Pb Transfer Preference of Arbuscular Mycorrhizal Fungus Rhizophagus irregularis in Morus alba under Different Light Intensities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Substrates, and AM Fungal Inoculum
2.2. Experimental Design
2.3. Plant Sampling, Growth Status, and AM Fungal Colonization
2.4. Concentrations of Pb
2.5. Photosynthesis
2.6. Gene Relative Expression
2.7. Statistical Analysis
3. Results
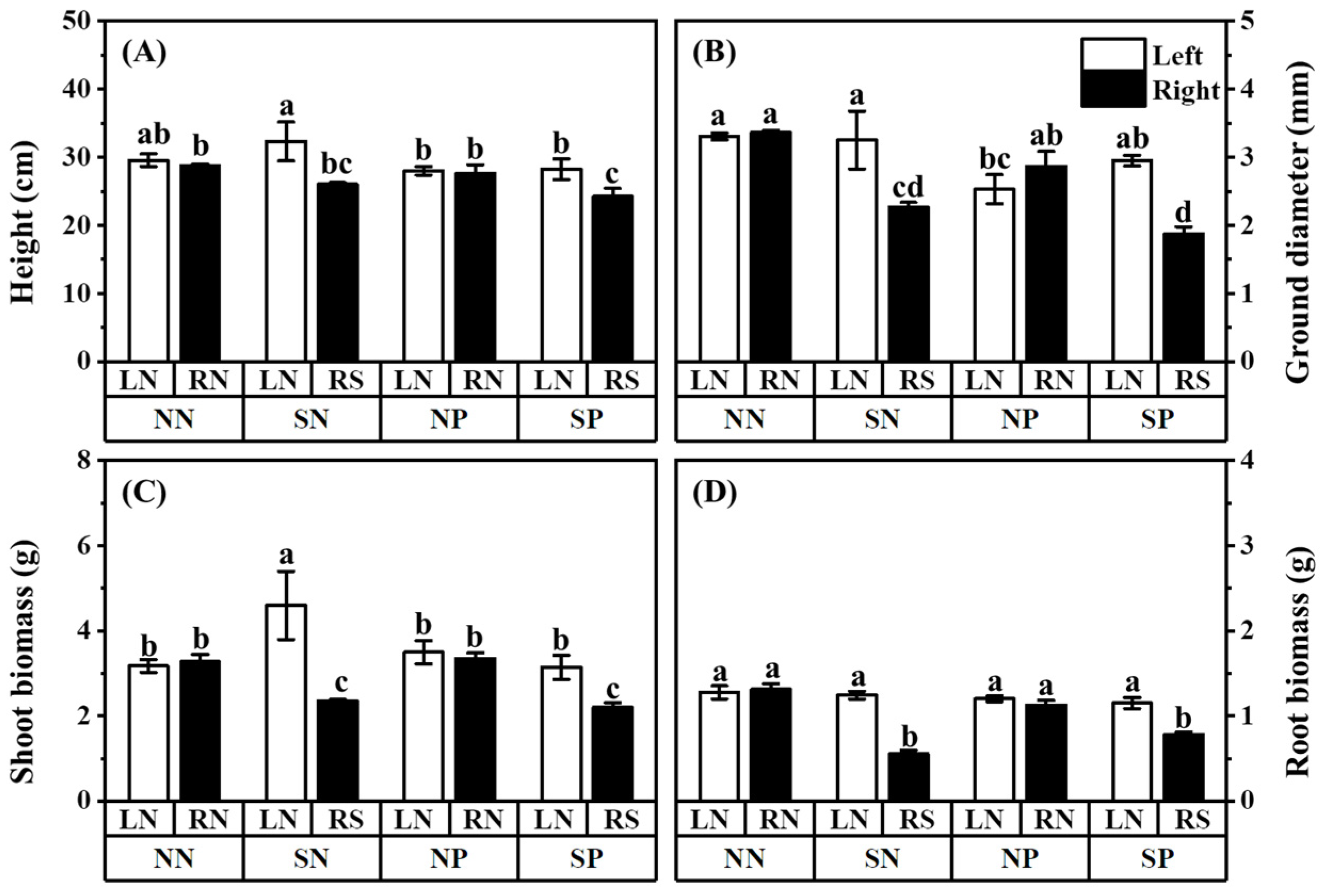
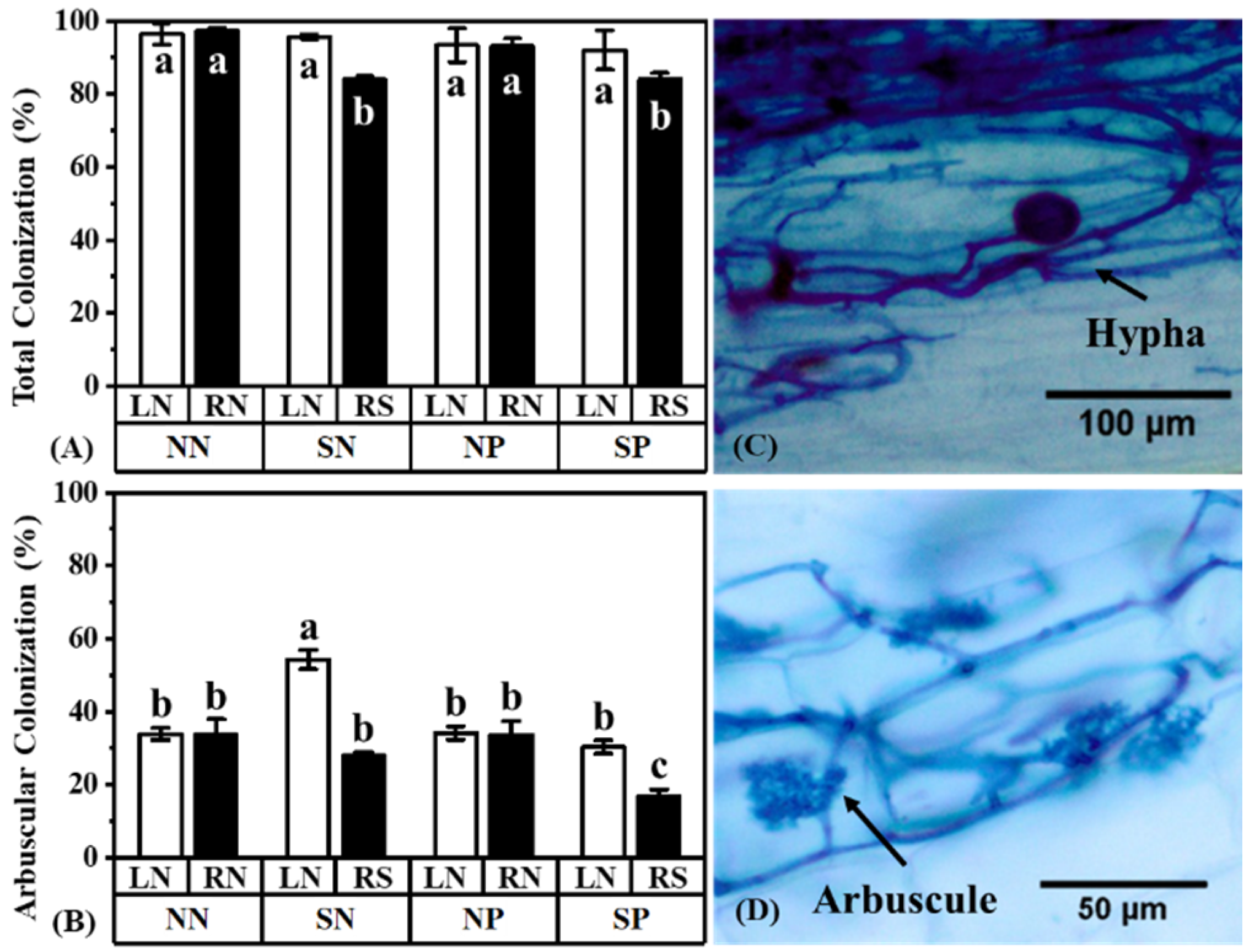
3.1. Negative Effects of Pb and Shading on Plant Growth and Colonization
3.2. Negative Effects of Pb and Shading on Plant Photosynthesis
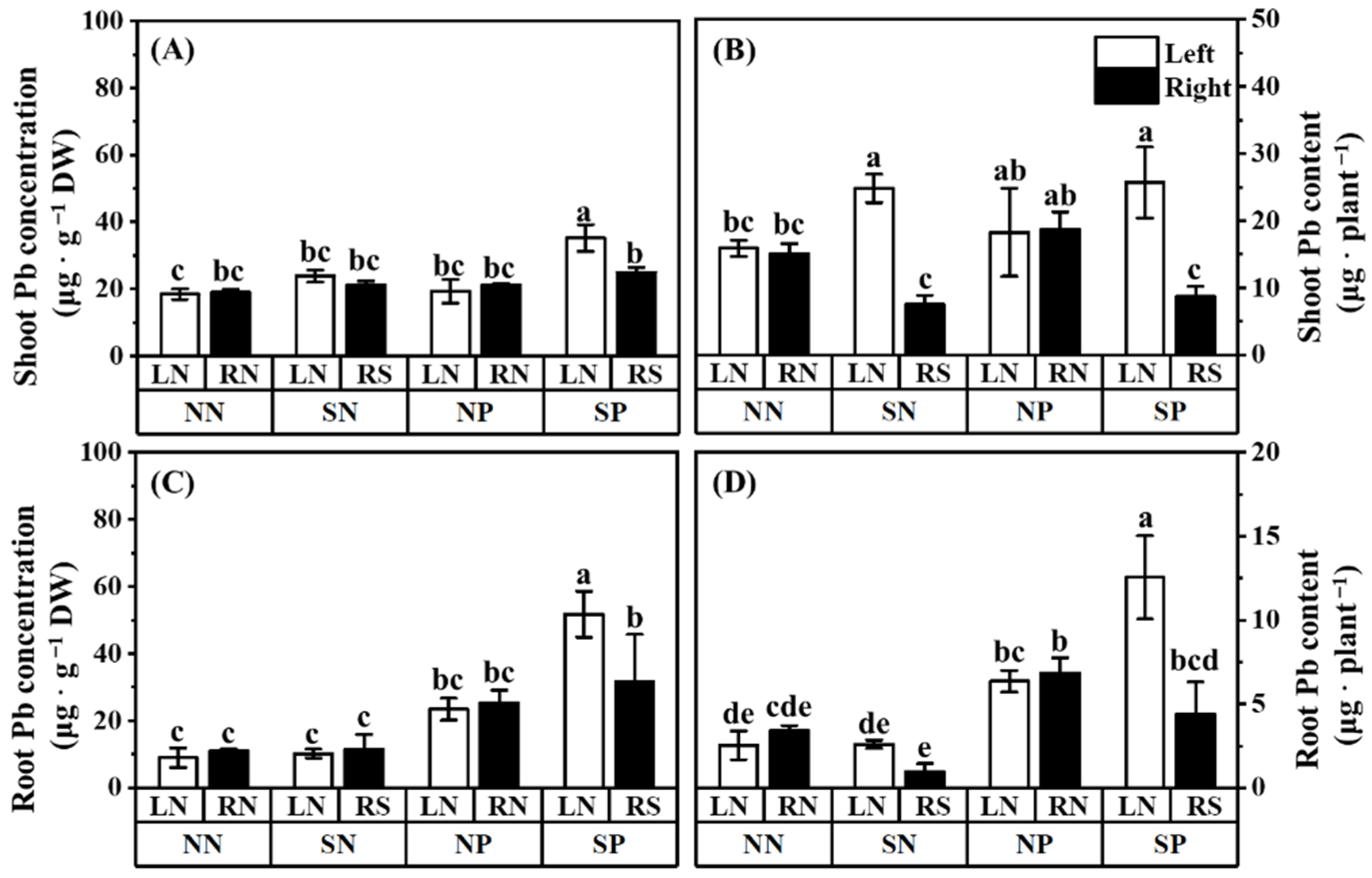
3.3. Pb Concentration and Content
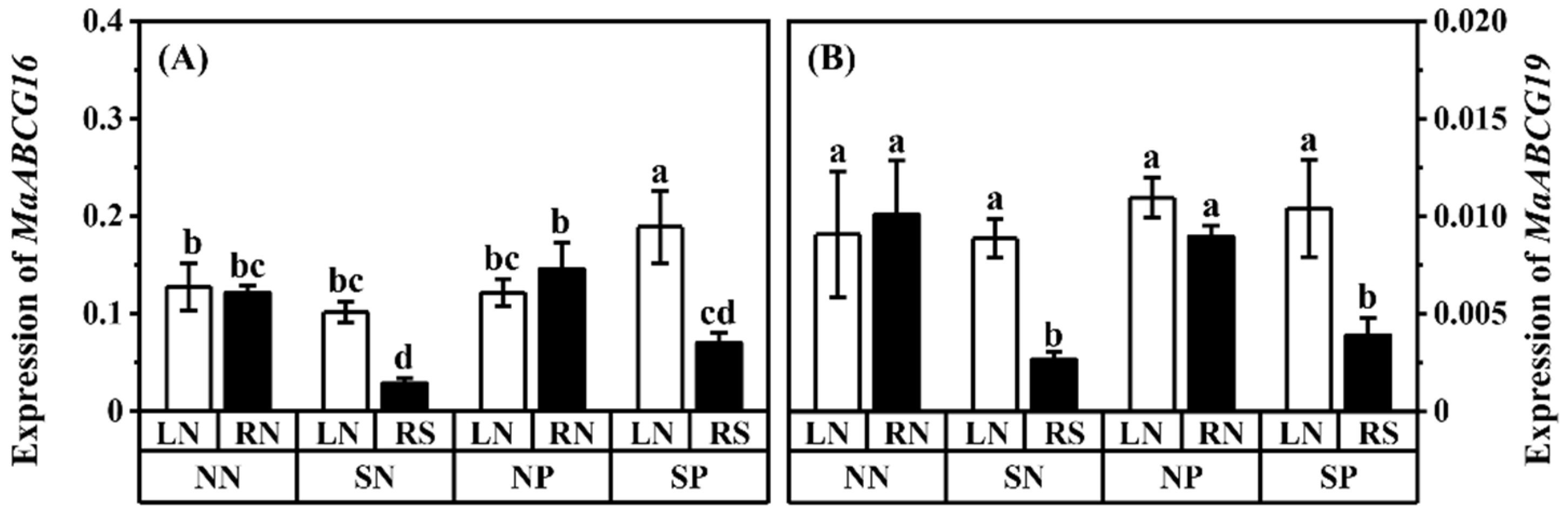
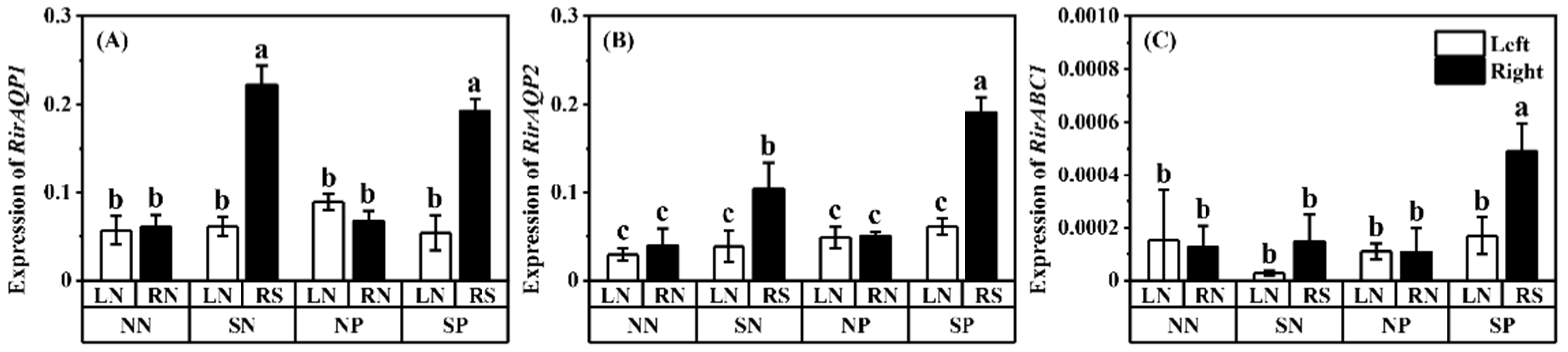
3.4. Relative Expressions of Related Genes
3.5. Correlation Analysis
4. Discussion
4.1. The AM Fungi Impact More on High-Light Mulberry than Low-Light Mulberry
4.2. The Status of Plant Light and Pb Effect AM Fungal Colonization
4.3. The AM Fungi Prefer to Transfer Pb to High-Light Mulberry Rather than Low-Light Mulberry
4.4. Effect of Shading and Pb on Related Gene
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baloch, S.; Kazi, T.G.; Baig, J.A.; Afridi, H.I.; Arain, M.B. Occupational exposure of lead and cadmium on adolescent and adult workers of battery recycling and welding workshops: Adverse impact on health. Sci. Total Environ. 2020, 720, 137549. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xu, Z.; Huang, Y.; Wang, T.; Zheng, S.; DePasquale, A.; Brueckner, C.; Lei, Y.; Li, B. Long-term continuous and real-time in situ monitoring of Pb(II) toxic contaminants in wastewater using solid-state ion selective membrane (S-ISM) Pb and pH auto-correction assembly. J. Hazard. Mater. 2020, 400, 123299. [Google Scholar] [CrossRef]
- Barea-Sepúlveda, M.; Espada-Bellido, E.; Ferreiro-González, M.; Bouziane, H.; López-Castillo, J.G.; Palma, M.; Barbero, F.G. Exposure to essential and toxic elements via consumption of Agaricaceae, Amanitaceae, Boletaceae, and Russulaceae mushrooms from Southern Spain and Northern Morocco. J. Fungi 2022, 8, 545. [Google Scholar] [CrossRef]
- Wang, C.; Rong, H.; Zhang, X.; Shi, W.; Hong, X.; Liu, W.; Cao, T.; Yu, X.; Yu, Q. Effects and mechanisms of foliar application of silicon and selenium composite sols on diminishing cadmium and lead translocation and affiliated physiological and biochemical responses in hybrid rice (Oryza sativa L.) exposed to cadmium and lead. Chemosphere 2020, 251, 126347. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Xu, X.; Zhu, Y.; Wang, Q.; Zhang, Y.; Huo, X. Pb and Cd exposure linked with Il-10 and Il-13 gene polymorphisms in asthma risk relevant immunomodulation in children. Chemosphere 2022, 294, 133656. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Freeman, J.L. Exposure to the heavy-metal lead induces DNA copy number alterations in zebrafish cells. Chem. Res. Toxicol. 2020, 33, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.H.; Harp, J.P.; Han, D.Y. Pb neurotoxicity: Neuropsychological effects of lead toxicity. Biomed. Res. Int. 2014, 2014, 840547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togao, M.; Nakayama, S.M.M.; Ikenaka, Y.; Mizukawa, H.; Makino, Y.; Kubota, A.; Matsukawa, T.; Yokoyama, K.; Hirata, T.; Ishizuka, M. Bioimaging of Pb and STIM1 in mice liver, kidney and brain using Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) and immunohistochemistry. Chemosphere 2020, 238, 124581. [Google Scholar] [CrossRef]
- Global No. 1 Business Data Platform. Available online: https://www.statista.com/statistics/273652/global-lead-reserves-by-selected-countries/ (accessed on 19 April 2022).
- Luo, X.; Wu, C.; Lin, Y.; Li, W.; Deng, M.; Tan, J.; Xue, S. Soil heavy metal pollution from Pb/Zn smelting regions in China and the remediation potential of biomineralization. J. Environ. Stud. 2023, 125, 662–677. [Google Scholar] [CrossRef]
- Wang, W.; Lu, N.; Pan, H.; Wang, Z.; Han, X.; Zhu, Z.; Guan, J. Heavy metal pollution and its prior pollution source identification in agricultural soil: A case study in the Qianguo Irrigation District, northeast China. Sustainability 2022, 14, 4494. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, X.; Liang, X.; Li, Z.; Wang, L.; He, Y.; Zhan, F. Arbuscular mycorrhizal fungi reduce cadmium leaching from sand columns by reducing availability and enhancing uptake by maize roots. J. Fungi 2022, 8, 866. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, B.; Xu, J.; Li, Z.; Tang, Z.; Wu, X. Heavy metal domestication enhances beneficial effects of arbuscular mycorrhizal fungi on lead (Pb) phytoremediation efficiency of Bidens parviflora through improving plant growth and root Pb accumulation. Environ. Sci. Pollut. Res. Int. 2022, 29, 32988–33001. [Google Scholar] [CrossRef]
- Soldi, E.; Casey, C.; Murphy, B.R.; Hodkinson, T.R. Fungal endophytes for grass based bioremediation: An endophytic consortium isolated from Agrostis stolonifera stimulates the growth of Festuca arundinacea in lead contaminated soil. J. Fungi 2020, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Blaudez, D.; Botton, B.; Chalot, M. Cadmium uptake and subcellular compartmentation in the ectomycorrhizal fungus Paxillus involutus. Microbiology 2000, 146, 1109–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faggioli, V.; Menoyo, E.; Geml, J.; Kemppainen, M.; Pardo, A.; Salazar, M.J.; Becerra, A.G. Soil lead pollution modifies the structure of arbuscular mycorrhizal fungal communities. Mycorrhiza 2019, 29, 363–373. [Google Scholar] [CrossRef]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef] [Green Version]
- Salazar, M.J.; Menoyo, E.; Faggioli, V.; Geml, J.; Cabello, M.; Rodriguez, J.H.; Marro, N.; Pardo, A.; Pignata, M.L.; Becerra, A.G. Pb accumulation in spores of arbuscular mycorrhizal fungi. Sci. Total Environ. 2018, 643, 238–246. [Google Scholar] [CrossRef]
- Field, K.J.; Bidartondo, M.I.; Rimington, W.R.; Hoysted, G.A.; Beerling, D.; Cameron, D.D.; Duckett, J.G.; Leake, J.R.; Pressel, S. Functional complementarity of ancient plant-fungal mutualisms: Contrasting nitrogen, phosphorus and carbon exchanges between Mucoromycotina and Glomeromycotina fungal symbionts of liverworts. New Phytol. 2019, 223, 908–921. [Google Scholar] [CrossRef] [Green Version]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef] [Green Version]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Wang, W.; Shi, J.; Xie, Q.; Jiang, Y.; Yu, N.; Wang, E. Nutrient exchange and regulation in arbuscular mycorrhizal symbiosis. Mol. Plant. 2017, 10, 1147–1158. [Google Scholar] [CrossRef] [Green Version]
- Gavito, M.E.; Jakobsen, I.; Mikkelsen, T.N.; Mora, F. Direct evidence for modulation of photosynthesis by an arbuscular mycorrhiza-induced carbon sink strength. New Phytol. 2019, 223, 896–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Mortimer, P.E.; Pérez-Fernández, M.A.; Valentine, A.J. The role of arbuscular mycorrhizal colonization in the carbon and nutrient economy of the tripartite symbiosis with nodulated Phaseolus vulgaris. Soil Biol. Biochem. 2008, 40, 1019–1027. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, H.; Song, Y.; Yang, Y.; Chen, H.; Tang, M. Subcellular compartmentalization and chemical forms of lead participate in lead tolerance of Robinia pseudoacacia L. with Funneliformis mosseae. Front. Plant Sci. 2017, 8, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Ren, W.; Zheng, Y.; Li, Y.; Zhu, M.; Tang, M. Arbuscular mycorrhizal fungi increase Pb uptake of colonized and non-colonized Medicago truncatula root and deliver extra Pb to colonized root segment. Microorganisms 2021, 9, 1203. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, W.; Tong, T.; Chen, G.; Zeng, F.; Jang, S.; Gao, W.; Li, Z.; Mak, M.; Deng, F.; et al. Molecular interaction and evolution of jasmonate signaling with transport and detoxification of heavy metals and metalloids in plants. Front. Plant Sci. 2021, 12, 665842. [Google Scholar] [CrossRef]
- Zhang, X.; Lou, X.; Zhang, H.; Ren, W.; Tang, M. Effects of sodium sulfide application on the growth of Robinia pseudoacacia, heavy metal immobilization, and soil microbial activity in Pb-Zn polluted soil. Ecotoxicol. Environ. Saf. 2020, 197, 110563. [Google Scholar] [CrossRef]
- Fuentes, A.; Almonacid, L.; Ocampo, J.A.; Arriagada, C. Synergistic interactions between a saprophytic fungal consortium and Rhizophagus irregularis alleviate oxidative stress in plants grown in heavy metal contaminated soil. Plant Soil 2016, 407, 355–366. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Xie, X.; Wu, Y.; Liang, F.; Tang, M. Arbuscular mycorrhizal fungi promote lead immobilization by increasing the polysaccharide content within pectin and inducing cell wall peroxidase activity. Chemosphere 2021, 267, 128924. [Google Scholar] [CrossRef]
- Rohela, G.K.; Shukla, P.; Muttanna; Kumar, R.; Chowdhury, S.R. Mulberry (Morus spp.): An ideal plant for sustainable development. Trees For. People 2020, 2, 100011. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) fruit-a review of characteristic components and health benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.P.; Senthilkumar, P.; Subburam, V. Mulberry-silkworm food chain-a templet to assess heavy metal mobility in terrestrial ecosystems. Environ. Monit. Assess. 2001, 69, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhao, Y.; Wang, S.; Han, S.; Liu, J. Lead in the soil-mulberry (Morus alba L.)-silkworm (Bombyx mori) food chain: Translocation and detoxification. Chemosphere 2015, 128, 171–177. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, S.; Yan, X.; Qin, Z.; Jia, C.; Li, Z.; Zhang, J.; Huang, R. The mobility of cadmium and lead in the soil-mulberry-silkworm system. Chemosphere 2020, 242, 125179. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Zhang, J.; Hussain, A.; Qiao, Y.; Zhou, J.; Wang, X. Accumulation and translocation of food chain in soil-mulberry (Morus alba L.)-silkworm (Bombyx mori) under single and combined stress of lead and cadmium. Ecotoxicol. Environ. Saf. 2021, 208, 111582. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y. Effects of biochar and am fungi on growth, mineral elements and cadmium uptake of mulberry under cadmium stress. IOP Conf. Ser. Earth Environ. Sci. 2021, 687, 012021. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [Green Version]
- Cooper, K.M.; Tinker, P.B. Translocation and transfer of nutrients in vesicular-arbuscular mycorrhizas: IV. Effect of environmental variables on movement of phosphorus. New Phytol. 1981, 88, 327–339. [Google Scholar] [CrossRef]
- Han, X.; Du, X.; Wu, Y.; Wei, M.; Gu, Y.; Aba, X.; Tang, M.; Zhang, H. Foliar-applied potassium improved mycorrhizal Goji (Lycium barbarum L.) growth of the potassium free-compartment in a compartmented culture system. Sci. Hortic. 2022, 293, 110681. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hijikata, N.; Ohtomo, R.; Handa, Y.; Kawaguchi, M.; Saito, K.; Masuta, C.; Ezawa, T. Aquaporin-mediated long-distance polyphosphate translocation directed towards the host in arbuscular mycorrhizal symbiosis: Application of virus-induced gene silencing. New Phytol. 2016, 211, 1202–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, L.; Li, H.; Song, Z.; Dong, B.; Cao, H.; Liu, T.; Du, T.; Yang, W.; Amin, R.; Wang, L.; et al. The functional analysis of ABCG transporters in the adaptation of pigeon pea (Cajanus cajan) to abiotic stresses. PeerJ 2021, 9, e10688. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, S. Structure and mechanism of ABC transporters. F1000prime Rep. 2015, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Yang, L.; Wu, X.; Ni, J.; Jiang, H.; Zhang, Q.; Fang, L.; Sheng, Y.; Ren, Y.; Cao, S. The PSE1 gene modulates lead tolerance in Arabidopsis. J. Exp. Bot. 2016, 67, 4685–4695. [Google Scholar] [CrossRef] [Green Version]
- Bhuiyan, M.S.U.; Min, S.R.; Jeong, W.J.; Sultana, S.; Choi, K.S.; Lee, Y.; Liu, J.R. Overexpression of AtATM3 in Brassica juncea confers enhanced heavy metal tolerance and accumulation. Plant Cell Tissue Organ Cult. 2011, 107, 69–77. [Google Scholar] [CrossRef]
- Gonzalez-Guerrero, M.; Benabdellah, K.; Valderas, A.; Azcon-Aguilar, C.; Ferrol, N. GintABC1 encodes a putative ABC transporter of the MRP subfamily induced by Cu, Cd, and oxidative stress in Glomus intraradices. Mycorrhiza 2010, 20, 137–146. [Google Scholar] [CrossRef]
- Konvalinkova, T.; Jansa, J. Lights off for arbuscular mycorrhiza: On its symbiotic functioning under light deprivation. Front. Plant Sci. 2016, 7, 782. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Wang, C.; Shen, Z.; Quan, Y.; Liu, X. Role of extrinsic arbuscular mycorrhizal fungi in heavy metal-contaminated wetlands with various soil moisture levels. Int. J. Phytoremediation 2015, 17, 208–214. [Google Scholar] [CrossRef]
- Guo, Y.; George, E.; Marschner, H. Contribution of an arbuscular mycorrhizal fungus to the uptake of cadmium and nickel in bean and maize plants. Plant Soil 1996, 184, 195–205. [Google Scholar] [CrossRef]
- Lee, Y.J.; George, E. Contribution of mycorrhizal hyphae to the uptake of metal cations by cucumber plants at two levels of phosphorus supply. Plant Soil 2005, 278, 361–370. [Google Scholar] [CrossRef]
- Sheng, M.; Chen, X.; Zhang, X.; Hamel, C.; Cui, X.; Chen, J.; Chen, H.; Tang, M. Changes in arbuscular mycorrhizal fungal attributes along a chronosequence of black locust (Robinia pseudoacacia) plantations can be attributed to the plantation-induced variation in soil properties. Sci. Total Environ. 2017, 599–600, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil, 2nd ed.; California Agricultural Experiment Station: Berkeley, CA, USA, 1950; Volume 347. [Google Scholar]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- McGonigle, G.T.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- GB/T 13080-2018; Determination of Lead in Feeds-Atomic Absorption Spectrometry. Standardization Administration of China. State Administration for Market Regulation: Beijing, China, 2018.
- Ma, Y.; He, J.; Ma, C.; Luo, J.; Li, H.; Liu, T.; Polle, A.; Peng, C.; Luo, Z.B. Ectomycorrhizas with Paxillus involutus enhance cadmium uptake and tolerance in Populus x canescens. Plant Cell Environ. 2014, 37, 627–642. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Q.; Mo, S.; Qian, Y.; Wu, X.; Jin, Y.; Ding, H. Transcriptome-wide modulation combined with morpho-physiological analyses of Typha orientalis roots in response to lead challenge. J. Hazard. Mater. 2020, 384, 121405. [Google Scholar] [CrossRef]
- Shi, S.M.; Chen, K.; Gao, Y.; Liu, B.; Yang, X.H.; Huang, X.Z.; Liu, G.X.; Zhu, L.Q.; He, X.H. Arbuscular mycorrhizal fungus species dependency governs better plant physiological characteristics and leaf quality of mulberry (Morus alba L.) seedlings. Front. Microbiol. 2016, 7, 1030. [Google Scholar] [CrossRef] [Green Version]
- Dhawi, F.; Datta, R.; Ramakrishna, W. Mycorrhiza and heavy metal resistant bacteria enhance growth, nutrient uptake and alter metabolic profile of sorghum grown in marginal soil. Chemosphere 2016, 157, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, Y.; Han, X.; Chiu, T.Y.; Ghosh, A.; Chen, H.; Tang, M. The roles of arbuscular mycorrhizal fungi (AMF) in phytoremediation and tree-herb interactions in Pb contaminated soil. Sci. Rep. 2016, 6, 20469. [Google Scholar] [CrossRef] [Green Version]
- Lang, M.; Li, X.; Zheng, C.; Li, H.; Zhang, J. Shading mediates the response of mycorrhizal maize (Zea mays L.) seedlings under varying levels of phosphorus. Appl. Soil Ecol. 2021, 166, 104060. [Google Scholar] [CrossRef]
- Zheng, C.; Ji, B.; Zhang, J.; Zhang, F.; Bever, J.D. Shading decreases plant carbon preferential allocation towards the most beneficial mycorrhizal mutualist. New Phytol. 2015, 205, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, M.; Tolosano, M.; Volpe, V.; Kopriva, S.; Bonfante, P. Identification and functional characterization of a sulfate transporter induced by both sulfur starvation and mycorrhiza formation in Lotus japonicus. New Phytol. 2014, 204, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Koegel, S.; Ait Lahmidi, N.; Arnould, C.; Chatagnier, O.; Walder, F.; Ineichen, K.; Boller, T.; Wipf, D.; Wiemken, A.; Courty, P.E. The family of ammonium transporters (AMT) in Sorghum bicolor: Two AMT members are induced locally, but not systemically in roots colonized by arbuscular mycorrhizal fungi. New Phytol. 2013, 198, 853–865. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Liu, J.; Cui, M.; Huang, Y.; Tian, Y.; Chen, A.; Xu, G. The potassium transporter SlHAK10 is involved in mycorrhizal potassium uptake. Plant Physiol. 2019, 180, 465–479. [Google Scholar] [CrossRef] [Green Version]
- Javot, H.; Penmetsa, R.V.; Terzaghi, N.; Cook, D.R.; Harrison, M.J. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2007, 104, 1720–1725. [Google Scholar] [CrossRef] [Green Version]
- Agren, G.I.; Wetterstedt, J.A.M.; Billberger, M.F.K. Nutrient limitation on terrestrial plant growth—modeling the interaction between nitrogen and phosphorus. New Phytol. 2012, 194, 953–960. [Google Scholar] [CrossRef]
- Sudová, R.; Vosátka, M. Differences in the effects of three arbuscular mycorrhizal fungal strains on P and Pb accumulation by maize plants. Plant Soil 2007, 296, 77–83. [Google Scholar] [CrossRef]
- Ruby, M.V.; Davis, A.; Nicholson, A. In situ formation of lead phosphates in soils as a method to immobilize lead. Environ. Sci. Technol. 1994, 28, 646–654. [Google Scholar] [CrossRef]
- Weissenhorn, I.; Leyval, C. Root colonization of maize by a Cd-sensitive and a Cd-tolerant Glomus mosseae and cadmium uptake in sand culture. Plant Soil 1995, 175, 233–238. [Google Scholar] [CrossRef]
- Chen, X.; Wu, C.; Tang, J.; Hu, S. Arbuscular mycorrhizae enhance metal lead uptake and growth of host plants under a sand culture experiment. Chemosphere 2005, 60, 665–671. [Google Scholar] [CrossRef]
- Yan, H.; Shang, A.; Peng, Y.; Yu, P.; Li, C. Covering middle leaves and ears reveals differential regulatory roles of vegetative and reproductive organs in root growth and nitrogen uptake in maize. Crop Sci. 2011, 51, 265–272. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Silva-Castro, G.A.; Sanchez, A.; Arriagada, C.; García-Romera, I. Effect of arbuscular mycorrhizal fungi and mycoremediated dry olive residue in lead uptake in wheat plants. Appl. Soil Ecol. 2021, 159, 103838. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, Y.; Hu, N.; Shi, Y.; Li, T.; Zhao, Z. Differential responses of 23 maize cultivar seedlings to an arbuscular mycorrhizal fungus when grown in a metal-polluted soil. Sci. Total Environ. 2021, 789, 148015. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, Y.; Chen, S.; Polle, A.; Rennenberg, H.; Luo, Z.B. Physiological and molecular mechanisms of heavy metal accumulation in nonmycorrhizal versus mycorrhizal plants. Plant Cell Environ. 2019, 42, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Kohzuma, K.; Froehlich, J.E.; Davis, G.A.; Temple, J.A.; Minhas, D.; Dhingra, A.; Cruz, J.A.; Kramer, D.M. The role of light-dark regulation of the chloroplast ATP synthase. Front. Plant Sci. 2017, 8, 1248. [Google Scholar] [CrossRef] [Green Version]
- Linné, J.A.; Jesus, M.V.; de Lima, V.T.; Reis, L.C.; Dresch, D.M.; de Paula Quintão Scalon, S.; Santos, C.C. Effects of shading on growth and photosynthetic metabolism in Dipteryx alata Vogel seedlings under flooding. Rev. Bras. Bot. 2021, 44, 629–638. [Google Scholar] [CrossRef]
- Xu, M.Y.; Wu, K.X.; Liu, Y.; Liu, J.; Tang, Z.H. Effects of light intensity on the growth, photosynthetic characteristics, and secondary metabolites of Eleutherococcus senticosus Harms. Photosynthetica 2020, 58, 881–889. [Google Scholar] [CrossRef]
- Hwang, J.U.; Song, W.Y.; Hong, D.; Ko, D.; Yamaoka, Y.; Jang, S.; Yim, S.; Lee, E.; Khare, D.; Kim, K.; et al. Plant ABC transporters enable many unique aspects of a terrestrial plant’s lifestyle. Mol. Plant 2016, 9, 338–355. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Hu, Y.J.; Hao, Z.P.; Li, H.; Wang, Y.S.; Chen, B.D. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, H.; Chen, H.; Tang, M. Arbuscular mycorrhizas influence Lycium barbarum tolerance of water stress in a hot environment. Mycorrhiza 2017, 27, 451–463. [Google Scholar] [CrossRef]
- Van’t Padje, A.; Werner, G.D.A.; Kiers, E.T. Mycorrhizal fungi control phosphorus value in trade symbiosis with host roots when exposed to abrupt ‘crashes’ and ‘booms’ of resource availability. New Phytol. 2021, 229, 2933–2944. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, W.; Zhang, H.; Jin, X.; Huang, H.; Zhou, L.; Xu, T.; Tang, M. Pb Transfer Preference of Arbuscular Mycorrhizal Fungus Rhizophagus irregularis in Morus alba under Different Light Intensities. J. Fungi 2022, 8, 1224. https://doi.org/10.3390/jof8111224
Ren W, Zhang H, Jin X, Huang H, Zhou L, Xu T, Tang M. Pb Transfer Preference of Arbuscular Mycorrhizal Fungus Rhizophagus irregularis in Morus alba under Different Light Intensities. Journal of Fungi. 2022; 8(11):1224. https://doi.org/10.3390/jof8111224
Chicago/Turabian StyleRen, Wei, Haoqiang Zhang, Xiaoxia Jin, Hongchao Huang, Linxi Zhou, Tingying Xu, and Ming Tang. 2022. "Pb Transfer Preference of Arbuscular Mycorrhizal Fungus Rhizophagus irregularis in Morus alba under Different Light Intensities" Journal of Fungi 8, no. 11: 1224. https://doi.org/10.3390/jof8111224





