Distinguishing Invasive from Chronic Pulmonary Infections: Host Pentraxin 3 and Fungal Siderophores in Bronchoalveolar Lavage Fluids
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design and Patient Selection
2.2. Criteria for Pulmonary Aspergillosis
2.3. Assays for Ptx3, CRP, CREA, IPA, and CPA Diagnosis Biomarker Monitoring
2.4. Liquid Chromatography and Mass Spectrometry of Siderophores
2.5. Statistical Analysis
3. Results
3.1. Chronic Obstructive Pulmonary Disease and Steroid Treatment Are Common Risk Factors for Pulmonary Aspergillosis
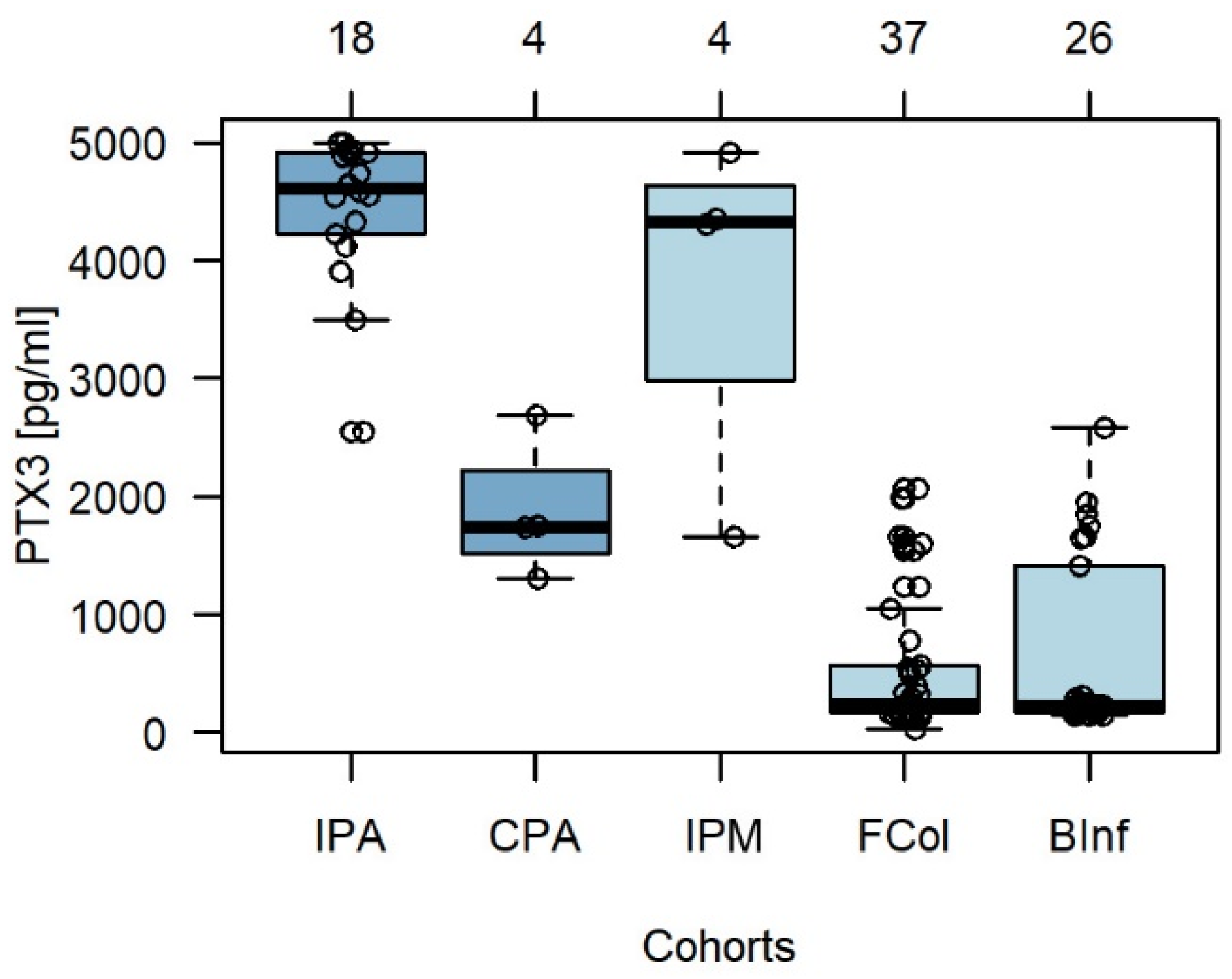
3.2. Pentraxin 3 in BALF Exhibits High Specificity for Invasive Aspergillosis and Mucormycosis
3.3. Fungal Siderophores in BALF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassetti, M.; Azoulay, E.; Kullberg, B.J.; Ruhnke, M.; Shoham, S.; Vazquez, J.; Giacobbe, D.R.; Calandra, T. EORTC/MSGERC Definitions of Invasive Fungal Diseases: Summary of Activities of the Intensive Care Unit Working Group. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 72, S121–S127. [Google Scholar] [CrossRef] [PubMed]
- Latge, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Dobias, R.; Jaworska, P.; Tomaskova, H.; Kanova, M.; Lyskova, P.; Vrba, Z.; Holub, C.; Svobodová, L.; Hamal, P.; Raska, M. Diagnostic value of serum galactomannan, (1,3)-β-d-glucan, and Aspergillus fumigatus-specific IgA and IgG assays for invasive pulmonary aspergillosis in non-neutropenic patients. Mycoses 2018, 61, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet. Infect. Dis. 2020, 21, e149–e162. [Google Scholar] [CrossRef]
- Meersseman, W.; Lagrou, K.; Maertens, J.; Wilmer, A.; Hermans, G.; Vanderschueren, S.; Spriet, I.; Verbeken, E.; Van Wijngaerden, E. Galactomannan in bronchoalveolar lavage fluid: A tool for diagnosing aspergillosis in intensive care unit patients. Am. J. Resp. Crit. Care Med. 2008, 177, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.J.; Li, Y.P.; Xie, L.M.; Zhang, H.L.; Qin, Y.S.; Guo, X.G. Diagnostic Accuracy of Bronchoalveolar Lavage Fluid Galactomannan for Invasive Aspergillosis. Biomed Res. Int. 2020, 2020, 5434589. [Google Scholar] [CrossRef]
- Škríba, A.; Pluháček, T.; Palyzová, A.; Nový, Z.; Lemr, K.; Hajdúch, M.; Petřík, M.; Havlíček, V. Early and Non-invasive Diagnosis of Aspergillosis Revealed by Infection Kinetics Monitored in a Rat Model. Front. Microbiol. 2018, 9, 2356. [Google Scholar] [CrossRef]
- Luptáková, D.; Pluháček, T.; Petřík, M.; Novák, J.; Palyzová, A.; Sokolová, L.; Škríba, A.; Šedivá, B.; Lemr, K.; Havlíček, V. Non-invasive and invasive diagnoses of aspergillosis in a rat model by mass spectrometry. Sci. Rep. 2017, 7, 16523. [Google Scholar] [CrossRef] [Green Version]
- Kabbani, D.; Bhaskaran, A.; Singer, L.G.; Bhimji, A.; Rotstein, C.; Keshavjee, S.; Liles, W.C.; Husain, S. Pentraxin 3 levels in bronchoalveolar lavage fluid of lung transplant recipients with invasive aspergillosis. J. Heart Lung Transplant. 2017, 36, 973–979. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Zhou, W.; Rui, Y.; He, B.; Shi, Y.; Su, X. Pentraxin 3 in bronchoalveolar lavage fluid and plasma in non-neutropenic patients with pulmonary aspergillosis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2019, 25, 504–510. [Google Scholar] [CrossRef]
- Dobiáš, R.; Škríba, A.; Pluháček, T.; Petřík, M.; Palyzová, A.; Káňová, M.; Čubová, E.; Houšť, J.; Novák, J.; Stevens, D.A.; et al. Noninvasive Combined Diagnosis and Monitoring of Aspergillus and Pseudomonas Infections: Proof of Concept. J. Fungi 2021, 7, 730. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutuja, H.P.; Luptáková, D.; Havlíček, V. Infection metallomics for critical care in the post-COVID era. Mass Spectrom. Rev. 2021. [Google Scholar] [CrossRef]
- Škríba, A.; Patil, R.H.; Hubáček, P.; Dobiáš, R.; Palyzová, A.; Marešová, H.; Pluháček, T.; Havlíček, V. Rhizoferrin Glycosylation in Rhizopus microsporus. J. Fungi 2020, 6, 89. [Google Scholar] [CrossRef]
- Novák, J.; Škríba, A.; Havlíček, V. CycloBranch 2: Molecular Formula Annotations Applied to imzML Data Sets in Bimodal Fusion and LC-MS Data Files. Anal. Chem. 2020, 92, 6844–6849. [Google Scholar] [CrossRef]
- R.C.T. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Thiele, C.; Hirschfeld, G. Cutpointr: Improved estimation and validation of optimal cutpoints in R. arXiv 2020, arXiv:2002.09209. [Google Scholar] [CrossRef]
- Narkhede, S. Understanding auc-roc curve. Towards Data Sci. 2018, 26, 220–227. [Google Scholar]
- Hoenigl, M.; Orasch, T.; Faserl, K.; Prattes, J.; Loeffler, J.; Springer, J.; Gsaller, F.; Reischies, F.; Duettmann, W.; Raggam, R.B.; et al. Triacetylfusarinine C: A urine biomarker for diagnosis of invasive aspergillosis. J. Infect. 2019, 78, 150–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asai, Y.; Hiratsuka, T.; Ueda, M.; Kawamura, Y.; Asamizu, S.; Onaka, H.; Arioka, M.; Nishimura, S.; Yoshida, M. Differential Biosynthesis and Roles of Two Ferrichrome-Type Siderophores, ASP2397/AS2488053 and Ferricrocin, in Acremonium persicinum. ACS Chem. Biol. 2022, 17, 207–216. [Google Scholar] [CrossRef]
- Pauwels, N.S.; Bracke, K.R.; Maes, T.; Van Pottelberge, G.R.; Garlanda, C.; Mantovani, A.; Joos, G.F.; Brusselle, G.G. Cigarette smoke induces PTX3 expression in pulmonary veins of mice in an IL-1 dependent manner. Respir. Res. 2010, 11, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurt, O.K.; Tosun, M.; Kurt, E.B.; Talay, F. Pentraxin 3 as a novel biomarker of inflammation in chronic obstructive pulmonary disease. Inflammation 2015, 38, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Thulborn, S.J.; Dilpazir, M.; Haldar, K.; Mistry, V.; Brightling, C.E.; Barer, M.R.; Bafadhel, M. Investigating the role of pentraxin 3 as a biomarker for bacterial infection in subjects with COPD. Int. J. Chron. Obst. Pulm. Dis. 2017, 12, 1199–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, T.; Haraguchi, G.; Kamiishi, T.; Tezuka, D.; Inagaki, H.; Isobe, M. Sensitive assessment of activity of Takayasu’s arteritis by pentraxin3, a new biomarker. J. Am. Coll. Cardiol. 2011, 57, 1712–1713. [Google Scholar] [CrossRef] [Green Version]
- Gamaletsou, M.N.; Denning, D.W. Gastroesophageal Reflux Disease and Pulmonary Diseases Associated with Aspergillosis: Is There a Connection? Mycopathologia 2017, 182, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Inforzato, A.; Bottazzi, B.; Garlanda, C.; Valentino, S.; Mantovani, A. Pentraxins in humoral innate immunity. Adv. Exp. Med. Biol. 2012, 946, 1–20. [Google Scholar] [CrossRef]

| PA (n = 22) | Non-PA (n = 67) | p | |
|---|---|---|---|
| Age (mean, range) | 59 (45–79) | 61 (24–85) | 0.361 |
| Male (ratio) | 13 (59%) | 43 (68%) | 0.800 |
| ICU | 14 (64%) | 11 (16%) | <0.001 |
| Underlying pulmonary diseases: | |||
| COPD | 10 (46%) | 2 (3%) | <0.001 |
| Bronchopneumonia | 3 (14%) | 22 (33%) | 0.104 |
| IPF | 0 (0%) | 10 (15%) | 0.062 |
| H1N1 influenza | 2 (9%) | 0 (0%) | 0.059 |
| Asthma | 0 (0%) | 11 (16%) | 0.059 |
| Bronchitis | 0 (0%) | 5 (8%) | 0.327 |
| Bronchiectasis | 0 (0%) | 3 (5%) | 0.572 |
| Mycobacteriosis | 0 (0%) | 1 (2%) | 1.000 |
| Sarcoidosis | 0 (0%) | 3 (5%) | 0.572 |
| COVID-19 | 1 (5%) | 1 (2%) | 0.436 |
| Extrapulmonary diseases: | |||
| SOM | 1 (5%) | 3 (5%) | 1.000 |
| Haemato-oncology | 2 (9%) | 2 (3%) | 0.254 |
| Cardiovascular disease | 0 (0%) | 1 (1.5%) | 1.000 |
| AKI | 1 (5%) | 0 (0%) | 0.247 |
| TPL | 1 (5%) | 2 (3%) | 1.000 |
| Polytrauma | 0 (0%) | 1 (2%) | 1.000 |
| Osteoarthritis | 1 (5%) | 0 (0%) | 0.247 |
| Steroid treatment | 11 (50%) | 16 (24%) | 0.038 |
| Fc/Crea (ratio *, >0) | TafC/Crea (ratio *, >0) | Ptx3 (≥2545 pg/mL) | CRP (>0.17 mg/L) | GM (PI ≥ 1) | |
|---|---|---|---|---|---|
| Pulmonary Aspergillosis | |||||
| IPA (n = 18) | 5 (28%) | 11 (61%) | 18 (100%) | 0 (0%) | 14 (78%) |
| CPA (n = 4) | 1 (25%) | 0 (0%) | 1 (25%) | 0 (0%) | 2 (50%) |
| Invasive Pulmonary Mucormycosis | |||||
| Rhizopus spp. (n = 4) | 0 (0%) | 0 (0%) | 4 (100%) | 0 (0%) | 0 (0%) |
| Ptx3 (≥2545 pg/mL) (CI %) | TafC/Crea (ratio †) (CI %) | Fc/Crea (ratio †) (CI %) | Ptx3-TafC * (CI %) | GM (PI ≥ 1) (CI %) | |
|---|---|---|---|---|---|
| Sensitivity (%) | 100 (81–100) | 61 (36–83) | 0 (0–6) | 81 | 71 (42–92) |
| Specificity (%) | 98 (91–100) | 98 (91–100) | 100 (81–100) | 98 | 97 (89–100) |
| PPV (%) | 95 (74–100) | 92 (62–100) | 0 (0–100) | 94 | 83 (52–98) |
| NPV (%) | 100 (94–100) | 90 (80–96) | 22 (14–33) | 95 | 94 (85–98) |
| AUC | 0.9991 | 0.8007 | 0.3668 | 0.8181 | 0.7051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobiáš, R.; Jaworská, P.; Skopelidou, V.; Strakoš, J.; Višňovská, D.; Káňová, M.; Škríba, A.; Lysková, P.; Bartek, T.; Janíčková, I.; et al. Distinguishing Invasive from Chronic Pulmonary Infections: Host Pentraxin 3 and Fungal Siderophores in Bronchoalveolar Lavage Fluids. J. Fungi 2022, 8, 1194. https://doi.org/10.3390/jof8111194
Dobiáš R, Jaworská P, Skopelidou V, Strakoš J, Višňovská D, Káňová M, Škríba A, Lysková P, Bartek T, Janíčková I, et al. Distinguishing Invasive from Chronic Pulmonary Infections: Host Pentraxin 3 and Fungal Siderophores in Bronchoalveolar Lavage Fluids. Journal of Fungi. 2022; 8(11):1194. https://doi.org/10.3390/jof8111194
Chicago/Turabian StyleDobiáš, Radim, Pavla Jaworská, Valeria Skopelidou, Jan Strakoš, Denisa Višňovská, Marcela Káňová, Anton Škríba, Pavlína Lysková, Tomáš Bartek, Ivana Janíčková, and et al. 2022. "Distinguishing Invasive from Chronic Pulmonary Infections: Host Pentraxin 3 and Fungal Siderophores in Bronchoalveolar Lavage Fluids" Journal of Fungi 8, no. 11: 1194. https://doi.org/10.3390/jof8111194
APA StyleDobiáš, R., Jaworská, P., Skopelidou, V., Strakoš, J., Višňovská, D., Káňová, M., Škríba, A., Lysková, P., Bartek, T., Janíčková, I., Kozel, R., Cwiková, L., Vrba, Z., Navrátil, M., Martinek, J., Coufalová, P., Krejčí, E., Ulmann, V., Raška, M., ... Havlíček, V. (2022). Distinguishing Invasive from Chronic Pulmonary Infections: Host Pentraxin 3 and Fungal Siderophores in Bronchoalveolar Lavage Fluids. Journal of Fungi, 8(11), 1194. https://doi.org/10.3390/jof8111194







