Bioactive Alkaloids from the Marine-Derived Fungus Metarhizium sp. P2100
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
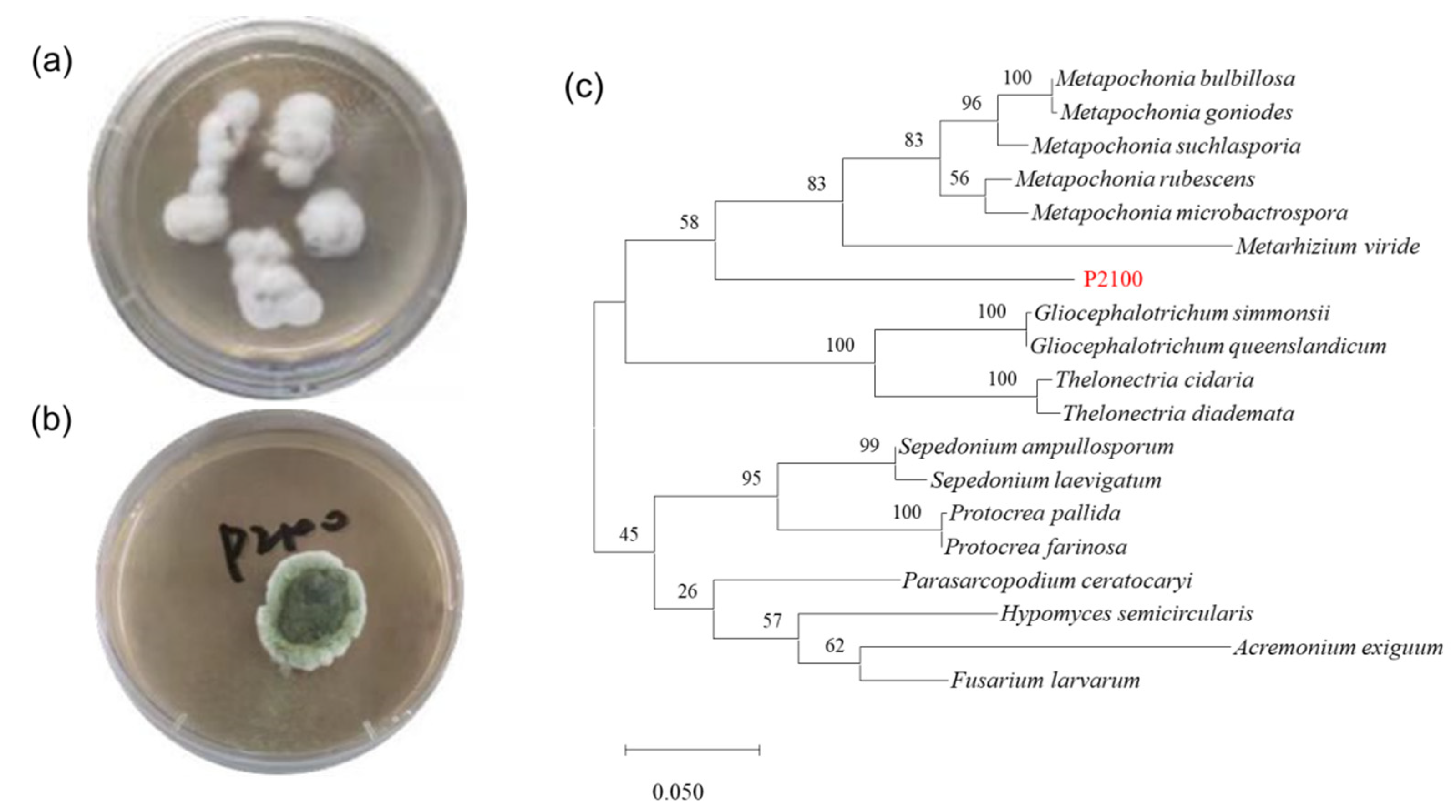
2.2. Isolation and Species Identification of Marine Fungus
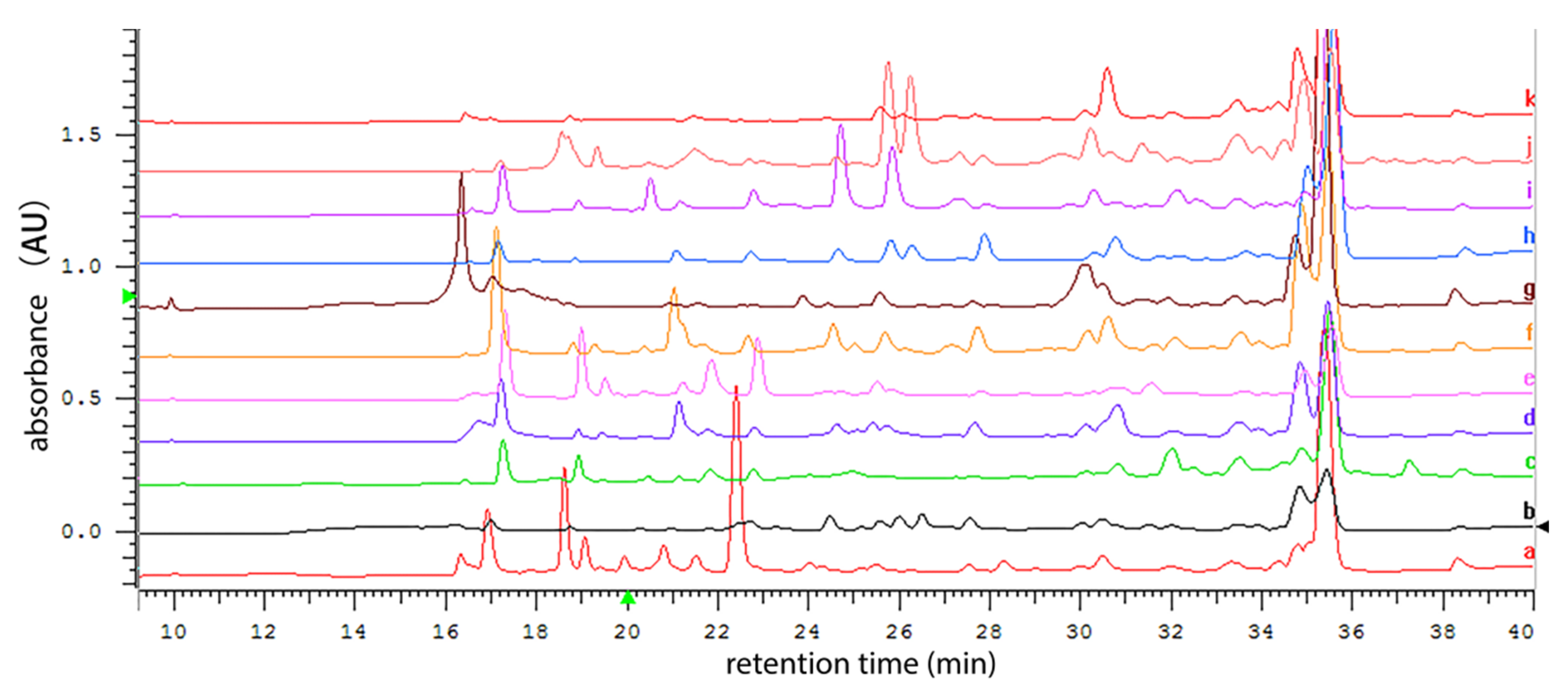
2.3. Fungal Fermentation and Metabolite Profile Analysis
2.4. Extraction and Isolation
2.5. Bioassay
3. Results
3.1. Strain Isolation and Species Identification
3.2. HPLC analysis of Secondary Metabolite Profile of P2100 Cultured in Multiple Media
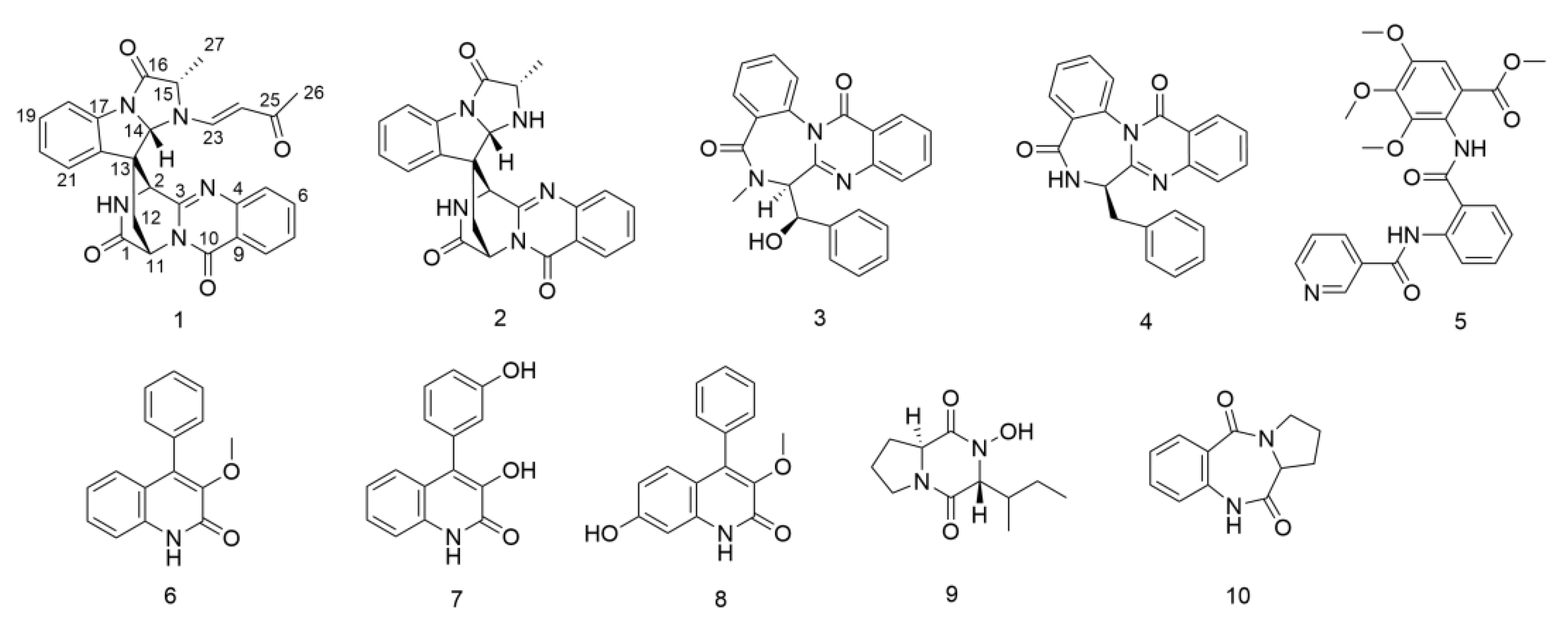
3.3. Elucidation of Chemical Structures
3.4. Spectroscopic and Spectrometric Data
3.5. Bioassays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Animal and Human Rights Statement
Conflicts of Interest
References
- Hou, X.M.; Wang, C.Y.; Gerwick, W.H.; Shao, C.L. Marine natural products as potential anti-tubercular agents. Eur. J. Med. Chem. 2019, 165, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Quan, C.; Hou, X.; Fan, S. Potential pharmacological resources: Natural bioactive compounds from marine-derived fungi. Mar. Drugs. 2016, 14, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altmann, K.H. Drugs from the oceans: Marine natural products as leads for drug discovery. Chimia 2017, 71, 646–652. [Google Scholar] [CrossRef]
- Sun, W.; Wu, W.; Liu, X.; Zaleta-Pinet, D.A.; Clark, B.R. Bioactive compounds isolated from marine-derived microbes in China: 2009–2018. Mar. Drugs 2019, 17, 339. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Frank, M.; Yu, X.; Yu, H.; Tran-Cong, N.M.; Gao, Y.; Proksch, P. Secondary metabolites from marine-derived fungi from China. Prog. Chem. Org. Nat. Prod. 2020, 111, 81–153. [Google Scholar] [PubMed]
- Song, Z.; Hou, Y.; Yang, Q.; Li, X.; Wu, S. Structures and biological activities of diketopiperazines from marine organisms: A review. Mar. Drugs 2021, 19, 403. [Google Scholar] [CrossRef]
- Natoli, M.; Herzig, P.; Pishali, B.E.; Buchi, M.; Ritschard, R.; Lloyd, G.K.; Mohanlal, R.; Tonra, J.R.; Huang, L.; Heinzelmann, V.; et al. Plinabulin, a distinct microtubule-targeting chemotherapy, promotes M1-like macrophage polarization and anti-tumor immunity. Front. Oncol. 2021, 11, 644608. [Google Scholar] [CrossRef]
- Song, F.; Ren, B.; Yu, K.; Chen, C.; Guo, H.; Yang, N.; Gao, H.; Liu, X.; Liu, M.; Tong, Y.; et al. Quinazolin-4-one coupled with pyrrolidin-2-iminium alkaloids from marine-derived fungus Penicillium aurantiogriseum. Mar. Drugs 2012, 10, 1297–1306. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Sun, W.; Deng, M.; Zhou, Q.; Wang, J.; Liu, J.; Chen, C.; Qi, C.; Luo, Z.; Xue, Y.; et al. Asperversiamides, linearly fused prenylated indole alkaloids from the marine-derived fungus Aspergillus versicolor. J. Org. Chem. 2018, 83, 8483–8492. [Google Scholar] [CrossRef]
- Zhang, P.; Mándi, A.; Li, X.M.; Du, F.Y.; Wang, J.N.; Li, X.; Kurtán, T.; Wang, B.G. Varioxepine A, a 3H-oxepine-containing alkaloid with a new oxa-cage from the marine algal-derived endophytic fungus Paecilomyces variotii. Org. Lett. 2014, 16, 4834–4837. [Google Scholar] [CrossRef]
- Wu, J.S.; Shi, X.H.; Yao, G.S.; Shao, C.L.; Fu, X.M.; Zhang, X.L.; Guan, H.S.; Wang, C.Y. New thiodiketopiperazine and 3,4-dihydroisocoumarin derivatives from the marine-derivedfungus Aspergillus terreus. Mar. Drugs 2020, 18, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.H.; Geng, C.; Zhang, X.W.; Zhu, H.J.; Shao, C.L.; Cao, F.; Wang, C.Y. Discovery of bioactive indole-diketopiperazines from the marine-derived fungus Penicillium brasilianum aided by genomic information. Mar. Drugs 2019, 17, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.Y.; Liu, X.H.; Zheng, Y.Y.; Ning, X.Y.; Zhang, Y.H.; Fu, X.M.; Li, X.; Shao, C.L.; Wang, C.Y. 2,5-Diketopiperazines from a sponge-derived fungus Aspergillus sclerotiorum. Front. Microbiol. 2022, 13, 808532. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.M.; Donzelli, B.G.; Krasnoff, S.B.; Keyhani, N.O. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat. Prod. Rep. 2014, 31, 1287–1305. [Google Scholar] [CrossRef]
- Wang, J.B.; St Leger, R.J.; Wang, C. Advances in genomics of entomopathogenic fungi. Adv. Genet. 2016, 94, 67–105. [Google Scholar]
- Liu, B.L.; Tzeng, Y.M. Development and applications of destruxins: A review. Biotechnol. Adv. 2012, 30, 1242–1254. [Google Scholar] [CrossRef]
- Wang, X.; Gong, X.; Li, P.; Lai, D.; Zhou, L. Structural diversity and biological activities of cyclic depsipeptides from fungi. Molecules 2018, 23, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozone, I.; Ueda, J.Y.; Watanabe, M.; Nogami, S.; Nagai, A.; Inaba, S.; Ohya, Y.; Takagi, M.; Shin-ya, K. Novel 24-membered macrolides, JBIR-19 and -20 isolated from Metarhizium sp. fE61. J. Antibiot. 2009, 62, 159–162. [Google Scholar] [CrossRef]
- Gupta, S.; Krasnoff, S.B.; Renwick, J.A.A.; Roberts, D.W.; Steiner, J.R.; Clardy, J. Viridoxins A and B: Novel toxins from the fungus Metarhizium flavoviride. J. Org. Chem. 1993, 58, 1062–1067. [Google Scholar] [CrossRef]
- Hou, X.M.; Xu, R.F.; Gu, Y.C.; Wang, C.Y.; Shao, C.L. Biological and chemical diversity of coral-derived microorganisms. Curr. Med. Chem. 2015, 22, 3707–3762. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, Y.Y.; Shao, C.L.; Wang, C.Y. Metabolites from marine invertebrates and their symbiotic microorganisms: Molecular diversity discovery, mining, and application. Mar. Life Sci. Technol. 2019, 1, 60–94. [Google Scholar] [CrossRef]
- Peng, X.Y.; Wu, J.T.; Shao, C.L.; Li, Z.Y.; Chen, M.; Wang, C.L. Co-culture: Stimulate the metabolic potential and explore the molecular diversity of natural products from microorganisms. Mar. Life Sci. Technol. 2021, 3, 363–374. [Google Scholar] [CrossRef]
- Hai, Y.; Cai, Z.M.; Li, P.J.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. Trends of antimalarial marine natural products: Progresses, challenges and opportunities. Nat. Prod. Rep. 2022, 39, 969–990. [Google Scholar] [CrossRef] [PubMed]
- Li, H.R.; Maimaitiming, M.; Zhou, Y.; Li, H.; Wang, P.; Liu, Y.; Schäberle, T.F.; Liu, Z.; Wang, C.Y. Discovery of marine natural products as promising antibiotics against Pseudomonas aeruginosa. Mar. Drugs 2022, 20, 192. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Yang, L.J.; Peng, X.Y.; Zhang, Y.H.; Liu, Z.Q.; Li, X.; Gu, Y.C.; Shao, C.L.; Han, Z.; Wang, C.Y. Antimicrobial and antioxidant polyketides from a deep-sea-derived fungus Aspergillus versicolor SH0105. Mar. Drugs 2020, 18, 636. [Google Scholar] [CrossRef]
- Aquino, R.; Morelli, S.; Lauro, M.R.; Abdo, S.; Saija, A.; Tomaino, A. Phenolic constituents and antioxidant activity of an extract of Anthurium versicolor leaves. J. Nat. Prod. 2001, 64, 1019–1023. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food. Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef]
- Xia, W.; Luo, P.; Hua, P.; Ding, P.; Li, C.; Xu, J.; Zhou, H.; Gu, Q. Discovery of a new pterocarpan-type antineuroinflammatory compound from Sophora tonkinensis through suppression of the TLR4/NFκB/MAPK signaling pathway with PU.1 as a potential target. ACS Chem. Neurosci. 2019, 10, 295–303. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta. 2008, 1780, 673–679. [Google Scholar] [CrossRef]
- Larsen, T.O.; Petersen, B.O.; Duus, J.Ø.; Sørensen, D.; Frisvad, J.C.; Hansen, M.E. Discovery of new natural products by application of X-hitting, a novel algorithm for automated comparison of full UV spectra, combined with structural determination by NMR spectroscopy. J. Nat. Prod. 2005, 68, 871–874. [Google Scholar] [CrossRef]
- Jang, J.P.; Jang, J.H.; Soung, N.K.; Kim, H.M.; Jeong, S.J.; Asami, Y.; Shin, K.S.; Kim, M.R.; Oh, H.; Kim, B.Y.; et al. Benzomalvin E, an indoleamine 2,3-dioxygenase inhibitor isolated from Penicillium sp. FN070315. J. Antibiot. 2012, 65, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Hosoe, T.; Itabashi, T.; Sato, F.; Wachi, H.; Nagase, H.; Yaguchi, T.; Kawai, K. Quinazolinobenzodiazepine derivatives, Novobenzomalvins A-C: Fibronectin expression regulators from Aspergillus novofumigatus. Sci. Pharm. 2011, 79, 937–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zheng, J.; Liu, P.; Wang, W.; Zhu, W. Three new compounds from Aspergillus terreus PT06-2 grown in a high salt medium. Mar. Drugs. 2011, 9, 1368–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Euch, I.Z.; Frese, M.; Sewald, N.; Smaoui, S.; Shaaban, M.; Mellouli, L. Bioactive secondary metabolites from new terrestrial Streptomyces sp. TN82 strain: Isolation, structure elucidation and biological activity. Med. Chem. Res. 2018, 27, 1085–1092. [Google Scholar] [CrossRef]
- Birkinshaw, J.H.; Luckner, M.; Mohammed, Y.S.; Mothes, K.; Stickings, C.E. Studies in the biochemistry of micro-organisms. 114. Viridicatol and cyclopenol, metabolites of Penicillium viridicatum westling and Penicillium cyclopium westling. Biochem. J. 1963, 89, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.C.; Ding, S.S.; Shi, W.S.; Cao, F.; Zhu, H.J.; Wen, M.L. A new quinolinone from freshwater lake-derived fungus Myrothecium verrucaria. Nat. Prod. Res. 2017, 31, 99–103. [Google Scholar] [CrossRef]
- Yang, B.; Dong, J.; Zhou, X.; Yang, X.; Lee, K.; Wang, L.; Zhang, S.; Liu, Y. Proline-containing dipeptides from a marine sponge of a Callyspongia species. Helv. Chim. Acta. 2009, 92, 1112–1117. [Google Scholar] [CrossRef]
- Nakatani, S.; Yamamoto, Y.; Hayashi, M.; Komiyama, K.; Ishibashi, M. Cycloanthranilylproline-derived constituents from a myxomycete Fuligo candida. Chem. Pharm. Bull. 2004, 52, 368–370. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, X.M.; Meng, L.H.; Wang, B.G. N-Formyllapatin A, a new N-formylspiroquinazoline derivative from the marine-derived fungus Penicillium adametzioides AS-53. Phytochem. Lett. 2014, 10, 145–148. [Google Scholar] [CrossRef]

| Position | δC Type | δH Mult (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 169.76 | |||
| 2 | 55.83 | 4.27 (d, J = 4.0 Hz, 1H) | NH | C-1, 3, 12, 13 |
| 3 | 151.22 | |||
| 4 | 147.60 | |||
| 5 | 127.12 | 7.57 (d, J = 8.2 Hz, 1H) | H-6 | C-7, 9 |
| 6 | 134.73 | 7.80 (ddd, J = 8.2, 7.1, 1.5 Hz, 1H) | H-5 | C-4, 8 |
| 7 | 127.12 | 7.52 (m, 1H) | H-8 | C-5, 6, 9 |
| 8 | 126.35 | 8.09 (dd, J = 8.2, 1.5 Hz, 1H) | H-7 | C-4, 6, 10 |
| 9 | 119.86 | |||
| 10 | 158.09 | |||
| 11 | 52.87 | 5.58 (t, J = 2.9 Hz, 1H) | H-12 | C-1, 3, 13 |
| 12 | 35.75 | 3.02 (dd, J = 15.3, 3.4 Hz, 1H), 2.75 (dd, J = 15.3, 2.4 Hz, 1H) | H-11 | C-11, 13, 14 |
| 13 | 51.79 | |||
| 14 | 85.36 | 5.91 (s, 1H) | C-2, 12, 16, 13, 23 | |
| 15 | 61.41 | 4.43 (q, J = 6.9 Hz, 1H) | H-27 | C-16, 23, 27 |
| 16 | 169.08 | |||
| 17 | 136.38 | |||
| 18 | 115.87 | 7.51 (m, 1H) | C-20, 22 | |
| 19 | 129.56 | 7.49(m, 1H) | H-20 | C-17, 21 |
| 20 | 126.07 | 7.35 (td, J = 7.5, 1.6 Hz, 1H) | H-19,21 | C-18, 22 |
| 21 | 126.16 | 7.19 (d, J = 7.5 Hz, 1H) | H-20 | C-13, 17, 19 |
| 22 | 136.99 | |||
| 23 | 146.24 | 6.74 (d, J = 13.9 Hz, 1H) | H-24 | C-14, 15, 25 |
| 24 | 103.41 | 4.74 (d, J = 13.9 Hz, 1H) | H-23 | C-26 |
| 25 | 193.39 | |||
| 26 | 25.31 | 1.27 (s, 3H) | C-25 | |
| 27 | 16.97 | 1.70 (d, J = 6.9 Hz, 3H) | H-15 | C-16 |
| NH | 9.26 (d, J = 4.0 Hz, 1H) | H-2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, G.-S.; Ma, Z.-L.; Zheng, Y.-Y.; Lv, L.; Mao, J.-Q.; Wang, C.-Y. Bioactive Alkaloids from the Marine-Derived Fungus Metarhizium sp. P2100. J. Fungi 2022, 8, 1218. https://doi.org/10.3390/jof8111218
Yao G-S, Ma Z-L, Zheng Y-Y, Lv L, Mao J-Q, Wang C-Y. Bioactive Alkaloids from the Marine-Derived Fungus Metarhizium sp. P2100. Journal of Fungi. 2022; 8(11):1218. https://doi.org/10.3390/jof8111218
Chicago/Turabian StyleYao, Guang-Shan, Zhong-Lian Ma, Yao-Yao Zheng, Ling Lv, Jun-Qiu Mao, and Chang-Yun Wang. 2022. "Bioactive Alkaloids from the Marine-Derived Fungus Metarhizium sp. P2100" Journal of Fungi 8, no. 11: 1218. https://doi.org/10.3390/jof8111218




