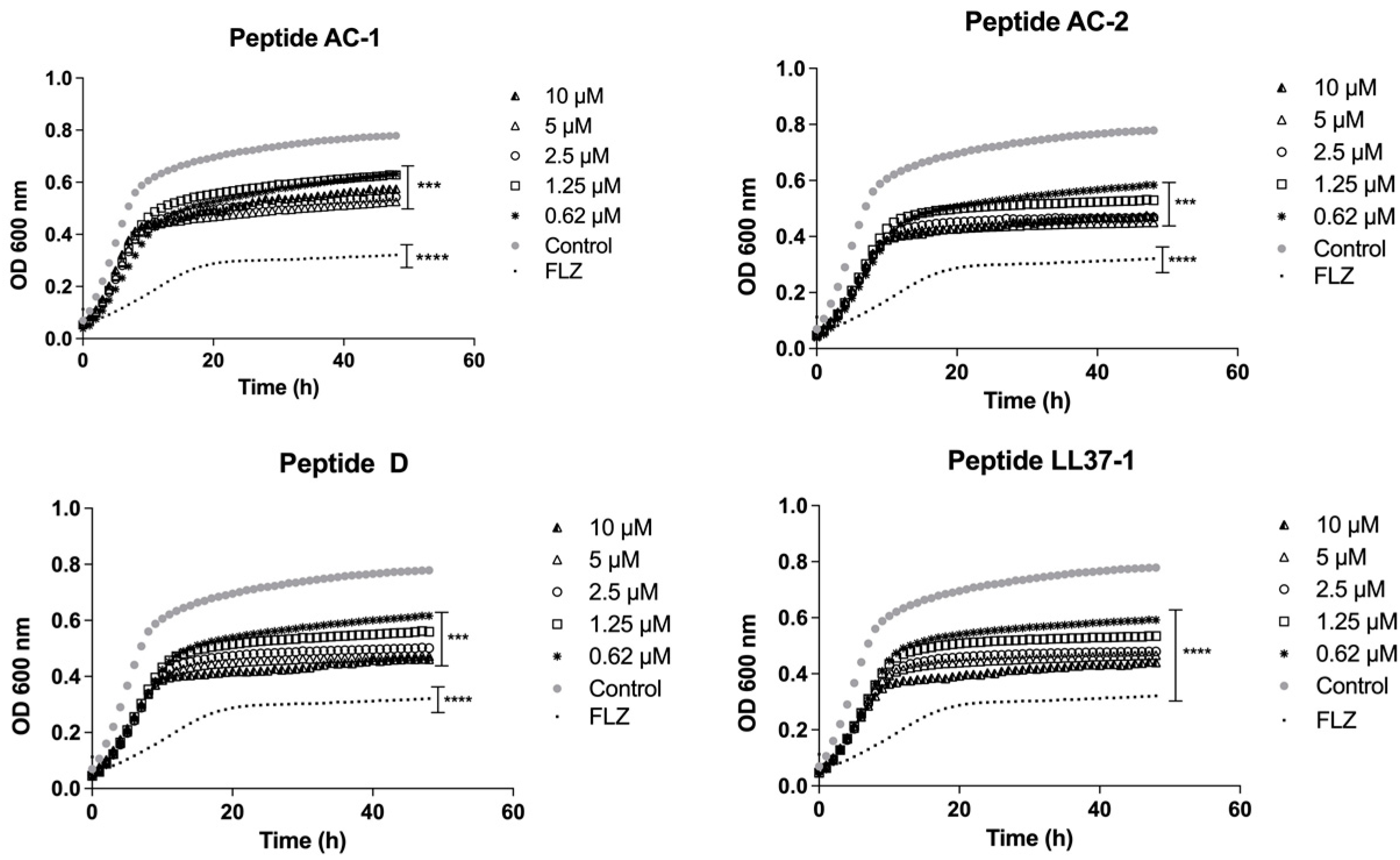
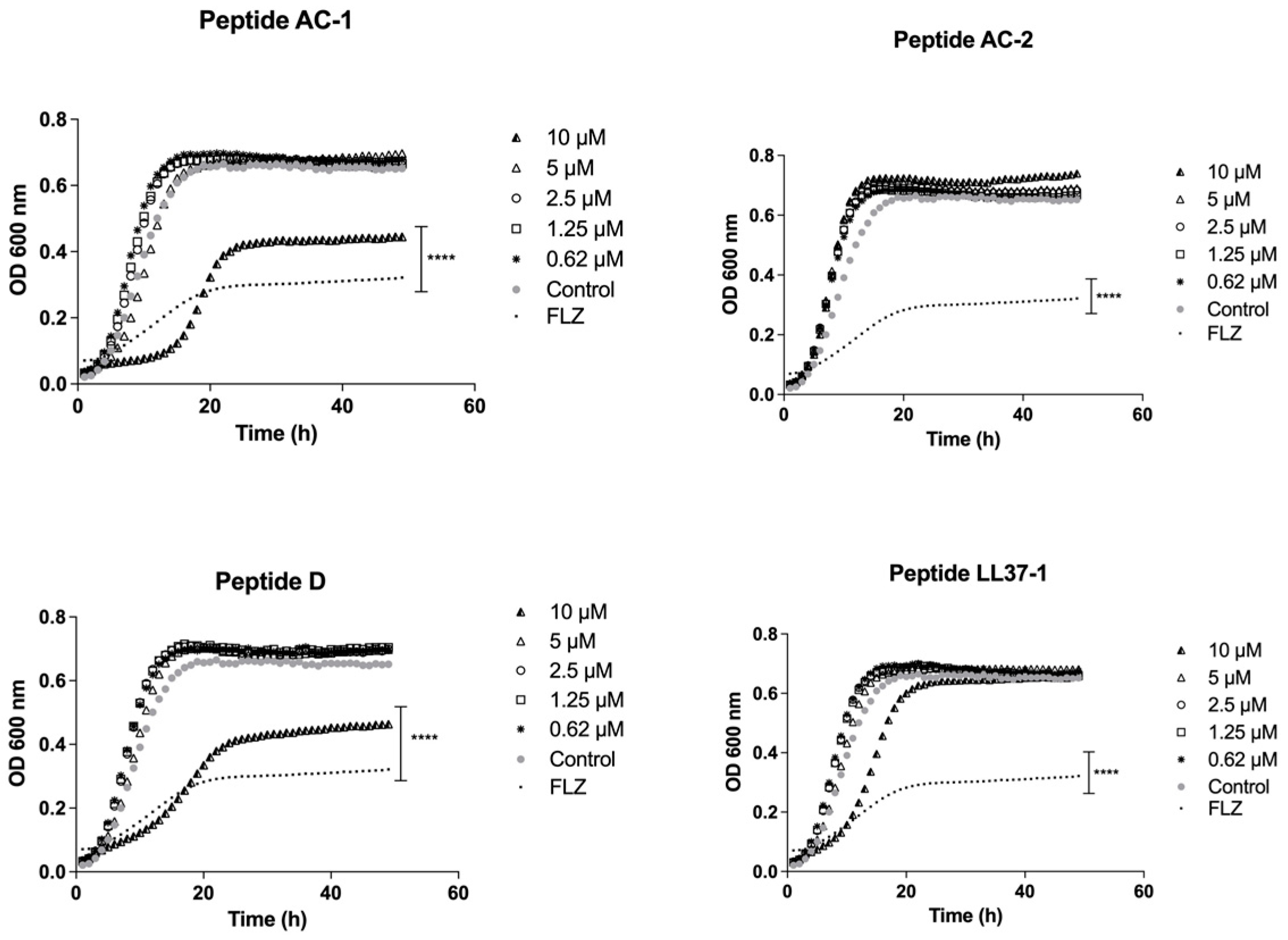
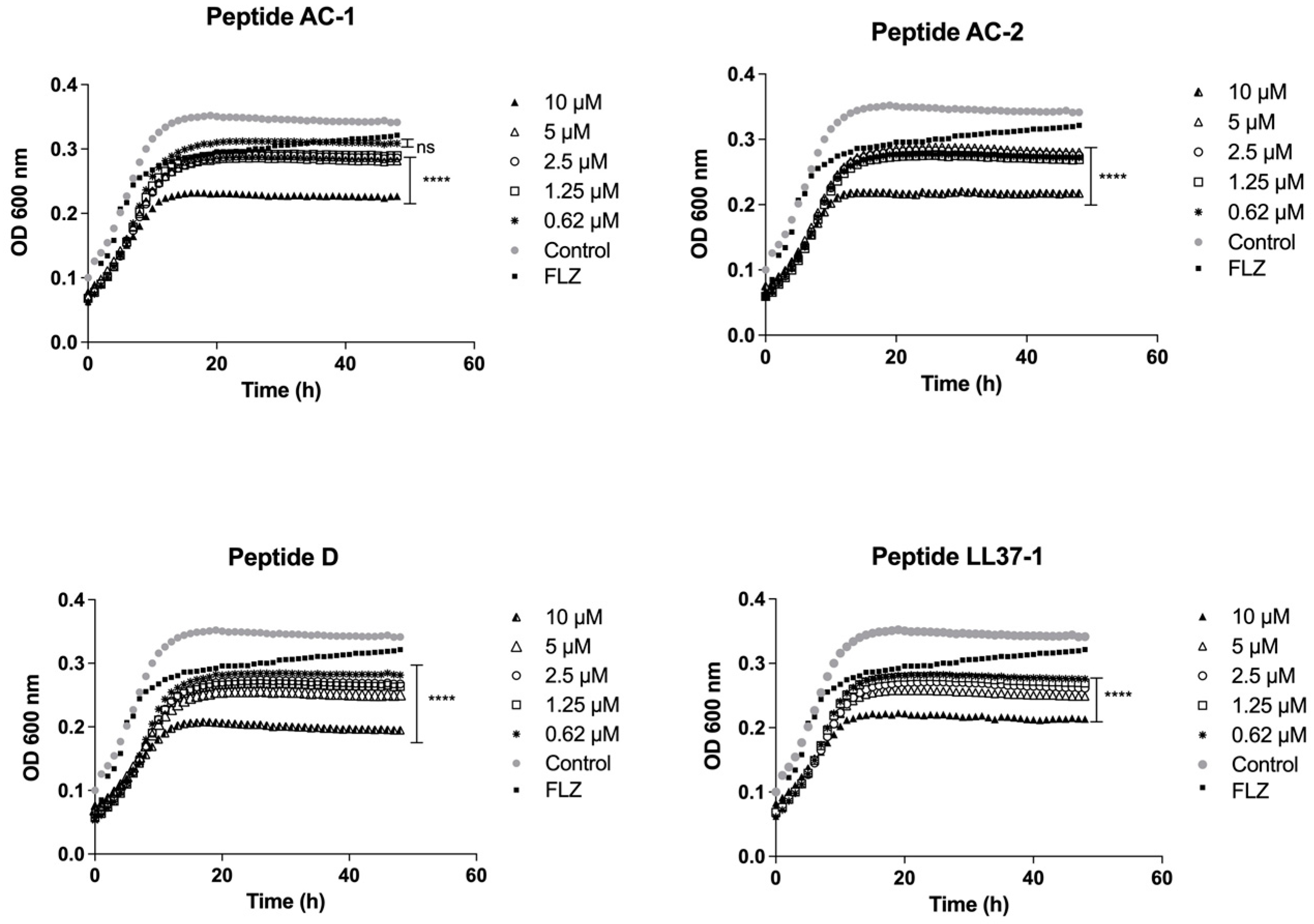
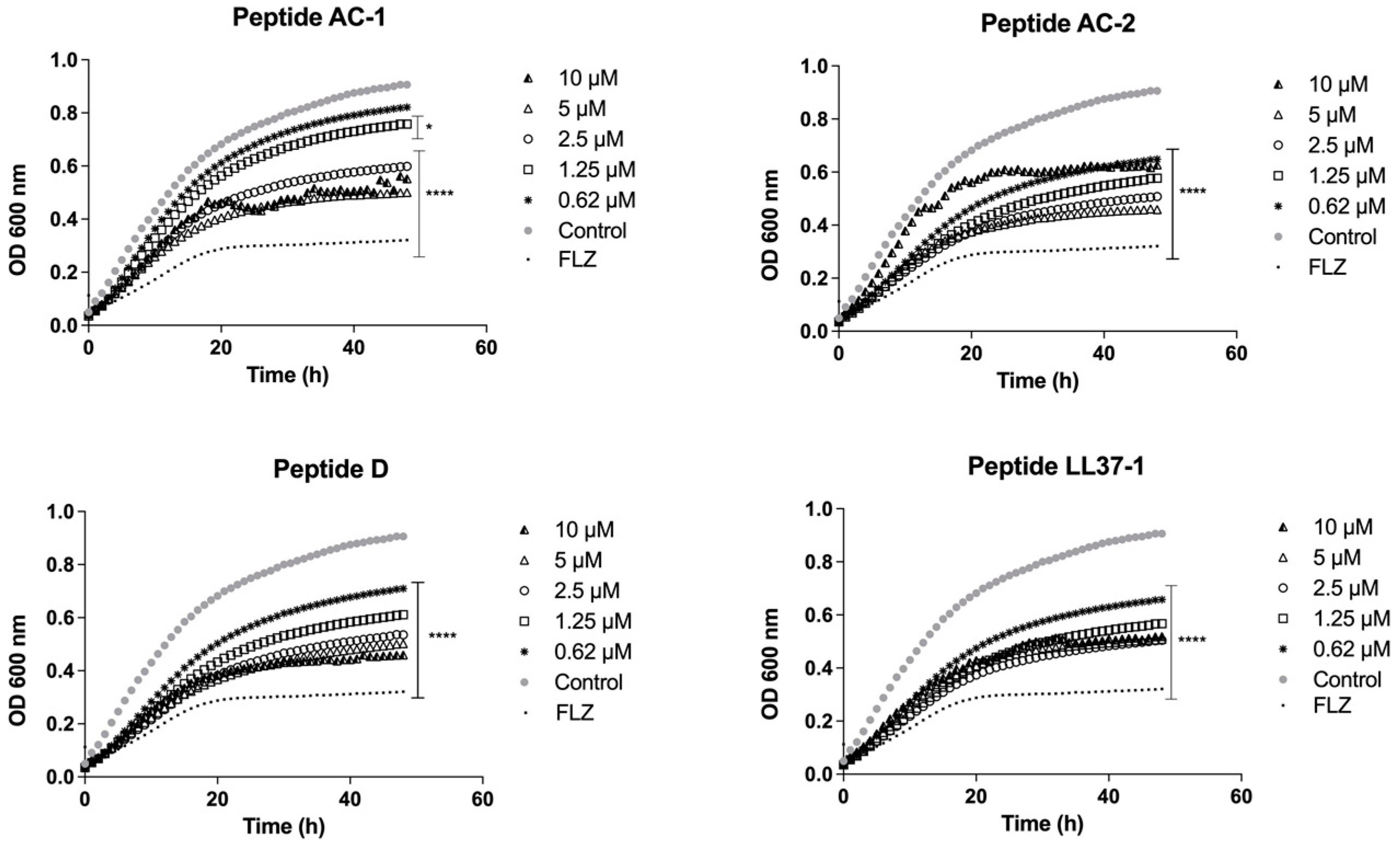
2.4. Determination of Growth Phases Using the LL-37-Derived Peptides
2.5. Preformed Biofilm Eradication Assay
2.6. Scanning Electron Microscopy (SEM)
2.7. Statistical Analysis
3. Results
3.1. Antifungal Susceptibility in Planktonic Cells of Candida spp.
3.2. Determination of Yeast Growth Phases Using LL-37 Analogue Peptides
3.3. Effect of LL-37 Analogue Peptides in the Biofilm Formation Process
3.4. Scanning Electron Microscopy (SEM)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Bedout, C.; Gómez, B.L. Candida y candidiasis invasora: Un reto continuo para su diagnóstico temprano. Infectio 2010, 14, 159–171. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Moreno, C.A.; Cortes, J.A.; Denning, D.W. Burden of fungal infections in Colombia. J. Fungi 2018, 4, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Moet, G.J.; Messer, S.A.; Jones, R.N.; Castanheira, M. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: Report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J. Clin. Microbiol. 2011, 49, 396–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front. Cell Infect Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef]
- De Smet, K.; Contreras, R. Human antimicrobial peptides: Defensins, cathelicidins and histatins. Biotechnol. Lett. 2005, 27, 1337–1347. [Google Scholar] [CrossRef]
- Dürr, U.H.N.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [Green Version]
- Duplantier, A.J.; van Hoek, M.L. The human cathelicidin antimicrobial peptide LL-37 as a potential treatment for polymicrobial infected wounds. Front. Immunol. 2013, 4, 143. [Google Scholar] [CrossRef] [Green Version]
- Tzitzilis, A.; Boura-Theodorou, A.; Michail, V.; Papadopoulos, S.; Krikorian, D.; Lekka, M.E.; Koukkou, A.; Sakarellos-Daitsiotis, M.; Panou-Pomonis, E. Cationic amphipathic peptide analogs of cathelicidin LL-37 as a probe in the development of antimicrobial/anticancer agents. J. Pept. Sci. 2020, 26, e3254. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Huang, L.C.; Romanowski, E.G.; Yates, K.A.; Proske, R.J.; McDermott, A.M. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr. Eye Res. 2005, 30, 385–394. [Google Scholar] [CrossRef]
- Tsai, P.; Yang, C.; Chang, H.; Lan, C. Human Antimicrobial Peptide LL-37 Inhibits Adhesion of Candida albicans by Interacting with Yeast Cell-Wall Carbohydrates. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crauwels, P.; Bank, E.; Walber, B.; Wenzel, U.A.; Agerberth, B.; Chanyalew, M.; Abebe, M.; König, R.; Ritter, U.; Reiling, N.; et al. Cathelicidin Contributes to the Restriction of Leishmania in Human Host Macrophages. Front. Immunol. 2019, 10, 2697. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A comprehensive summary of LL-37, the factoctum human cathelicidin peptide. Cell Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Santiago, B.; Sada, E.; Hernández-Pando, R.; Tsutsumi, V. Péptidos antimicrobianos en la inmunidad innata de enfermedades infecciosas. Salud Publica Mex. 2006, 48, 62–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agerberth, B.; Charo, J.; Werr, J.; Olsson, B.; Idali, F.; Lindbom, L.; Kiessling, R.; Jörnvall, H.; Wigzell, H.; Gudmundsson, G.H. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000, 96, 3086–3093. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Nagaoka, I.; Okumura, K.; Ogawa, H. The Human β-Defensins (-1, -2, -3, -4) and Cathelicidin LL-37 Induce IL-18 Secretion through p38 and ERK MAPK Activation in Primary Human Keratinocytes. J. Immunol. 2005, 175, 1776–1784. [Google Scholar] [CrossRef] [Green Version]
- Rico-Mata, R.; De Leon-Rodriguez, L.M.; Avila, E.E. Effect of antimicrobial peptides derived from human cathelicidin LL-37 on Entamoeba histolytica trophozoites. Exp. Parasitol. 2013, 133, 300–306. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Chau, J.K.; Perry, N.A.; de Boer, L.; Zaat, S.A.J.; Vogel, H.J. Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. PLoS ONE 2010, 5, e12684. [Google Scholar] [CrossRef] [Green Version]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Ramage, G.; Lopez-ribot, J.L. A simple and reproducible 96 well plate-based methos for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- de la Fuente-Núñez, C.; Silva, O.N.; Lu, T.K.; Franco, O.L. Antimicrobial peptides: Role in human disease and potential as immunotherapies. Pharmacol. Ther. 2017, 178, 132–140. [Google Scholar] [CrossRef]
- Malmsten, M. Interactions of Antimicrobial Peptides with Bacterial Membranes and Membrane Components. Curr. Top. Med. Chem. 2016, 16, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Takamura, Y.; Kawakami, T.; Aimoto, S.; Saito, H.; Mukai, T. Effect of amino acid distribution of amphipathic helical peptide derived from human apolipoprotein A-I on membrane curvature sensing. FEBS Lett. 2013, 587, 510–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollmann, A.; Martínez, M.; Noguera, M.E.; Augusto, M.T.; Disalvo, A.; Santos, N.C.; Semorile, L.; Maffía, P.C. Role of amphipathicity and hydrophobicity in the balance between hemolysis and peptide-membrane interactions of three related antimicrobial peptides. Colloids Surf. B Biointerfaces 2016, 141, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Alvares, D.S.; Wilke, N.; Ruggiero Neto, J. Effect of N-terminal acetylation on lytic activity and lipid-packing perturbation induced in model membranes by a mastoparan-like peptide. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.; Cheng, Y.; Hsieh, W.; Lan, C. Responses of Candida albicans to the Human Antimicrobial Peptide LL-37. J. Microbiol. 2014, 52, 581–589. [Google Scholar] [CrossRef]
- Nuijens, T.; Piva, E.; Kruijtzer, J.A.W.; Rijkers, D.T.S.; Liskamp, R.M.J.; Quaedflieg, P.J.L.M. Enzymatic C-terminal amidation of amino acids and peptides. Tetrahedron Lett. 2012, 53, 3777–3779. [Google Scholar] [CrossRef]
- Kodedová, M.; Sychrová, H. Synthetic antimicrobial peptides of the halictines family disturb the membrane integrity of Candida cells. Biochim. Biophys. Acta-Biomembr. 2017, 1859, 1851–1858. [Google Scholar] [CrossRef]
- Pierce, C.G.; Vila, T.; Romo, J.A.; Montelongo-Jauregui, D.; Wall, G.; Ramasubramanian, A.; Lopez-Ribot, J.L. The Candida albicans biofilm matrix: Composition, structure and function. J. Fungi. 2017, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, challenges, and promising strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Polonelli, L.; Ciociola, T.; Elviri, L.; Zanello, P.P.; Giovati, L.; Arruda, D.C.; Muñoz, J.; Mortara, R.; Morace, G.; Borghi, E.; et al. A Naturally Occurring Antibody Fragment Neutralizes Infectivity of Diverse Infectious Agents. Sci. Rep. 2016, 6, 35018. [Google Scholar] [CrossRef] [Green Version]
- Ridyard, K.E.; Overhage, J. The Potential of Human Peptide LL-37 as an Antimicrobial and Anti-Biofilm Agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- León-Buitimea, A.; Garza-Cervantes, J.A.; Gallegos-Alvarado, D.Y.; Osorio-Concepción, M.; Morones-Ramírez, J.R. Nanomaterial-based antifungal therapies to combat fungal diseases aspergillosis, coccidioidomycosis, mucormycosis, and candidiasis. Pathogens 2021, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Wani, I.A.; Ahmad, T. Size and shape dependant antifungal activity of gold nanoparticles: A case study of Candida. Colloids Surf. Biointerfaces 2013, 101, 162–170. [Google Scholar] [CrossRef] [PubMed]



| MIC of LL-37 Analogue Peptides | |||||||
|---|---|---|---|---|---|---|---|
| Peptide | C. albicans ATCC 10231 | C. albicans SC5314 | C. albicans 256 (Clinical Strain) | C. parapsilosis ATCC 22019 | C. krusei ATCC 6558 | C. tropicalis ATCC 750 | 20 Vulvovaginal Candidiasis Clinical Isolates |
| AC-1 | 1.25–0.31 µM | 10 µM | 5 µM | 0.15 µM | 0.15 µM | 0.15 µM | 10–2.5 µM |
| AC-2 | 0.31–0.07 µM | 5 µM | 5 µM | 2.5 µM | 0.15 µM | 0.07 µM | 10–2.5 µM |
| D | 2.5–1.25 µM | 5 µM | 5 µM | 5 µM | 10 µM | 0.15 µM | 5–2.5 µM |
| LL37-1 | 0.31–0.07 µM | 1.25 µM | 2.5–5 µM | 1.25 µM | 1.25 µM | 0.07 µM | 5–2.5 µM |
| FLZ | 0.25 µg/mL | 0.25 µg/mL | 8–16 µg/mL | 0.5 µg/mL | 16 µg/mL | 0.5 µg/mL | 2–0.5 µg/mL |
| AMB | 0.5–1 µg/mL | 0.5–1 µg/mL | 4 µg/mL | 0.5 µg/mL | 0.5 µg/mL | 2 µg/mL | 2–4 µg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinilla, G.; Coronado, Y.T.; Chaves, G.; Muñoz, L.; Navarrete, J.; Salazar, L.M.; Taborda, C.P.; Muñoz, J.E. In Vitro Antifungal Activity of LL-37 Analogue Peptides against Candida spp. J. Fungi 2022, 8, 1173. https://doi.org/10.3390/jof8111173
Pinilla G, Coronado YT, Chaves G, Muñoz L, Navarrete J, Salazar LM, Taborda CP, Muñoz JE. In Vitro Antifungal Activity of LL-37 Analogue Peptides against Candida spp. Journal of Fungi. 2022; 8(11):1173. https://doi.org/10.3390/jof8111173
Chicago/Turabian StylePinilla, Gladys, Yenifer Tatiana Coronado, Gabriel Chaves, Liliana Muñoz, Jeannette Navarrete, Luz Mary Salazar, Carlos Pelleschi Taborda, and Julián E. Muñoz. 2022. "In Vitro Antifungal Activity of LL-37 Analogue Peptides against Candida spp." Journal of Fungi 8, no. 11: 1173. https://doi.org/10.3390/jof8111173
APA StylePinilla, G., Coronado, Y. T., Chaves, G., Muñoz, L., Navarrete, J., Salazar, L. M., Taborda, C. P., & Muñoz, J. E. (2022). In Vitro Antifungal Activity of LL-37 Analogue Peptides against Candida spp. Journal of Fungi, 8(11), 1173. https://doi.org/10.3390/jof8111173








