Characteristics and Outcomes of Cryptococcosis among Patients with and without COVID-19
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
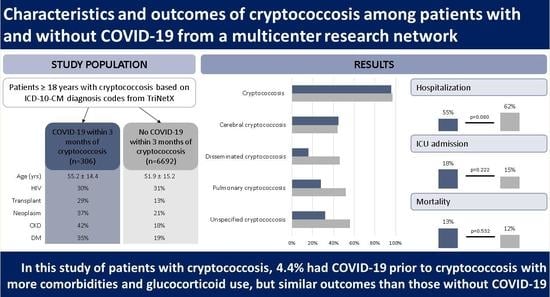
3.1. Demographic Characteristics
3.2. Comorbidity- and Medication-Related Risk Factors
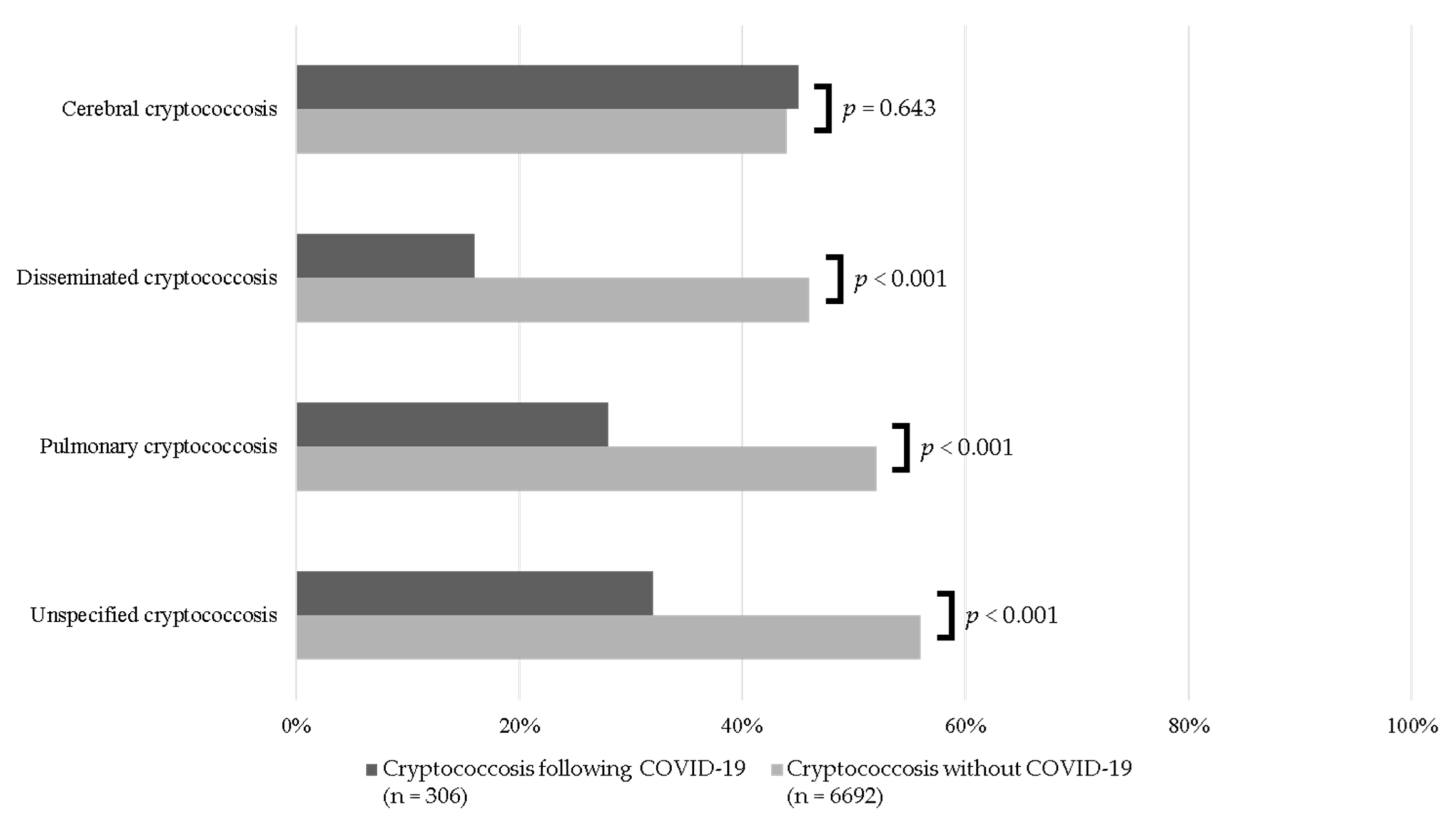
3.3. Sites of Infection
3.4. Outcomes
3.5. Subgroup Analysis of Cases and Controls without HIV
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [PubMed]
- George, I.A.; Spec, A.; Powderly, W.G.; Santos, C.A.Q. Comparative Epidemiology and Outcomes of Human Immunodeficiency virus (HIV), Non-HIV Non-transplant, and Solid Organ Transplant Associated Cryptococcosis: A Population-Based Study. Clin. Infect. Dis. 2018, 66, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Hevey, M.A.; George, I.A.; Raval, K.; Powderly, W.G.; Spec, A. Presentation and Mortality of Cryptococcal Infection Varies by Predisposing Illness: A Retrospective Cohort Study. Am. J. Med. 2019, 132, 977–983.e971. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Sun, Y.; Spec, A.; Lu, N.; Panackal, A.; Bennett, J.; Pappas, P.; Ostrander, D.; Datta, K.; Zhang, S.X.; et al. A Multicenter, Longitudinal Cohort Study of Cryptococcosis in Human Immunodeficiency Virus-negative People in the United States. Clin. Infect. Dis. 2020, 70, 252–261. [Google Scholar] [CrossRef]
- Kula, B.E.; Clancy, C.J.; Hong Nguyen, M.; Schwartz, I.S. Invasive mould disease in fatal COVID-19: A systematic review of autopsies. Lancet Microbe 2021, 2, e405–e414. [Google Scholar] [CrossRef]
- Baddley, J.W.; Thompson, G.R., 3rd; Chen, S.C.; White, P.L.; Johnson, M.D.; Nguyen, M.H.; Schwartz, I.S.; Spec, A.; Ostrosky-Zeichner, L.; Jackson, B.R.; et al. Coronavirus Disease 2019-Associated Invasive Fungal Infection. Open Forum Infect. Dis. 2021, 8, ofab510. [Google Scholar] [CrossRef]
- Garg, D.; Muthu, V.; Sehgal, I.S.; Ramachandran, R.; Kaur, H.; Bhalla, A.; Puri, G.D.; Chakrabarti, A.; Agarwal, R. Coronavirus Disease (COVID-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature. Mycopathologia 2021, 186, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Chastain, D.B.; Henao-Martínez, A.F.; Dykes, A.C.; Steele, G.M.; Stoudenmire, L.L.; Thomas, G.M.; Kung, V.; Franco-Paredes, C. Missed opportunities to identify cryptococcosis in COVID-19 patients: A case report and literature review. Ther. Adv. Infect. Dis. 2022, 9, 20499361211066363. [Google Scholar] [CrossRef] [PubMed]
- Chastain, D.B.; Kung, V.M.; Golpayegany, S.; Jackson, B.T.; Franco-Paredes, C.; Vargas Barahona, L.; Thompson, G.R., 3rd; Henao-Martínez, A.F. Cryptococcosis among hospitalised patients with COVID-19: A multicentre research network study. Mycoses 2022, 65, 815–823. [Google Scholar] [CrossRef]
- Topaloglu, U.; Palchuk, M.B. Using a Federated Network of Real-World Data to Optimize Clinical Trials Operations. JCO Clin. Cancer Inform. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Henao-Martínez, A.F.; Gross, L.; McNair, B.; McCollister, B.; DeSanto, K.; Montoya, J.G.; Shapiro, L.; Beckham, J.D. Risk Factors for Cryptococcal Meningitis: A Single United States Center Experience. Mycopathologia 2016, 181, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Kashef Hamadani, B.H.; Franco-Paredes, C.; McCollister, B.; Shapiro, L.; Beckham, J.D.; Henao-Martínez, A.F. Cryptococcosis and cryptococcal meningitis: New predictors and clinical outcomes at a United States academic medical centre. Mycoses 2018, 61, 314–320. [Google Scholar] [CrossRef] [PubMed]
- CDC. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 8 November 2022).
- Austin, P.C. A comparison of 12 algorithms for matching on the propensity score. Stat. Med. 2014, 33, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Bratton, E.W.; El Husseini, N.; Chastain, C.A.; Lee, M.S.; Poole, C.; Stürmer, T.; Juliano, J.J.; Weber, D.J.; Perfect, J.R. Comparison and Temporal Trends of Three Groups with Cryptococcosis: HIV-Infected, Solid Organ Transplant, and HIV-Negative/Non-Transplant. PLoS ONE 2012, 7, e43582. [Google Scholar] [CrossRef]
- George, I.A.; Santos, C.A.Q.; Olsen, M.A.; Powderly, W.G. Epidemiology of Cryptococcosis and Cryptococcal Meningitis in a Large Retrospective Cohort of Patients After Solid Organ Transplantation. Open Forum Infect. Dis. 2017, 4, ofx004. [Google Scholar] [CrossRef]
- Liang, C.; Ogilvie, R.P.; Doherty, M.; Clifford, C.R.; Chomistek, A.K.; Gately, R.; Song, J.; Enger, C.; Seeger, J.; Lin, N.D.; et al. Trends in COVID-19 patient characteristics in a large electronic health record database in the United States: A cohort study. PLoS ONE 2022, 17, e0271501. [Google Scholar] [CrossRef]
- Pyrgos, V.; Seitz, A.E.; Steiner, C.A.; Prevots, D.R.; Williamson, P.R. Epidemiology of cryptococcal meningitis in the US: 1997-2009. PLoS ONE 2013, 8, e56269. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, A.A.; Somayaji, R.; Myers, R.; Mody, C.H. Epidemiology and trends of cryptococcosis in the United States from 2000 to 2007: A population-based study. Int. J. STD AIDS 2018, 29, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnezhad, A.; Rapose, A. Cryptococccal meningoencephalitis after H1N1 influenza. BMJ Case Rep. 2012, 2012, bcr1120115224. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, H.; Lan, C.; Zou, S.; Zhang, H.; Wang, X.; Weng, H. Concomitant severe influenza and cryptococcal infections: A case report and literature review. Medicine 2019, 98, e15544. [Google Scholar] [CrossRef]
- Flerlage, T.; Boyd, D.F.; Meliopoulos, V.; Thomas, P.G.; Schultz-Cherry, S. Influenza virus and SARS-CoV-2: Pathogenesis and host responses in the respiratory tract. Nat. Rev. Microbiol. 2021, 19, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, K.L.; Murphy, J.W. What makes Cryptococcus neoformans a pathogen? Emerg. Infect. Dis. 1998, 4, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Stins, M.F.; McCaffery, M.J.; Miller, G.F.; Pare, D.R.; Dam, T.; Paul-Satyaseela, M.; Kim, K.S.; Kwon-Chung, K.J. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect. Immun. 2004, 72, 4985–4995. [Google Scholar] [CrossRef] [PubMed]
- Seoane, P.I.; Taylor-Smith, L.M.; Stirling, D.; Bell, L.C.K.; Noursadeghi, M.; Bailey, D.; May, R.C. Viral infection triggers interferon-induced expulsion of live Cryptococcus neoformans by macrophages. PLoS Pathog. 2020, 16, e1008240. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Haselow, D.; Lloyd, S.; Lockhart, S.; Moulton-Meissner, H.; Lester, L.; Wheeler, G.; Gladden, L.; Garner, K.; Derado, G.; et al. Cluster of Cryptococcus neoformans Infections in Intensive Care Unit, Arkansas, USA, 2013. Emerg. Infect. Dis. 2015, 21, 1719–1724. [Google Scholar] [CrossRef]
- Chastain, D.B.; Stitt, T.M.; Ly, P.T.; Henao-Martinez, A.F.; Franco-Paredes, C.; Osae, S.P. Countermeasures to Coronavirus Disease 2019: Are Immunomodulators Rational Treatment Options-A Critical Review of the Evidence. Open Forum Infect. Dis. 2020, 7, ofaa219. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e1003. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Regalla, D.; VanNatta, M.; Alam, M.; Malek, A.E. COVID-19-associated Cryptococcus infection (CACI): A review of literature and clinical pearls. Infection 2022, 50, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, S.; Robicsek, A.; Scranton, R.; Zuckerman, D.; Solomon, D.H. Veteran’s affairs hospital discharge databases coded serious bacterial infections accurately. J. Clin. Epidemiol. 2007, 60, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Luciano, S.; Geddes, J.R.; Harrison, P.J. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry 2021, 8, 130–140. [Google Scholar] [CrossRef]
- Taquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry 2022, 9, 815–827. [Google Scholar] [CrossRef]
- Tang, K.; Seo, J.; Tiu, B.C.; Le, T.K.; Pahalyants, V.; Raval, N.S.; Semenov, Y.R.; Ugwu-Dike, P.O.; Zubiri, L.; Vivek, N.; et al. Association of Cutaneous Immune-Related Adverse Events With Increased Survival in Patients Treated With Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Therapy. JAMA Dermatol. 2022, 158, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Mandadi, S.; Pulluru, H.; Annie, F. Comparative outcomes of combined corticosteroid and remdesivir therapy with corticosteroid monotherapy in ventilated COVID-19 patients. PLoS ONE 2022, 17, e0264301. [Google Scholar] [CrossRef]

| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Variable | Cryptococcosis Following COVID-19 (n = 306) | Cryptococcosis without COVID-19 (n = 6692) | p Value | Cryptococcosis Following COVID-19 (n = 296) | Cryptococcosis without COVID-19 (n = 296) | p Value |
| Demographics | ||||||
| Age at index event (years), mean (SD) | 55.2 (14.4) | 51.9 (15.2) | <0.001 | 55.1 (14.4) | 55.3 (14.8) | 0.890 |
| Male sex | 68 (208) | 69 (4291) | 0.705 | 68 (200) | 68 (201) | 0.930 |
| White | 175 (57) | 3421 (55) | 0.454 | 57 (169) | 58 (172) | 0.803 |
| Black or African American | 79 (26) | 1586 (26) | 0.917 | 26 (78) | 23 (68) | 0.640 |
| Asian | 10 (3) | 102 (2) | 0.032 | 3 (10) | 3 (10) | 1 |
| Unknown race | 44 (14) | 1080 (17) | 0.177 | 14 (41) | 16 (48) | 0.421 |
| Hispanic or Latino | 51 (17) | 787 (13) | 0.041 | 16 (48) | 16 (47) | 0.911 |
| Non-Hispanic | 218 (71) | 3945 (63) | 0.006 | 72 (212) | 63 (186) | 0.023 |
| Underlying comorbidities | ||||||
| HIV | 92 (30) | 1954 (31) | 0.618 | 31 (92) | 32 (95) | 0.791 |
| Transplanted organs or tissues | 89 (29) | 806 (13) | <0.001 | 27 (80) | 29 (86) | 0.583 |
| Neoplastic diseases | 114 (37) | 1329 (21) | <0.001 | 37 (109) | 27 (80) | 0.011 |
| Immunodeficiency with predominantly antibody defects | 10 (3) | 69 (1) | 0.001 | 3 (10) | 3 (10) | 1 |
| Combined immunodeficiencies | 0 (0) | 10 (<1) | 0.483 | 0 (0) | 0 (0) | – |
| Common variable immunodeficiency | 10 (3) | 17 (<1) | <0.001 | 3 (10) | 3 (10) | 1 |
| Other immunodeficiencies * | 70 (23) | 300 (5) | <0.001 | 21 (61) | 20 (59) | 0.838 |
| Sarcoidosis | 14 (5) | 141 (2) | 0.010 | 5 (14) | 3 (10) | 0.405 |
| Systemic connective tissue disorders | 19 (6) | 256 (4) | 0.075 | 6 (19) | 5 (15) | 0.480 |
| Rheumatoid arthritis | 10 (3) | 19 (<1) | <0.001 | 3 (10) | 3 (10) | 1 |
| Noninfective enteritis and colitis | 30 (10) | 428 (7) | 0.051 | 10 (28) | 11 (31) | 0.681 |
| Hepatic fibrosis and cirrhosis | 19 (6) | 396 (6) | 0.912 | 6 (17) | 7 (21) | 0.502 |
| Type 2 diabetes mellitus | 106 (35) | 1158 (19) | <0.001 | 34 (10) | 28 (82) | 0.109 |
| Heart failure | 62 (20) | 514 (8) | <0.001 | 19 (57) | 12 (35) | 0.013 |
| Chronic kidney disease | 127 (42) | 1096 (18) | <0.001 | 40 (119) | 29 (86) | 0.004 |
| Laboratory values | ||||||
| Leukocytes (K/μL), mean (SD) | 18.7 (147.5) | 19.3 (213) | 0.982 | 19 (149.6) | 7.5 (6.6) | 0.304 |
| Lymphocytes (K/μL), mean (SD) | 6.4 (22.3) | 6.8 (41.8) | 0.916 | 6.6 (22.7) | 5.1 (36.8) | 0.703 |
| CD4 cells (cells/μL), mean (SD) | 108 (141) | 175 (306) | 0.332 | 108 (141) | 151 (243) | 0.485 |
| Serum creatinine (mg/dL), mean (SD) | 1.7 (1.9) | 1.5 (1.5) | 0.033 | 1.7 (1.9) | 1.6 (1.5) | 0.554 |
| Hemoglobin A1C (%), mean (SD) | 6.9 (2.2) | 6.5 (1.8) | 0.110 | 6.9 (2.3) | 7.0 (1.9) | 0.849 |
| Ferritin (ng/mL), mean (SD) | 2240 (9887) | 1004 (1815) | 0.020 | 2296 (10,176) | 617 (645) | 0.476 |
| C-reactive protein (mg/dL), mean (SD) | 49.5 (62.7) | 36.4 (57.9) | 0.078 | 50.7 (63.2) | 34.9 (51.3) | 0.238 |
| Lactate dehydrogenase (units/L), mean (SD) | 551 (1034) | 356 (497) | 0.004 | 553 (1047) | 292 (172) | 0.113 |
| Before Matching | After Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Cryptococcosis Following COVID-19 (n = 306) | Cryptococcosis without COVID-19 (n = 6219) | OR (95% CI) | p Value | Cryptococcosis Following COVID-19 (n = 296) | Cryptococcosis without COVID-19 (n = 296) | OR (95% CI) | p Value |
| ED visit | 88 (29) | 1442 (23) | 1.337 (1.037–1.725) | 0.025 | 86 (29) | 69 (23) | 1.347 (0.932, 1.947) | 0.112 |
| Hospitalization | 169 (55) | 3529 (57) | 0.940 (0.746, 1.184) | 0.601 | 163 (55) | 184 (62) | 0.746 (0.537, 1.036) | 0.080 |
| ICU admission | 56 (18) | 678 (11) | 1.8 (1.355, 2.472) | <0.001 | 54 (18) | 43 (15) | 1.313 (0.848, 2.034) | 0.222 |
| Mechanical ventilation | 36 (12) | 646 (10) | 1.150 (0.805, 1.644) | 0.442 | 35 (12) | 30 (10) | 1.189 (0.709, 1.993) | 0.511 |
| Death | 44 (14) | 653 (11) | 1.431 (1.030, 1.990) | 0.032 | 39 (13) | 34 (12) | 1.169 (0.76, 1.911) | 0.532 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chastain, D.B.; Kung, V.M.; Vargas Barahona, L.; Jackson, B.T.; Golpayegany, S.; Franco-Paredes, C.; Thompson, G.R., III; Henao-Martínez, A.F. Characteristics and Outcomes of Cryptococcosis among Patients with and without COVID-19. J. Fungi 2022, 8, 1234. https://doi.org/10.3390/jof8111234
Chastain DB, Kung VM, Vargas Barahona L, Jackson BT, Golpayegany S, Franco-Paredes C, Thompson GR III, Henao-Martínez AF. Characteristics and Outcomes of Cryptococcosis among Patients with and without COVID-19. Journal of Fungi. 2022; 8(11):1234. https://doi.org/10.3390/jof8111234
Chicago/Turabian StyleChastain, Daniel B., Vanessa M. Kung, Lilian Vargas Barahona, Brittany T. Jackson, Sahand Golpayegany, Carlos Franco-Paredes, George R. Thompson, III, and Andrés F. Henao-Martínez. 2022. "Characteristics and Outcomes of Cryptococcosis among Patients with and without COVID-19" Journal of Fungi 8, no. 11: 1234. https://doi.org/10.3390/jof8111234
APA StyleChastain, D. B., Kung, V. M., Vargas Barahona, L., Jackson, B. T., Golpayegany, S., Franco-Paredes, C., Thompson, G. R., III, & Henao-Martínez, A. F. (2022). Characteristics and Outcomes of Cryptococcosis among Patients with and without COVID-19. Journal of Fungi, 8(11), 1234. https://doi.org/10.3390/jof8111234