Transcriptomic Analyses Reveals Molecular Regulation of Photosynthesis by Epichloë endophyte in Achnatherum inebrians under Blumeria graminis Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Measurement of Photosynthetic Data
2.3. RNA Extraction and Transcriptome Analysis
2.4. Meta-Analysis Data Collection
2.5. Statistical Analyses
3. Results
3.1. Plant Growth and Biomass
3.2. Photosynthesis Index
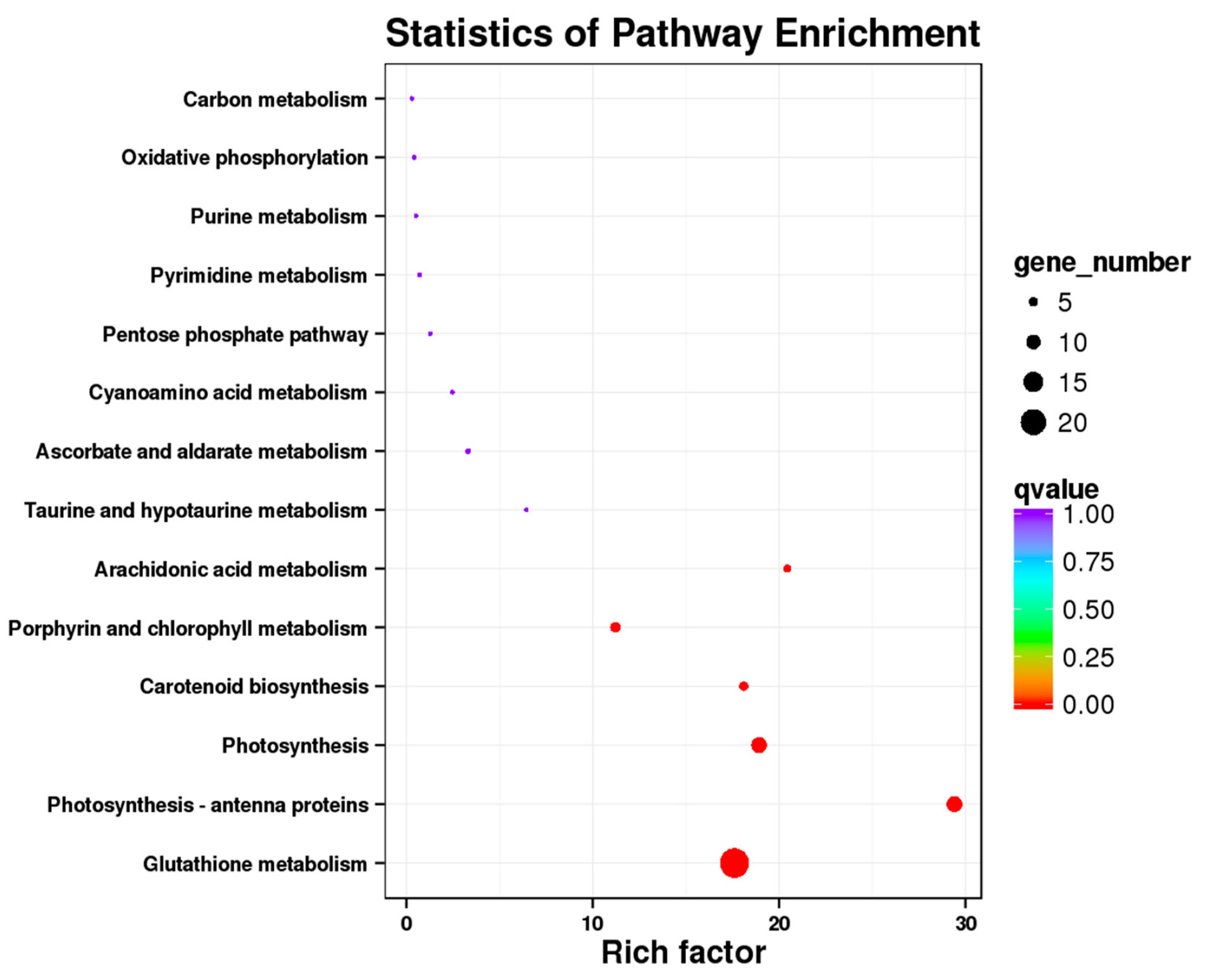
3.3. Kegg Pathway Enrichment Analysis of the DEGs
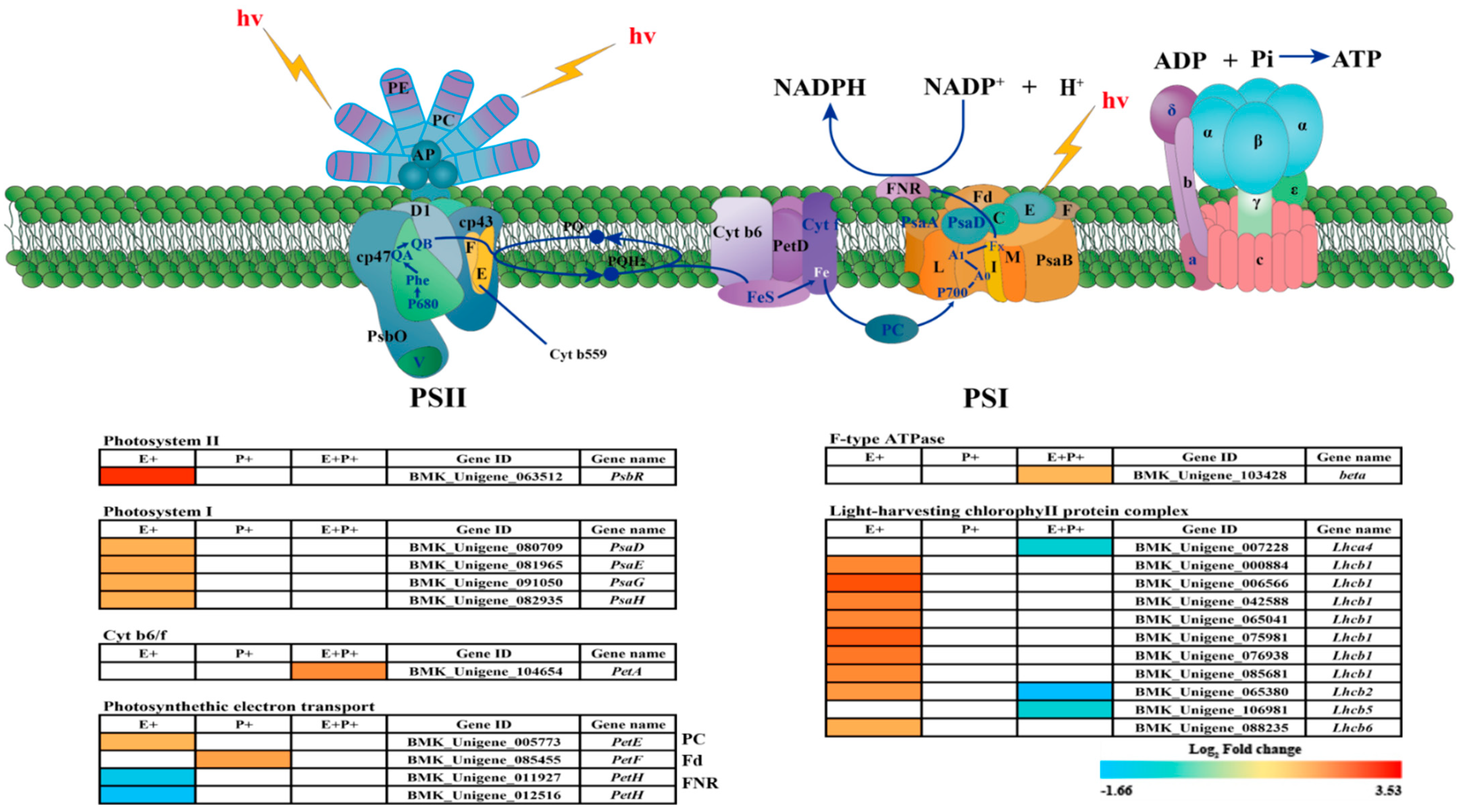
3.4. DEGs of Regulatory Pathways Related to Photosynthesis
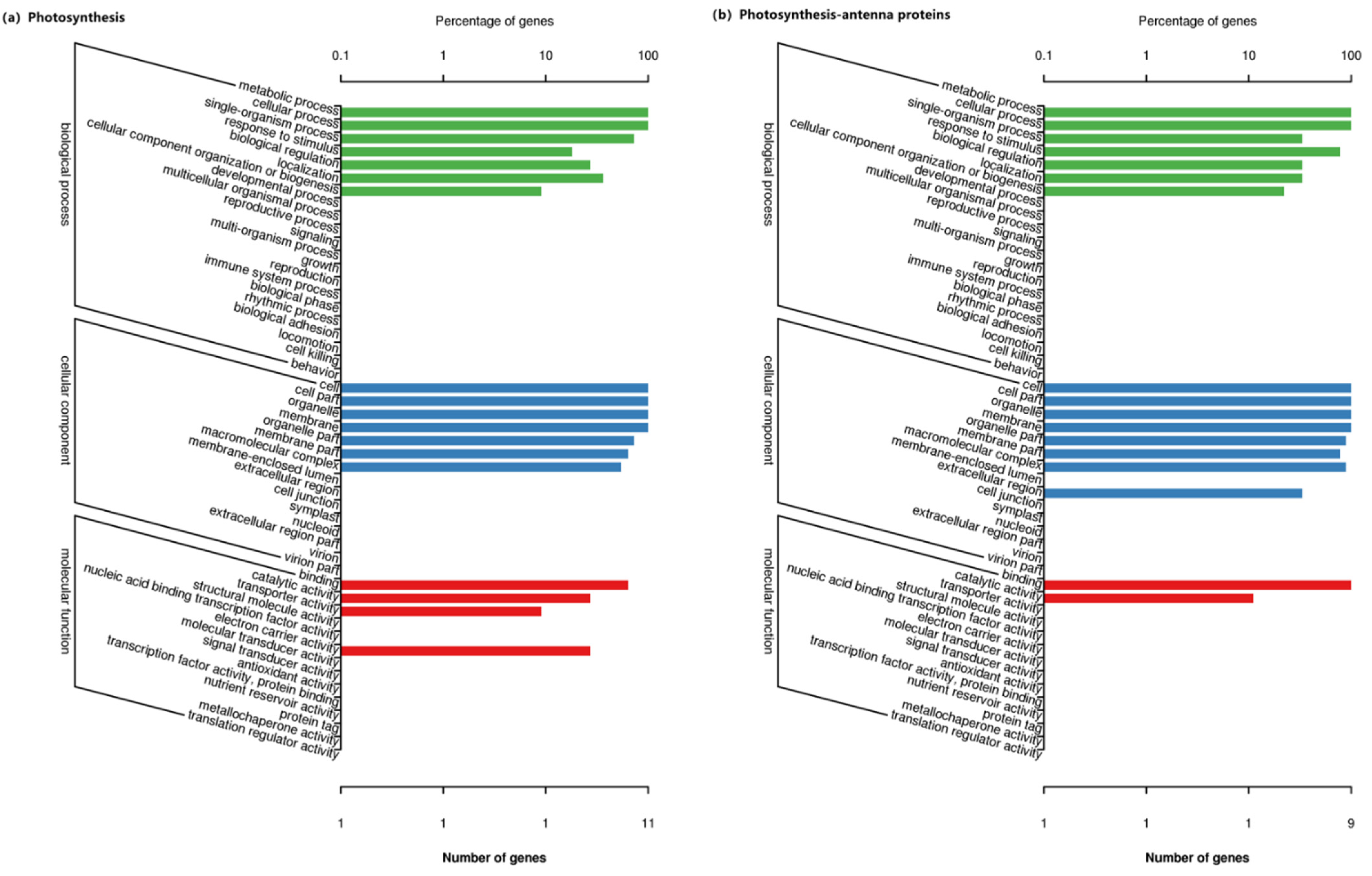
3.5. Go Functional Enrichment Analysis of the DEGs
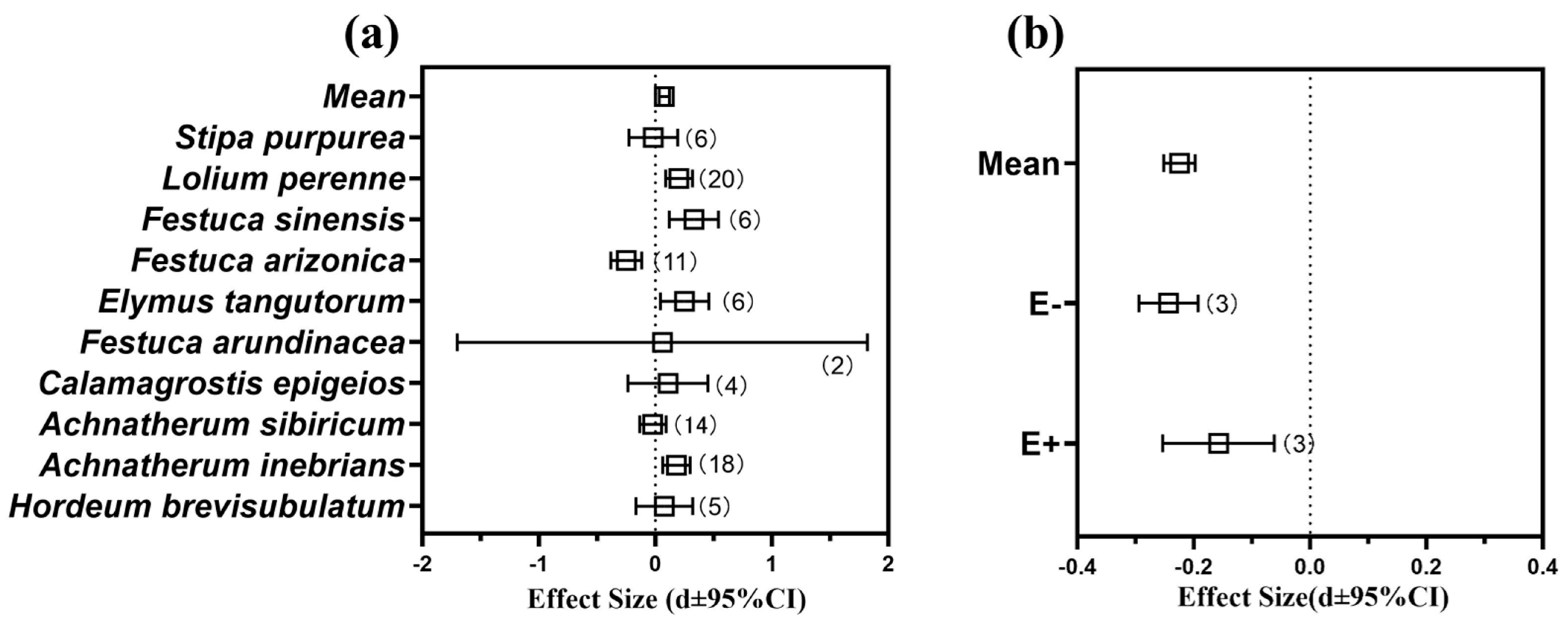
3.6. Meta-Analysis
4. Discussion
4.1. Effects of Biotic and Abiotic Stresses on Photosynthesis
4.2. Effects of Epichloë endophyte on Photosynthesis
4.3. Presence of Epichloë endophyte Alters Photosynthetic Genes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Inuma, T.; Khodaparast, S.A.; Takamatsu, S. Multilocus phylogenetic analyses within Blumeria graminis, a powdery mildew fungus of cereals. Mol. Phylogenetics Evol. 2007, 44, 741–751. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology: Top 10 fungal pathogens. Mol. Plant Path. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Glawe, D.A. The powdery mildews: A review of the world’s most familiar (yet poorly known) plant pathogens. Annu. Rev. Phytopathol. 2008, 46, 27–51. [Google Scholar] [CrossRef]
- Edwards, H.H. Development of primary germ tubes by conidia of Blumeria graminis f.sp. hordei on leaf epidermal cells of Hordeum vulgare. Can. J. Bot. 2002, 80, 1121–1125. [Google Scholar] [CrossRef]
- Yamaoka, N.; Matsumoto, I.; Nishiguchi, M. The role of primary germ tubes (PGT) in the life cycle of Blumeria graminis: The stopping of PGT elongation is necessary for the triggering of appressorial germ tube (AGT) emergence. Physiol. Mol. Plant. Pathol. 2006, 69, 153–159. [Google Scholar] [CrossRef]
- Zhu, M.; Riederer, M.; Hildebrandt, U. Very-long-chain aldehydes induce appressorium formation in ascospores of the wheat powdery mildew fungus Blumeria graminis. Fungal Biol. 2017, 121, 716–728. [Google Scholar] [CrossRef]
- Hansjakob, A.; Riederer, M.; Hildebrandt, U. Appressorium morphogenesis and cell cycle progression are linked in the grass powdery mildew fungus Blumeria graminis. Fungal Biol. 2012, 116, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Akhkha, A.; Clarke, D.D.; Dominy, P.J. Relative tolerances of wild and cultivated barley to infection by Blumeria graminis f. sp. hordei (Syn. Erysiphe graminis f. sp. hordei). II-the effects of infection on photosynthesis and respiration. Physiol. Mol. Plant Pathol. 2003, 62, 347–354. [Google Scholar] [CrossRef]
- Cao, A.; Xing, L.; Wang, X.; Wang, W.; Sun, Y.; Qian, C.; Ni, J.; Chen, Y.; Liu, D.; Wang, X.; et al. Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc. Natl. Acad. Sci. USA 2011, 108, 7727–7732. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.R.; Yao, D.M.; Duan, X.Y.; Liu, W.; Fan, J.R.; Ding, K.J.; Zhou, Y.L. Effects of powdery mildew on 1000-kernel weight, crude protein content and yield of winter wheat in three consecutive growing seasons. J. Integr. Agric. 2014, 13, 1530–1537. [Google Scholar] [CrossRef]
- Liu, N.; Lei, Y.; Gong, G.; Zhang, M.; Wang, X.; Zhou, Y.; Qi, X.; Chen, H.; Yang, J.; Chang, X.; et al. Temporal and spatial dynamics of wheat powdery mildew in Sichuan Province, China. Crop Prot. 2015, 74, 150–157. [Google Scholar] [CrossRef]
- Leuchtmann, A. Systematics, distribution, and host specificity of grass endophytes. Nat. Toxins 1993, 1, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Craven, K.D.; Speakman, S.; Stromberg, A.; Lindstrom, A.; Yoshida, R. A novel test for host-symbiont codivergence indicates ancient origin of fungal endophytes in grasses. Syst. Biol. 2008, 57, 483–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuchtmann, A.; Bacon, C.W.; Schardl, C.L.; White, J.F., Jr.; Tadych, M. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia 2014, 106, 202–215. [Google Scholar] [CrossRef]
- Vignale, M.V.; Astiz-Gassó, M.M.; Novas, M.V.; Iannone, L.J. Epichloë endophytes confer resistance to the smut Ustilago bullata in the wild grass Bromus auleticus (Trin.). Biol. Control. 2013, 67, 1–7. [Google Scholar] [CrossRef]
- Bastías, D.A.; Alejandra Martínez-Ghersa, M.; Newman, J.A.; Card, S.D.; Mace, W.J.; Gundel, P.E. The plant hormone salicylic acid interacts with the mechanism of anti-herbivory conferred by fungal endophytes in grasses: Effects of salicylic acid on fungal endophytes. Plant Cell Environ. 2018, 41, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.I.; Gundel, P.E.; Zabalgogeazcoa, I.; Omacini, M. An ecological framework for understanding the roles of Epichloë endophytes on plant defenses against fungal diseases. Fungal Biol. Rev. 2020, 34, 115–125. [Google Scholar] [CrossRef]
- Christensen, M.J.; Bennett, R.J.; Ansari, H.A.; Koga, H.; Johnson, R.D.; Bryan, G.T.; Simpson, W.R.; Koolaard, J.P.; Nickless, E.M.; Voisey, C.R. Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genet. Biol. 2008, 45, 84–93. [Google Scholar] [CrossRef]
- Li, C.J.; Nan, Z.B.; Paul, V.; Dapprich, P.; Liu, Y. A new Neotyphodium species symbiotic with drunken horse grass (Achnatherum inebrians) in China. Mycotaxon 2004, 90, 141–147. [Google Scholar]
- Chen, L.; Li, X.Z.; Li, C.J.; Swoboda, G.A.; Young, C.A.; Sugawara, K.; Leuchtmann, A.; Schardl, C.L. Two distinct Epichloë species symbiotic with Achnatherum inebrians, drunken horse grass. Mycologia 2015, 107, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.Z. Several poisonous weeds in grassland of northwest. J. Gansu Agric. Univ. 1954, 2, 9–16. [Google Scholar]
- Shi, Z.C. Important Poisonous Plants in Chinese Grassland; World Book Publishing Company: Beijing, China, 1997; pp. 166–176. [Google Scholar]
- Nan, Z.B.; Li, C.J. Neotyphodium in native grasses in China and observations on endophyte/host interactions. In Proceedings of the 4th International Neotyphodium/Grass Interactions Symposium, Soest, Germany, 27–29 September 2000. [Google Scholar]
- Liu, Z.P. Animal Toxicology; China Agricultural Press: Beijing, China, 2006; pp. 152–154. [Google Scholar]
- Zhang, X.X.; Li, C.J.; Nan, Z.B. Effects of salt and drought stress on alkaloid production in endophyte-infected drunken horse grass (Achnatherum inebrians). Biochem. Syst. Ecol. 2011, 39, 471–476. [Google Scholar] [CrossRef]
- Zhang, X.X.; Nan, Z.B.; Li, C.J.; Gao, K. Cytotoxic effect of ergot alkaloids in Achnatherum inebrians infected by the Neotyphodium gansuense endophyte. J. Agric. Food. Chem. 2014, 62, 7419–7422. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Li, C.; Nan, Z.B.; Li, F. Effects of feeding drunken horse grass infected with Epichloë gansuensis endophyte on animal performance, clinical symptoms and physiological parameters in sheep. BMC Vet. Res. 2017, 13, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, C.; Li, N.N.; Zhang, Y.W.; Li, C.J.; Zhang, X.X.; Nan, Z.B. Role of Epichloë endophytes in defense responses of cool-season grasses to pathogens: A review. Plant Dis. 2018, 102, 2061–2073. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.R.; Kou, M.Z.; Zhong, R.; Xia, C.; Christensen, M.J.; Zhang, X.X. Transcriptome analysis revealed plant hormone biosynthesis and response pathway modification by Epichloë gansuensis in Achnatherum inebrians under different soil moisture avail-ability. J. Fungi 2021, 7, 640. [Google Scholar] [CrossRef]
- Zhong, R.; Bastías, D.A.; Zhang, X.X.; Li, C.J.; Nan, Z.B. Vertically transmitted Epichloë systemic endophyte enhances drought tolerance of Achnatherum inebrians host plants through promoting pbhotosynthesis and biomass accumulation. J. Fungi 2022, 8, 512. [Google Scholar] [CrossRef]
- Wang, J.F.; Tian, P.; Christensen, M.J.; Zhang, X.X.; Li, C.J.; Nan, Z.B. Effect of Epichloë gansuensis endophyte on the activity of enzymes of nitrogen metabolism, nitrogen use efficiency and photosynthetic ability of Achnatherum inebrians under various NaCl concentrations. Plant Soil 2019, 435, 57–68. [Google Scholar] [CrossRef]
- Chen, N.; He, R.; Chai, Q.; Li, C.J.; Nan, Z.B. Transcriptomic analyses giving insights into molecular regulation mechanisms involved in cold tolerance by Epichloë endophyte in seed germination of Achnatherum inebrians. Plant Growth Regul. 2016, 80, 367–375. [Google Scholar] [CrossRef]
- Zhang, X.X.; Fan, X.M.; Li, C.J.; Nan, Z.B. Effects of cadmium stress on seed germination, seedling growth and antioxidative enzymes in Achnatherum inebrians plants infected with a Neotyphodium endophyte. Plant Growth Regul. 2010, 60, 91–97. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.J.; Nan, Z.B. Effects of cadmium stress on seed germination and seedling growth of Elymus dahuricus infected with the Neotyphodium endophyte. Sci. China Life Sci. 2012, 55, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zhang, X.X.; Christensen, M.J.; Nan, Z.B.; Li, C.J. Epichloë endophyte affects the ability of powdery mildew (Blumeria graminis) to colonise drunken horse grass (Achnatherum inebrians). Fungal Ecol. 2015, 16, 26–33. [Google Scholar] [CrossRef]
- Xia, C.; Li, N.N.; Zhang, X.X.; Feng, Y.; Christensen, M.J.; Nan, Z.B. An Epichloë endophyte improves photosynthetic ability and dry matter production of its host Achnatherum inebrians infected by Blumeria graminis under various soil water conditions. Fungal Ecol. 2016, 22, 26–34. [Google Scholar] [CrossRef]
- Kou, M.Z.; Bastías, D.A.; Christensen, M.J.; Zhong, R.; Nan, Z.B.; Zhang, X.X. The plant salicylic acid signalling pathway regulates the infection of a biotrophic pathogen in grasses associated with an Epichloë endophyte. J. Fungi 2021, 7, 633. [Google Scholar] [CrossRef]
- Doubnerová, V.; Ryšlavá, H. What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Sci. 2011, 180, 575–583. [Google Scholar] [CrossRef]
- Freschi, L.; Mercier, H. Connecting environmental stimuli and crassulacean acid metabolism expression: Phytohormones and other signaling molecules. Prog. Bot. 2012, 73, 231–255. [Google Scholar]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Todorova, D.; Aleksandrov, V.; Anev, S.; Sergiev, I. Photosynthesis alterations in wheat plants induced by herbicide, soil drought or flooding. Agronomy 2022, 12, 390. [Google Scholar] [CrossRef]
- Kissana, S.N.; Mujahid, Y.M.; Mustafa, Z.S. A Technical Report to Apprise the Issues and Future Strategies; National Agricultural Research Center, Pakistan Agricultural Research Council: Islamabad, Pakistan, 2003; pp. 2002–2003. [Google Scholar]
- Carretero, R.; Bancal, M.O.; Miralles, D.J. Effect of leaf rust (Puccinia triticina) on photosynthesis and related processes of leaves in wheat crops grown at two contrasting sites and with different nitrogen levels. Eur. J. Agron. 2011, 35, 237–246. [Google Scholar] [CrossRef]
- Zhang, H.J.; Li, X.; White, J.F.; Wei, X.; He, Y.; Li, C.J. Epichloë endophyte improves ergot disease resistance of host (Achnatherum inebrians) by regulating leaf senescence and photosynthetic capacity. J. Plant. Growth. Regul. 2022, 41, 808–817. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Amorim, L.; Filho, A.B.; Hau, B.; Berger, R.D. Accounting for photosynthetic efficiency of bean leaves with rust, angular leaf spot and anthracnose to assess crop damage: Photosynthesis of diseased bean leaves and crop damage. Plant Path. 2001, 50, 443–452. [Google Scholar] [CrossRef]
- Robert, C.; Bancal, M.; Ney, B.; Lannou, C. Wheat leaf photosynthesis loss due to leaf rust, with respect to lesion development and leaf nitrogen status. New Phytol. 2005, 165, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.Z.; Christensen, M.J.; Nan, Z.B. Effects of the endophyte Epichloë festucae var. lolii of perennial ryegrass (Lolium perenne) on indicators of oxidative stress from pathogenic fungi during seed germination and seedling growth. Eur. J. Plant Pathol. 2015, 141, 571–583. [Google Scholar] [CrossRef]
- Ambrose, K.V.; Belanger, F.C. SOLiD-SAGE of endophyte-infected red fescue reveals numerous effects on host transcriptome and an abundance of highly expressed fungal secreted proteins. PLoS ONE 2012, 7, e53214. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.; Zhang, L.; Zhang, X.X. Allelopathic Effects of Foliar Epichloë Endophytes on Belowground Arbuscular Mycorrhizal Fungi: A Meta-Analysis. Agriculture 2022, 12, 1768. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Gurevitch, J.; Curtis, P.S.; Jones, M.H. Meta-analysis in ecology. Adv. Ecol. Res. 2001, 32, 199–247. [Google Scholar]
- Saibo, N.J.M.; Lourenço, T.; Oliveira, M.M. Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses. Ann. Bot. 2009, 103, 609–623. [Google Scholar] [CrossRef] [Green Version]
- Rahnama, A.; Poustini, K.; Tavakkol-Afshari, R.; Tavakoli, A. Growth and stomatal responses of bread wheat genotypes in tolerance to salt stress. Int. J. Biol. Sci. 2010, 6, 216–221. [Google Scholar]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulias, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: The stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Karaba, A.; Dixit, S.; Greco, R.; Aharoni, A.; Trijatmiko, K.R.; Marsch-Martinez, N.; Krishnan, A.; Nataraja, K.N.; Udayakumar, M.; Pereira, A. Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc. Natl. Acad. Sci. USA 2007, 104, 15270–15275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, M.C.; Brüggemann, W. Limitations of photosynthesis in Phaseolus vulgaris under drought stress: Gas exchange, chlorophyll fluorescence and Calvin cycle enzymes. Photosynthetica 2010, 48, 96–102. [Google Scholar] [CrossRef]
- Dias, M.C.; Brüggemann, W. Water-use efficiency in Flaveria species under drought-stress conditions. Photosynthetica 2010, 48, 469–473. [Google Scholar] [CrossRef]
- Ayres, P.G. Patterns of stomatal behaviour, transpiration, and CO2 exchange in pea following infection by powdery mildew (Erysiphe pisi). J. Exp. Bot. 1976, 27, 1196–1205. [Google Scholar] [CrossRef]
- Moriondo, M.; Orlandini, S.; Giuntoli, A.; Bindi, M. The effect of downy and powdery mildew on grapevine (Vitis vinifera L.) leaf gas exchange. J. Phytopathol. 2005, 153, 350–357. [Google Scholar] [CrossRef]
- Xia, C.; Christensen, M.J.; Zhang, X.X.; Nan, Z.B. Effect of Epichloë gansuensis endophyte and transgenerational effects on the water use efficiency, nutrient and biomass accumulation of Achnatherum inebrians under soil water deficit. Plant Soil 2018, 424, 555–571. [Google Scholar] [CrossRef]
- Soleimani, M.; Hajabbasi, M.A.; Afyuni, M.; Mirlohi, A.; Borggaard, O.K.; Holm, P.E. Effect of endophytic fungi on cadmium tolerance and bioaccumulation by Festuca arundinacea and Festuca pratensis. Int. J. Phytoremediation 2010, 12, 535–549. [Google Scholar] [CrossRef]
- Sabzalian, M.R.; Mirlohi, A.; Sharifnabi, B. Reaction to powdery mildew fungus, Blumeria graminis in endophyte-infected and endophyte-free tall and meadow fescues. Australas. Plant Path. 2012, 41, 565–572. [Google Scholar] [CrossRef]
- Gundel, P.E.; Irisarri, J.G.N.; Fazio, L.; Casas, C.; Pérez, L.I. Inferring field performance from drought experiments can be misleading: The case of symbiosis between grasses and Epichloë fungal endophytes. J. Arid. Environ. 2016, 132, 60–62. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Bauerle, T.L. A new currency for mutualism? Fungal endophytes alter antioxidant activity in hosts responding to drought. Fungal Divers. 2012, 54, 39–49. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Ling, N.; Li, W.; Xu, G.; Qi, Z.; Ji, C.; Liu, X.; Cui, D.; Sun, Y. Transcriptomic sequencing reveals the response of Dunaliella salina to copper stress via the increased photosynthesis and carbon mechanism. Mol. Omics 2021, 17, 769–782. [Google Scholar] [CrossRef]
- Rozpądek, P.; Wężowicz, K.; Nosek, M.; Ważny, R.; Tokarz, K.; Lembicz, M.; Miszalski, Z.; Turnau, K. The fungal endophyte Epichloë typhina improves photosynthesis efficiency of its host orchard grass (Dactylis glomerata). Planta 2015, 242, 1025–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Ma, J.; He, Y.; Yu, S.; Lin, Z.; Xiong, Y.; Rafique, F.; Jiang, F.; Sun, L.; Ma, M.; et al. Comparative transcriptomic and proteomic analyses of the green and white parts of chimeric leaves in Ananas comosus var. bracteatus. PeerJ 2019, 7, e7261. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.A.; Kovalenko, I.B.; Khruschev, S.S.; Ustinin, D.M.; Antal, T.K.; Riznichenko, G.Y.; Rubin, A.B. Comparative analysis of plastocyanin-cytochrome f complex formation in higher plants, green algae and cyanobacteria. Physiol. Plant. 2019, 166, 320–335. [Google Scholar] [CrossRef]
- Su, X.; Ma, J.; Pan, X.; Zhao, X.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Antenna arrangement and energy transfer pathways of a green algal photosystem-I-LHCI supercomplex. Nat. Plants 2019, 5, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.S. Effect of the Hemiparasitic Plant Pedicularis Kansuensis on Growth and Photosynthetic Properties of Stipa Purpurea-Epichloë symbiosis; Lanzhou University: Lanzhou, China, 2015. [Google Scholar]
- Bao, G.S.; Song, M.L.; Wang, Y.Q.; Li, C.J. Effects of parasitic Artemisia annua from Gansu on the photosynthetic characteristics of endophytic fungal symbiont in grasses. Acta Microbiol Sin. 2020, 60, 294–305. [Google Scholar]
- Bao, G.S. Study of Epichloë endophyte-grass symbionts on photosynthetic characteristics of hemiparasitic plant. Chin. Qinghai J. Anim. Vet. Sci. 2020, 50, 1–7. [Google Scholar]
- Cao, Y. Effect of Interaction between Jasmonic Acid and Endophytic Fungi on Wild Barely under Salt Stress; Lanzhou University: Lanzhou, China, 2018. [Google Scholar]
- Chen, T.X.; Johnson, R.; Chen, S.H.; Li, C.J. Infection by the fungal endophyte Epichloë bromicola enhances the tolerance of wild barley (Hordeum brevisubulatum) to salt and alkali stresses. Plant Soil 2018, 428, 353–370. [Google Scholar] [CrossRef]
- Cui, X.L.; Xia, C. Effect of exogenous abscisic acid on seedling establishment of Epichloë gansuensis-Achnatherum inebrians symbiont. Acta Prataculture Sin. 2020, 29, 70–80. [Google Scholar]
- Deng, J. Mechanisms of the Effects of Arbuscular Mycorrhizal Fungus and Grass Endophyte on Left Spot of Perennial Ryegrass; Lanzhou University: Lanzhou, China, 2021. [Google Scholar]
- Deng, J.; Li, F.; Duan, T.Y. Effects of AM fungus and grass endophyte on the infection of Lolium perenne by the pathogen Biopolaris sorokinianum in a greenhouse. Acta Prataculture Sin. 2019, 28, 103–113. [Google Scholar]
- Du, Y.J. Stress Resistance Research of Endophyte in Wild Festuca L and Their Association; Beijing Forestry University: Beijing, China, 2010. [Google Scholar]
- Du, Y.J.; Sun, X.B.; Han, L.B. Effect of Neotyphodium starrii infected on photosynthetic and orphological characteristics of tall fescue under high temperature. J. Cent. South Univ. For. Technol. 2010, 30, 41–47. [Google Scholar]
- Du, Y.J.; Wang, Q.; Han, L.B. Endophytic fungus Neotyphodium typhinum infection effects on photosynthetic characteristics of tall fescue. Ecol. Environ. Sci. 2009, 18, 45–47. [Google Scholar]
- Fang, A.G. Effect of Neotyphodium Endophyte and AM Fungi on Growth of Hordeum Brevisubulatum under Salt and Phosphorus Stress Conditions; Lanzhou: Lanzhou University, 2013. [Google Scholar]
- Guo, Y.E. Effect of Phouphorus, Claroideoglomus etunicatum and Grass Endophte on Perennial Ryegrass Left Spot Disease Caused by Bipolaris zeae; Lanzhou University: Lanzhou, China, 2018. [Google Scholar]
- Guo, Y.E.; Gao, P.; Li, F. Effects of AM fungi and grass endophytes on perennial ryegrass Bipolaris sorokiniana leaf spot disease under limited soil nutrients. Eur. J. Plant Path. 2019, 154, 659–671. [Google Scholar] [CrossRef]
- Guo, Y.E.; Li, F.; Wang, X.Y.; Wang, Z.G.; Duan, T.Y. Effects of phosphorus, AM fungi and grass endophytes on leaf spot of perennial ryegrass. Cao Xue 2018, 4, 17–26, 72. [Google Scholar]
- Guo, Y.E.; Li, Y.D.; Gao, P.; Wang, Z.G.; Duan, T.Y. Effects of Claroideoglomus etunicatum and grass endophyte on the growth of Lolium perenne under different phosphorus levels. Acta Prataculture Sin. 2018, 26, 1458–1466. [Google Scholar]
- Han, R.; Li, X.; Ren, A.Z.; Gao, Y.B. Physiological ecological effect of endophyte infection on Achnatherum sibiricum under drought stress. Acta. Ecol. Sin. 2011, 31, 2115–2123. [Google Scholar]
- Jia, T. Effect of Endophytic Species, Host Genotype and Water/Nutrient Supply on the Performance of Grass-Endophyte Symbionts; Nankai Universitg: Tianjing, China, 2014. [Google Scholar]
- Jia, T.; Ren, A.Z.; Gao, Y.B. Host genotype overrides endophyte infection effects on growth, physiology, and nutrient content of a native grass, Achnatherum sibiricum. Plant Ecol. 2014, 215, 875–887. [Google Scholar] [CrossRef]
- Jia, T.; Ren, A.Z.; Wang, S.; Gao, Y.B. Effect of Endophytic fungi on growth and photosynthetic characteristics of Achnatherum sibiricum. Acta. Ecol. Sin. 2011, 31, 4811–4817. [Google Scholar]
- Li, C.; Li, X.; Ren, A.Z.; Gao, Y.B. Effect of endophyte infection on Zn resistance of Achnatherum sibiricum. Acta Sci. Nat. Univ. Nankaiensis 2013, 46, 29–35. [Google Scholar]
- Li, C.; Ren, A.Z.; Gao, Y.B. Effect of endophyte intection on Zn resistance of tall fescue. Acta. Ecol. Sin. 2010, 30, 1684–1690. [Google Scholar]
- Li, F.; Deng, J.; Nzabanita, C. Growth and physiological responses of perennial ryegrass to an AMF and an Epichloë endophyte under different soil water contents. Symbiosis 2019, 79, 151–161. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.Z.; Duan, T.Y. Effects of interactions between a grass endophyte and an arbuscular mycorrhizal fungus on perennial ryegrass growth. Acta Pratculturae Sin. 2017, 26, 132–140. [Google Scholar]
- Li, M.M. Effect of Epichloë Endophyte on Drought Resistance of Different Ecotypes Festuca sinensis; Lanzhou University: Lanzhou, China, 2019. [Google Scholar]
- Li, N.N. Effect of Interaction of Endophyte and Blumeria graminis on the Achnatherum inebrians in Different Water Conditions; Lanzhou University: Lanzhou, China, 2018. [Google Scholar]
- Li, X.; Zhou, Y.; Mace, W.; Qin, J.; Liu, H.; Chen, W.; Ren, A.Z.; Gao, Y.B. Endophyte species influence the biomass production of the native grass Achnatherum sibiricum (L.) Keng under high nitrogen availability. Ecol. Evol. 2016, 6, 8595–8606. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, Y.B.; Chen, S.P.; Ren, A.Z. Effects of endophyte infection on photosynthesis, transpiration and water use efficiency of Lolium perenen L. under drought stress. Acta Phytoecol. Sin. 2001, 5, 537–543. [Google Scholar]
- Lin, F.; Li, C.; Zhang, X.; Bao, X.Y.; Ren, A.Z.; Gao, Y.B. Effect of endophytic fungi on photosynthetic characteristics of three Achnatherum sibiricum populations in Inner Mongolia Steppe. Bull. Botanical. Res. 2009, 29, 61–68. [Google Scholar]
- Liu, B.H. Biological Properties of Endophtes/Calamagrostis Symbiota; Naning Agricultural University: Naning, China, 2012. [Google Scholar]
- Liu, L. Response of Epichloë gansuensis-Achnatherum Inebrians Symbiont on Stresses of Cold and Powdery Mildew Disease Treated by SA and ABA; Lanzhou University: Lanzhou, China, 2016. [Google Scholar]
- Ma, M.Z. Disease Resistance of Perennial Ryegrass (Lolium perenne)-Epichloë festucae var. lolii Endophyte Symbiont and Their Resistance Mechanism to Bipolaris sorokiniana; Lanzhou University: Lanzhou, China, 2015. [Google Scholar]
- Ma, M.Z.; Nan, Z.B. Effect of fungal endophytes against rust disease of perennial ryegrass (Lolium perenne) on growth and physiological indices. Acta Pratculturae Sin. 2011, 20, 150–156. [Google Scholar]
- Morse, L.J.; Day, T.A.; Faeth, S.H. Effect of Neotyphodium endophyte infection on growth and leaf gas exchange of Arizona fescue under contrasting water availability regimes. Environ. Exp. Bot. 2002, 48, 257–268. [Google Scholar] [CrossRef]
- Qin, J.H.; Lu, Y.; Li, Z.; Zhou, Y.; Ren, A.Z.; Gao, Y.B. Effects of methyl jasmonate treatments and endophyte infection on growth of Achnatherum sibiricum. Chin. J. Appl. Ecol. 2015, 26, 1145–1152. [Google Scholar]
- Shi, Z.B.; Zhou, Y.; Li, X.; Ren, A.Z.; Gao, Y.B. Physioecological effects of endophyte infection on the host grass with elevated CO2. Acta. Ecol. Sin. 2013, 33, 6135–6141. [Google Scholar]
- Wang, J.L.; Gao, Y.B.; Ren, A.Z.; Wang, W.; Zhao, N.X. Effects of endophyte infection on photosynthesis, transpiration and biomass of Lolium perenne L. at different nitrogen levels. Chin. Bull. Bot. 2004, 5, 539–546. [Google Scholar]
- Xia, C. Responses of Epichloë gansuensis-Achnatherum inebrians Symbiont to Drought Stress; Lanzhou University: Lanzhou, China, 2018. [Google Scholar]
- Yan, Z.C.; Li, Y.D.; Cheng, W.J.; Gao, P.; Guo, Y.; Duan, T.Y. Effects of AM fungi and grass endophyte on the growth of ryegrass under different salt concentrations. Grassland. Turf. 2018, 38, 63–70. [Google Scholar]
- Zhang, H.J. The Effect of Pathogenic Ergot bacteria on the Epichloë Endophyte Symbionts of Achnatherum sibiricum; Lanzhou University: Lanzhou, China, 2021. [Google Scholar]
- Zhang, J.F. Effect of Fungal Endophyte on Intraspecific Competition of Elymus Nutans under Water Stress; Lanzhou University: Lanzhou, China, 2013. [Google Scholar]
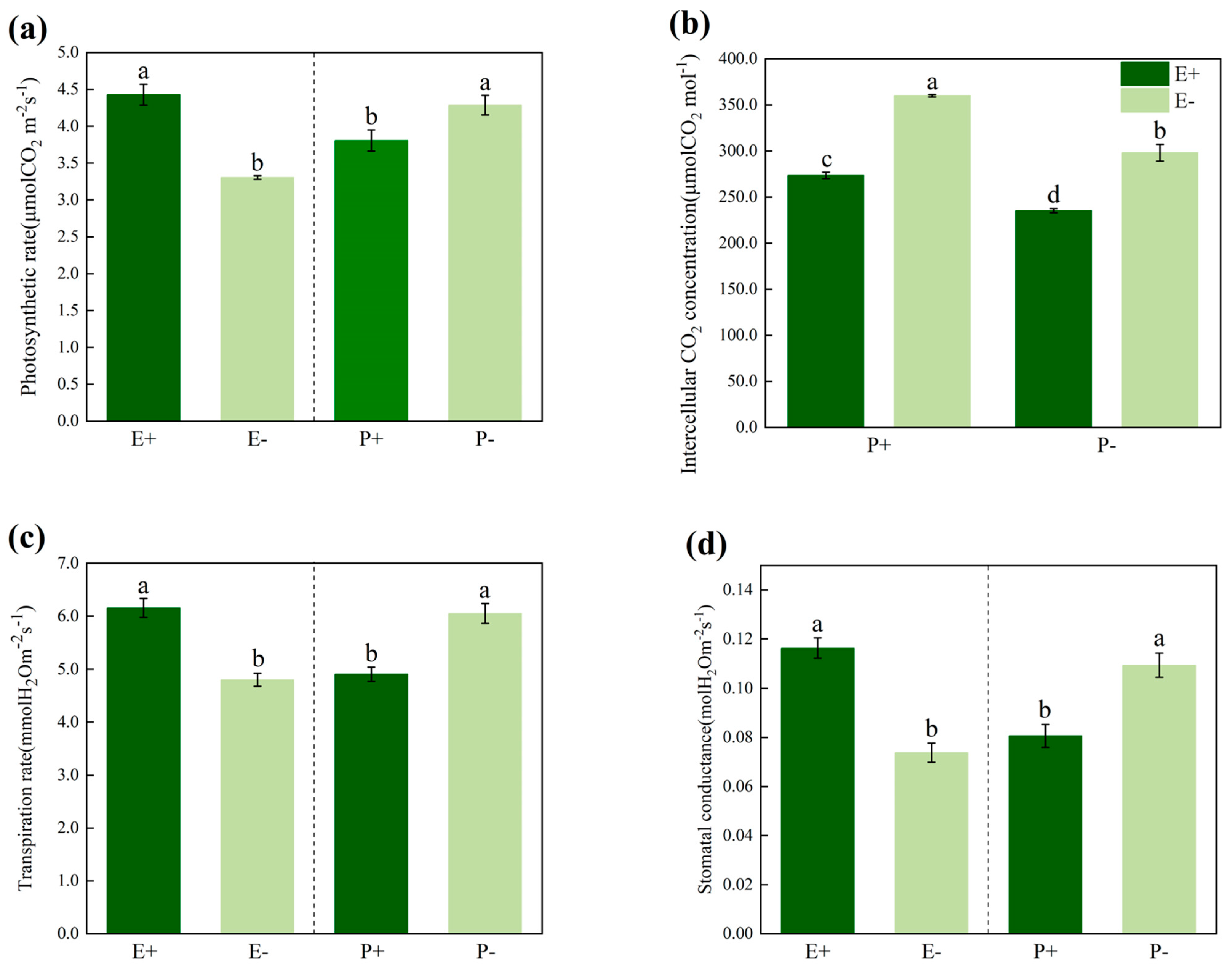
| Treatments | df | Photosynthetic Rate | Intercellular CO2 Concentration | Transpiration Rate | Stomatal Conductance | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | ||
| P | 1 | 106.787 | 0.000 | 98.877 | 0.000 | 46.759 | 0.000 | 40.290 | 0.000 |
| E | 1 | 211.323 | 0.000 | 219.716 | 0.000 | 65.380 | 0.000 | 88.861 | 0.000 |
| P × E | 1 | 3.628 | 0.061 | 5.547 | 0.021 | 3.235 | 0.077 | 0.181 | 0.672 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Zhu, S.; Zhang, F.; Zhao, Z.; Christensen, M.J.; Nan, Z.; Zhang, X. Transcriptomic Analyses Reveals Molecular Regulation of Photosynthesis by Epichloë endophyte in Achnatherum inebrians under Blumeria graminis Infection. J. Fungi 2022, 8, 1201. https://doi.org/10.3390/jof8111201
Zhu Y, Zhu S, Zhang F, Zhao Z, Christensen MJ, Nan Z, Zhang X. Transcriptomic Analyses Reveals Molecular Regulation of Photosynthesis by Epichloë endophyte in Achnatherum inebrians under Blumeria graminis Infection. Journal of Fungi. 2022; 8(11):1201. https://doi.org/10.3390/jof8111201
Chicago/Turabian StyleZhu, Yue, Shibo Zhu, Fang Zhang, Zhenrui Zhao, Michael J. Christensen, Zhibiao Nan, and Xingxu Zhang. 2022. "Transcriptomic Analyses Reveals Molecular Regulation of Photosynthesis by Epichloë endophyte in Achnatherum inebrians under Blumeria graminis Infection" Journal of Fungi 8, no. 11: 1201. https://doi.org/10.3390/jof8111201










