Metagenomics of Toenail Onychomycosis in Three Victorian Regions of Australia
Abstract
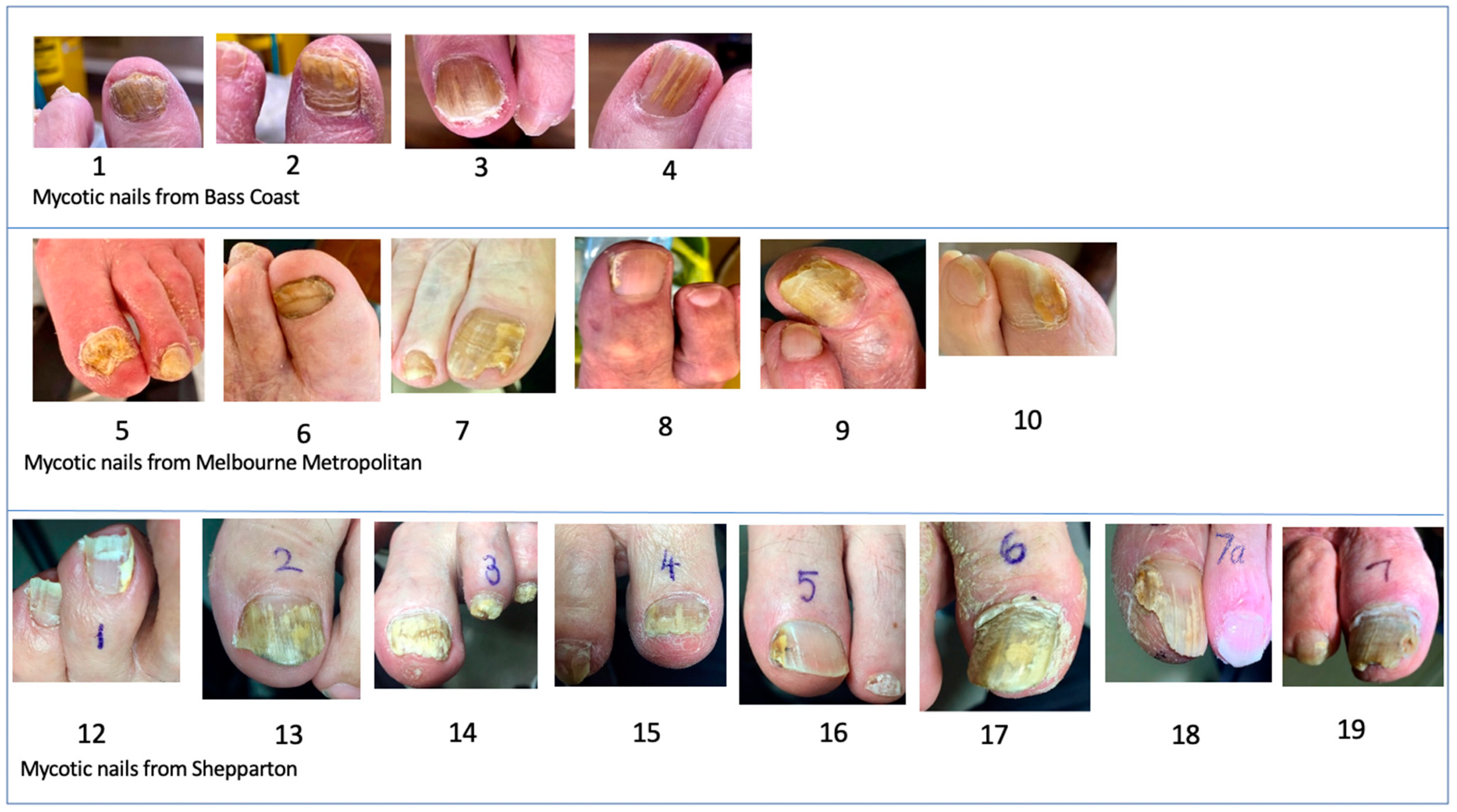
:1. Introduction
2. Materials and Methods
3. Results
3.1. Fungi
3.2. NDMs and Yeasts
3.2.1. Bacteria
3.2.2. Combined Overview of Microflora
4. Discussion
4.1. Most Likely Infecting Agents (MLIAs)
4.2. Mixed Infections
4.3. Significance of NDMs, Yeasts and Bacteria in Nails
4.4. Limitations of This Study
4.5. Estimated Costs of Metagenomic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghannoum, M.A.; Salem, I.; Christensen, L. Epidemiology of onychomycosis. In Onychomycosis: Diagnosis and Effective Management; Wiley: New York, NY, USA, 2018; pp. 13–20. [Google Scholar]
- Kovitwanichkanont, T.; Chong, A.H. Superficial fungal infections. Aust. J. Gen. Pract. 2019, 48, 706–711. [Google Scholar]
- Eba, M.; Njunda, A.L.; Mouliom, R.N.; Kwenti, E.T.; Fuh, A.N.; Nchanji, G.T.; Atashili, J. Onychomycosis in diabetic patients in Fako Division of Cameroon: Prevalence, causative agents, associated factors and antifungal sensitivity patterns. BMC Res. Notes 2016, 9, 494. [Google Scholar]
- Ilkit, M.; Durdu, M. Tinea pedis: The etiology and global epidemiology of a common fungal infection. Crit. Rev. Microbiol. 2015, 41, 374–388. [Google Scholar]
- Bickers, D.R.; Lim, H.W.; Margolis, D.; Weinstock, M.A.; Goodman, C.; Faulkner, E.; Gould, C.; Gemmen, E.; Dall, T. The burden of skin diseases: 2004: A joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J. Am. Acad. Dermatol. 2006, 55, 490–500. [Google Scholar]
- Tamer, F.; Yuksel, M.E. Onychomycosis due to mixed infection with non-dermatophyte molds and yeasts. Dermatol. Online J. 2019, 10, 267–269. [Google Scholar]
- Gupta, A.K.; Taborda, V.B.; Taborda, P.R.; Shemer, A.; Summerbell, R.C.; Nakrieko, K.-A. High prevalence of mixed infections in global onychomycosis. PLoS ONE 2020, 15, e0239648. [Google Scholar]
- Westerberg, D.P.; Voyack, M.J. Onychomycosis: Current trends in diagnosis and treatment. Am. Fam. Physician 2013, 88, 762–770. [Google Scholar]
- Freedman, J.B.; Tosti, A. Fungi and the Nails. In Onychomycosis; Springer: Cham, Switzerland, 2017; pp. 3–10. [Google Scholar]
- Gupta, A.K.; Versteeg, S.G.; Shear, N.H. Onychomycosis in the 21st Century: An Update on Diagnosis, Epidemiology, and Treatment. J. Cutan. Med. Surg. 2017, 21, 525–539. [Google Scholar]
- Reinel, D. Non-dermatophyte fungi in onychomycosis—Epidemiology and consequences for clinical practice. Mycoses 2021, 64, 694–700. [Google Scholar]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51, 2–15. [Google Scholar]
- Singh, A.; Masih, A.; Khurana, A.; Singh, P.K.; Gupta, M.; Hagen, F.; Meis, J.F.; Chowdhary, A. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the Squalene epoxidase (SQLE) gene. Mycoses 2018, 61, 477–484. [Google Scholar]
- Aghamirian, M.R.; Ghiasian, S.A. Onychomycosis in Iran: Epidemiology, causative agents and clinical features. J. Nippon Ishinkin Gakkai Zasshi 2010, 51, 23–29. [Google Scholar]
- Yamada, T.; Maeda, M.; Alshahni, M.M.; Tanaka, R.; Yaguchi, T.; Bontems, O.; Salamin, K.; Fratti, M.; Monod, M. Terbinafine resistance of Trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob. Agents Chemother. 2017, 61, e00115-17. [Google Scholar]
- Joyce, A.; Gupta, A.K.; Koenig, L.; Wolcott, R.; Carviel, J. Fungal Diversity and Onychomycosis: An Analysis of 8816 Toenail Samples Using Quantitative PCR and Next-Generation Sequencing. J. Am. Podiatr. Med. Assoc. 2019, 109, 57–63. [Google Scholar]
- Ebrahimi, M.; Zarrinfar, H.; Naseri, A.; Najafzadeh, M.J.; Fata, A.; Parian, M.; Khorsand, I.; Babič, M.N. Epidemiology of dermatophytosis in northeastern Iran; A subtropical region. Curr. Med. Mycol. 2019, 5, 16. [Google Scholar]
- Liu, X.; Tan, J.; Yang, H.; Gao, Z.; Cai, Q.; Meng, L.; Yang, L. Characterization of skin microbiome in tinea pedis. Indian J. Microbiol. 2019, 59, 422–427. [Google Scholar]
- English, M.P. Nails and fungi. Br. J. Dermatol. 1976, 94, 697–701. [Google Scholar]
- Harada, T. Tinea Unguium. Med. Mycol. J. 2011, 52, 77–95. [Google Scholar]
- Pang, S.M.; Pang, J.Y.Y.; Fook-Chong, S.; Tan, A.L. Tinea unguium onychomycosis caused by dermatophytes: A ten-year (2005–2014) retrospective study in a tertiary hospital in Singapore. Singap. Med. J. 2018, 59, 524. [Google Scholar]
- Monod, M.; Méhul, B. Recent findings in onychomycosis and their application for appropriate treatment. J. Fungus 2019, 5, 20. [Google Scholar]
- Jesus-Silva, M.A.; Roldan-Marin, R.; Asz-Sigall, D.; Arenas, R. Dermoscopy. In Onychomycosis; Springer: Cham, Switzerland, 2017; pp. 131–140. [Google Scholar]
- Leung, A.K.; Lam, J.M.; Leong, K.F.; Hon, K.L.; Barankin, B.; Leung, A.A.; Wong, A.H. Onychomycosis: An Updated Review. Recent Pat. Inflamm. Allergy Drug Discov. 2020, 14, 32–45. [Google Scholar]
- Mügge, C.; Haustein, U.F.; Nenoff, P. Causative agents of onychomycosis—A retrospective study. JDDG J. Dtsch. Dermatol. Ges. 2006, 4, 218–228. [Google Scholar]
- Ellis, D.; Watson, A.; Marley, J.; Williams, T. Non-dermatophytes in onychomycosis of the toenails. Br. J. Dermatol. 1997, 136, 490–493. [Google Scholar]
- Pihet, M.; Le Govic, Y. Reappraisal of conventional diagnosis for dermatophytes. Mycopathologia 2017, 182, 169–180. [Google Scholar]
- Gupta, A.K.; Nakrieko, K.-A. Molecular Determination of Mixed Infections of Dermatophytes and Nondermatophyte Molds in Individuals with Onychomycosis. J. Am. Podiatr. Med. Assoc. 2014, 104, 330–336. [Google Scholar]
- Gupta, A.; Summerbell, R.; Venkataraman, M.; Quinlan, E. Nondermatophyte mould onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1628–1641. [Google Scholar]
- Gupta, C.; Tripathi, K.; Tiwari, S.; Rathore, Y.; Nema, S.; Dhanvijay, A. Current trends of clinicomycological profile of dermatophytosis in Central India. IOSR-JDMS 2014, 13, 23–26. [Google Scholar]
- Carrascal-Correa, D.F.; Zuluaga, A.; González, A. Species distribution of the main aetiologic agents causing skin dermatophytosis in Colombian patients: A 23-year experience at a Mycological Reference Center. Mycoses 2020, 63, 494–499. [Google Scholar]
- Sherman, S.; Goshen, M.; Treigerman, O.; Ben-Zion, K.; Carp, M.J.; Maisler, N.; Ehrenreich, I.B.; Kimchi, A.; Lifshitz, S.; Smollan, G.; et al. Evaluation of multiplex real-time PCR for identifying dermatophytes in clinical samples—A multicentre study. Mycoses 2018, 61, 119–126. [Google Scholar]
- De Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Göker, M.; et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar]
- Koo, S.H.; Teoh, Y.L.; Koh, W.L.; Ochi, H.; Tan, S.K.; Sim, D.M.F.; Jiang, B.; Tan, A.L.; Tan, T.Y.; Lim, S.P.R. Development and validation of a real-time multiplex PCR assay for the detection of dermatophytes and Fusarium spp. J. Med. Microbiol. 2019, 68, 1641–1648. [Google Scholar]
- Kupsch, C.; Ohst, T.; Pankewitz, F.; Nenoff, P.; Uhrlaß, S.; Winter, I.; Gräser, Y. The agony of choice in dermatophyte diagnostics—Performance of different molecular tests and culture in the detection of Trichophyton rubrum and Trichophyton interdigitale. Clin. Microbiol. Infect. 2016, 22, 735.e11–735.e17. [Google Scholar]
- Iwanaga, T.; Ushigami, T.; Anzawa, K.; Mochizuki, T. Pathogenic dermatophytes survive in nail lesions during oral terbinafine treatment for tinea unguium. Mycopathologia 2017, 182, 673–679. [Google Scholar]
- Iwanaga, T.; Ushigami, T.; Anzawa, K.; Mochizuki, T. Viability of pathogenic dermatophytes during a 4-week treatment with 1% topical luliconazole for tinea pedis. Med. Mycol. 2020, 58, 401–403. [Google Scholar]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar]
- Castaner, O.; Goday, A.; Park, Y.-M.; Lee, S.-H.; Magkos, F.; Shiow, S.-A.T.E.; Schröder, H. The gut microbiome profile in obesity: A systematic review. Int. J. Endocrinol. 2018, 2018, 4095789. [Google Scholar]
- Vaghef-Mehrabany, E.; Maleki, V.; Behrooz, M.; Ranjbar, F.; Ebrahimi-Mameghani, M. Can psychobiotics “mood” ify gut? An update systematic review of randomized controlled trials in healthy and clinical subjects, on anti-depressant effects of probiotics, prebiotics, and synbiotics. Clin. Nutr. 2020, 39, 1395–1410. [Google Scholar]
- Adamczyk, K.; Garncarczyk, A.; Antończak, P.; Wcisło-Dziadecka, D. The foot microbiome. J. Cosmet. Dermatol. 2020, 19, 1039–1043. [Google Scholar]
- Cruz-Correa, O.F.; Ramírez-Hobak, L.; Arenas, R.; Soberón, X. Metagenómica en onicomicosis. El análisis de un caso que revela el posible papel de Malassezia globosa en la patogénesis. Dermatol. Cosmét. Méd. Quir. 2016, 14, 245–248. [Google Scholar]
- Gupta, A.K.; Ryder, J.E.; Baran, R.; Summerbell, R.C. Non-dermatophyte onychomycosis. Dermatol. Clin. 2003, 21, 257–268. [Google Scholar]
- Watanabe, S.; Ishida, K. Molecular diagnostic techniques for onychomycosis: Validity and potential application. Am. J. Clin. Dermatol. 2017, 18, 281–286. [Google Scholar]
- Ebihara, M.; Makimura, K.; Sato, K.; Abe, S.; Tsuboi, R. Molecular detection of dermatophytes and nondermatophytes in onychomycosis by nested polymerase chain reaction based on 28S ribosomal RNA gene sequences. Br. J. Dermatol. 2009, 161, 1038–1044. [Google Scholar]
- Gupta, A.K.; Drummond-Main, C.; Cooper, E.A.; Brintnell, W.; Piraccini, B.M.; Tosti, A. Systematic review of nondermatophyte mold onychomycosis: Diagnosis, clinical types, epidemiology, and treatment. J. Am. Acad. Dermatol. 2012, 66, 494–502. [Google Scholar]
- Nenoff, P.; Verma, S.B.; Vasani, R.; Burmester, A.; Hipler, U.C.; Wittig, F.; Krüger, C.; Nenoff, K.; Wiegand, C.; Saraswat, A.; et al. The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes—A molecular study. Mycoses 2019, 62, 336–356. [Google Scholar]
- Gupta, A.; Nakrieko, K.A. Trichophyton rubrum DNA strain switching increases in patients with onychomycosis failing antifungal treatments. Br. J. Dermatol. 2015, 172, 74–80. [Google Scholar]
- Torres-Rodríguez, J.; Madrenys-Brunet, N.; Siddat, M.; López-Jodra, O.; Jimenez, T. Aspergillus versicolor as cause of onychomycosis: Report of 12 cases and susceptibility testing to antifungal drugs. J. Eur. Acad. Dermatol. Venereol. 1998, 11, 25–31. [Google Scholar]
- Veraldi, S.; Chiaratti, A.; Harak, H. Onychomycosis caused by Aspergillus versicolor. Mycoses 2010, 53, 363–365. [Google Scholar]
- Richardson, M. Effect of Lamisil® and azole antifungals in experimental nail infection. Dermatology 1997, 194 (Suppl. S1), 27–31. [Google Scholar]
- Richardson, M.; Edward, M. Model systems for the study of dermatophyte and non-dermatophyte invasion of human keratin. Dep. Bacteriol. Immunol. 2000, 14, 669. [Google Scholar]
- Aspiroz, C.; Rezusta, A.; Paz-Cristóbal, P.; Domínguez-Luzón, F.; Gilaberte, Y. Photodynamic therapy for onychomycosis. Case report and review of the literature. Rev. Iberoam. Micol. 2011, 28, 191–193. [Google Scholar]
- Summerbell, R.C.; Scott, J.A. Acremonium. In Molecular Biology of Food and Water Borne Mycotoxigenic and Mycotic Fungi; Paterson, R.R.M., Lima, N., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 115–128. [Google Scholar]
- Simmons, B.; Griffith, R.; Falto-Aizpurua, L.; Nouri, K. An update on photodynamic therapies in the treatment of onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1275–1279. [Google Scholar]
- Gräfenhan, T.; Schroers, H.-J.; Nirenberg, H.; Seifert, K. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Stud. Mycol. 2011, 68, 79–113. [Google Scholar]
- Gharib, S.J.; Abdullah, S.K. Onychomycosis due to Fusarium oxysporum in Sulumaniyah City, Iraq. J. Clin. Diagn. Res. 2020, 14, 1–3. [Google Scholar]
- Uemura, E.V.G.; Barbosa, M.D.S.; Simionatto, S.; Al-Harrasi, A.; Al-Hatmi, A.M.; Rossato, L. Onychomycosis Caused by Fusarium Species. J. Fungi 2022, 8, 360. [Google Scholar]
- Veiga, F.F.; de Castro-Hoshino, L.V.; Sato, F.; Bombassaro, A.; Vicente, V.A.; Mendes, V.; Baesso, M.L.; Negri, M.; Svidzinski, T.I. Fusarium oxysporum is an onychomycosis etiopathogenic agent. Future Microbiol. 2018, 13, 1745–1756. [Google Scholar]
- Nowicka, D.; Nawrot, U.; Włodarczyk, K.; Pajączkowska, M.; Patrzałek, A.; Pęcak, A.; Mozdyniewicz, P.; Fleischer, M. Detection of dermatophytes in human nail and skin dust produced during podiatric treatments in people without typical clinical signs of mycoses. Mycoses 2016, 59, 379–382. [Google Scholar]
- Gupta, A.; Elewski, B. Nondermatophyte causes of onychomycosis and superficial mycoses. Curr. Top. Med. Mycol. 1996, 7, 87–97. [Google Scholar]
- Jessup, C.; Ghannoum, M.; Ryder, N. An evaluation of the in vitro activity of terbinafine. Med. Mycol. 2000, 38, 155–159. [Google Scholar]
- Mikx, F.; de Jong, M. Keratinolytic activity of cutaneous and oral bacteria. Infect. Immun. 1987, 55, 621–625. [Google Scholar]
- De Almeida, H.L., Jr.; de Castro, L.A.; Rocha, N.E.; Abrantes, V.L. Ultrastructure of pitted keratolysis. Int. J. Dermatol. 2000, 39, 698–701. [Google Scholar]



| Nail Number | Dominant Fungus | Dermatophyte Trace |
|---|---|---|
| 1 | 98.11% T. rubrum | 0.65% T. interdigitale/mentagrophytes |
| 3 | 99.93% T. rubrum | 0.02% T. interdigitale/mentagrophytes |
| 4 | 99.13% T. interdigitale/mentagrophytes | 0.01% T. rubrum |
| 7 | 99.45% T. interdigitale/mentagrophytes | 0.02% T. rubrum |
| 10 | 86.27% F. oxysporum | 12.81% T. interdigitale/mentagrophytes 0.01% T. rubrum |
| 11 (control) | 95.77% F. oxysporum | 0.08% T. rubrum 0.06% T. interdigitale/mentagrophytes |
| 12 | 94.73% T. interdigitale/mentagrophytes | 0.02% T. rubrum |
| 20 (control) | 93.57% Malassezia slooffiae | 3.89% T. interdigitale/mentagrophytes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hainsworth, S.; Lawrie, A.C.; Vanniasinkam, T.; Grando, D. Metagenomics of Toenail Onychomycosis in Three Victorian Regions of Australia. J. Fungi 2022, 8, 1198. https://doi.org/10.3390/jof8111198
Hainsworth S, Lawrie AC, Vanniasinkam T, Grando D. Metagenomics of Toenail Onychomycosis in Three Victorian Regions of Australia. Journal of Fungi. 2022; 8(11):1198. https://doi.org/10.3390/jof8111198
Chicago/Turabian StyleHainsworth, Steven, Ann C. Lawrie, Thiru Vanniasinkam, and Danilla Grando. 2022. "Metagenomics of Toenail Onychomycosis in Three Victorian Regions of Australia" Journal of Fungi 8, no. 11: 1198. https://doi.org/10.3390/jof8111198
APA StyleHainsworth, S., Lawrie, A. C., Vanniasinkam, T., & Grando, D. (2022). Metagenomics of Toenail Onychomycosis in Three Victorian Regions of Australia. Journal of Fungi, 8(11), 1198. https://doi.org/10.3390/jof8111198





