The Multifaceted Gene 275 Embedded in the PKS-PTS Gene Cluster Was Involved in the Regulation of Arthrobotrisin Biosynthesis, TCA Cycle, and Septa Formation in Nematode-Trapping Fungus Arthrobotrys oligospora
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Culture Conditions
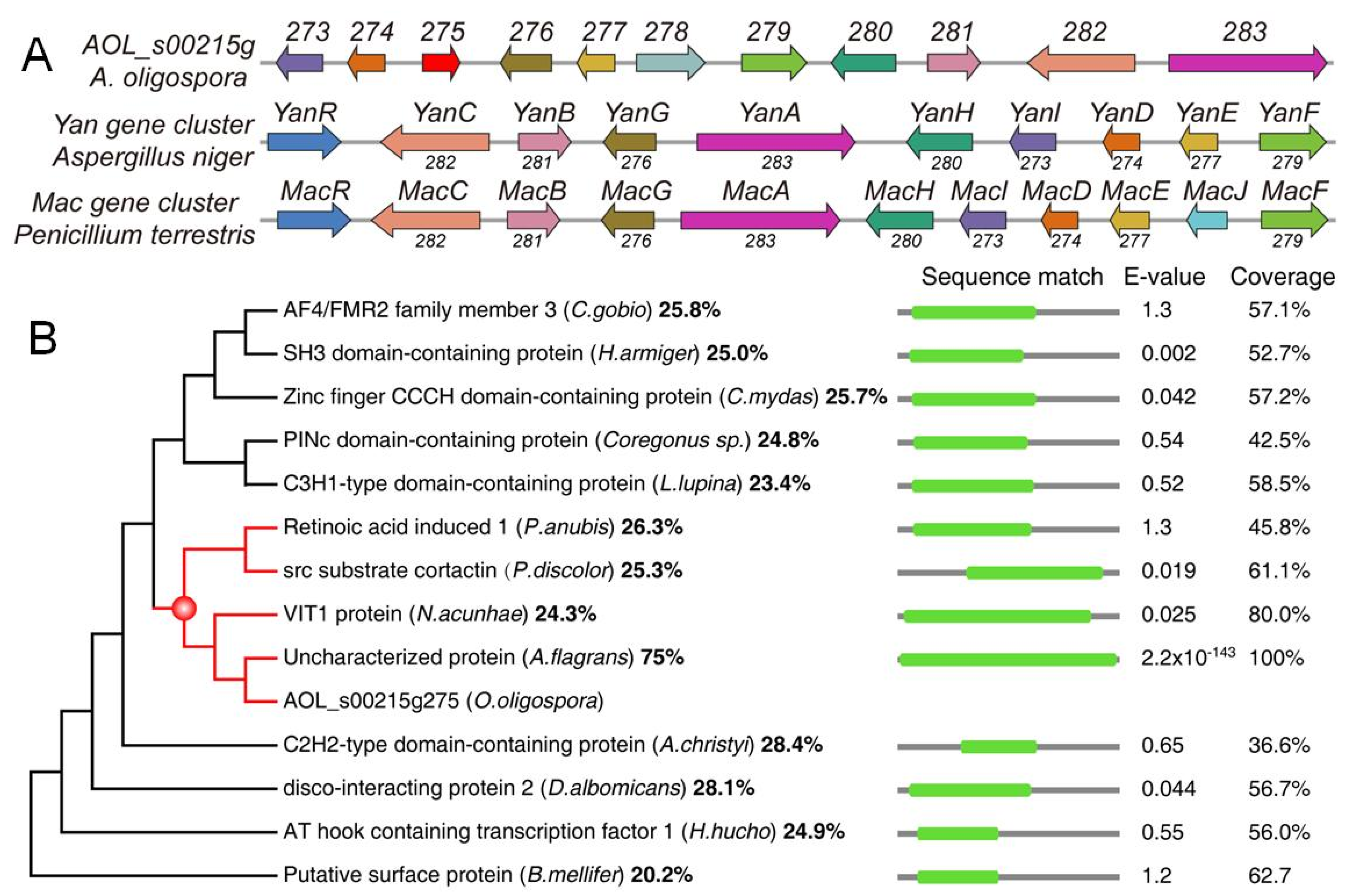
2.2. Sequence and Phylogenetic Analysis of 275 in A. oligospora
2.3. Construction of Mutant Δ275 and OE-275
2.4. Quantitative Real-Time PCR Analysis
2.5. Ultrahigh-Performance Liquid Chromatography–Mass Spectrometry (UPLC−MS) Analysis
2.6. Fungal Colony Growth and Morphology
2.7. Fungal Conidial Production and Germination
2.8. Multi-Stress Assays
2.9. Trap Formation and Nematicidal Activity Assays
2.10. Endocytosis Assay
2.11. Transmission Electron Microscopy (TEM)
2.12. Quantitative Analysis of Arthrobotrisins and TCA Metabolites and Amino Acids
2.13. Transcriptomic Analysis
2.14. Protein Modeling and Docking
2.15. Statistical Analysis
3. Results and discussion
3.1. Sequence and Phylogenetic Analyses of 275
3.2. Function of 275 in Regulating Fungal Growth and Morphology
3.3. Roles of 275 in Conidial Production, Germination, Trap Formation, and Nematicidal Activity
3.4. The Association of 275 with Multiple Stress Responses
3.5. Transcriptional Link of 275 to the PKS-PTS Hybrid Pathway, TCA Cycle, Membrane Transport, and Redox Homeostasis
3.6. 275 Regulated the Production of Unique PKS-PTS Hybrid Metabolites, TCA Metabolites, and Amino Acids
3.7. Vital Role of 275 in Endocytosis, Septum Formation, and Mitochondrial Homeostasis
3.8. Docking of 275 with the PKS-PTS Hybrid Metabolites
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Keller, N.P. Translating biosynthetic gene clusters into fungal armor and weaponry. Nat. Chem. Biol. 2015, 11, 671–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zou, C.G.; Xu, J.P.; Ji, X.L.; Niu, X.M.; Yang, J.K.; Huang, X.K.; Zhang, K.Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Wang, L.; Ji, X.L.; Feng, Y.; Li, X.M.; Zou, C.G.; Xu, J.P.; Ren, Y.; Mi, Q.L.; Wu, J.L.; et al. Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS Pathog. 2011, 7, e1002179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, G.H.; Zou, C.G.; Ji, X.L.; Liu, T.; Zhao, P.J.; Liang, L.M.; Xu, J.P.; An, Z.Q.; Zheng, X.; et al. Bacteria can mobilize nematode-trapping fungi to kill nematodes. Nat. Commun. 2014, 5, 5776. [Google Scholar] [CrossRef] [Green Version]
- Stadler, M.; Sterner, O.; Anke, H. New biologically active compounds from the nematode-trapping fungus Arthrobotrys oligospora Fresen. Z. Nat. 1993, 48, 843–850. [Google Scholar]
- Anderson, M.G.; Jarman, T.B.; Rickards, R.W. Structures and absolute configurations of antibiotics of the oligosporon group from the nematode-trapping fungus Arthrobotrys oligospora. J. Antibiot. 1995, 48, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.X.; Zhang, H.X.; Tan, J.L.; Chu, Y.S.; Li, N.; Xue, H.X.; Wang, Y.L.; Niu, X.M.; Zhang, Y.; Zhang, K.Q. Arthrobotrisins A-C, oligosporons from the nematode-trapping fungus Arthrobotrys oligospora. J. Nat. Prod. 2011, 74, 1526–1530. [Google Scholar] [CrossRef]
- Zhang, H.X.; Tan, J.L.; Wei, L.X.; Wang, Y.L.; Zhang, C.P.; Wu, D.K.; Zhu, C.Y.; Zhang, Y.; Zhang, K.Q.; Niu, X.M. Morphology regulatory metabolites from Arthrobotrys oligospora. J. Nat. Prod. 2012, 75, 1419–1423. [Google Scholar] [CrossRef]
- Holm, D.K.; Petersen, L.M.; Klitgaard, A.; Knudsen, P.B.; Jarczynska, Z.D.; Nielsen, K.F.; Gotfredsen, C.H.; Larsen, T.O.; Mortensen, U.H. Molecular and chemical characterization of the biosynthesis of the 6-MSA-derived meroterpenoid yanuthone D in Aspergillus niger. Chem. Biol. 2014, 21, 519–529. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.C.; Cui, X. Late-stage terpene cyclization by an integral membrane cyclase in the biosynthesis of isoprenoid epoxycyclohexenone natural products. Org. Lett. 2017, 19, 5376–5379. [Google Scholar] [CrossRef]
- Xu, Z.F.; Chen, Y.H.; Song, T.Y.; Zeng, Z.J.; Yan, N.; Zhang, K.Q.; Niu, X.M. Nematicidal key precursors for the biosynthesis of morphological regulatory arthrosporols in the nematode-trapping fungus Arthrobotrys oligospora. J. Agric. Food Chem. 2016, 64, 7949–7956. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.M.; Zhang, K.Q. Arthrobotrys oligospora: A model organism for understanding the interaction between fungi and nematodes. Mycology 2011, 2, 59–78. [Google Scholar] [CrossRef]
- He, Z.Q.; Tan, J.L.; Li, N.; Zhang, H.X.; Chen, Y.H.; Wang, L.J.; Zhang, K.Q.; Niu, X.M. Sesquiterpenyl epoxy-cyclohexenoids and their signaling functions in nematode-trapping fungus Arthrobotrys oligospora. J. Agric. Food Chem. 2019, 67, 3061–3072. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.L.; Song, T.Y.; Xu, Z.F.; Liu, X.; Dai, R.; Chen, Y.H.; Li, S.H.; Zhang, K.Q.; Niu, X.M. Selected mutations revealed intermediates and key precursors in the biosynthesis of polyketide–terpenoid hybrid sesquiterpenyl epoxy-cyclohexenoids. Org. Lett. 2017, 19, 3923–3926. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Q.; Wang, L.J.; Wang, Y.J.; Chen, Y.H.; Wen, Y.; Zhang, K.Q.; Niu, X.M. Polyketide synthase–terpenoid synthase hybrid pathway regulation of trap formation through ammonia metabolism controls soil colonization of predominant nematode-trapping fungus. J. Agric. Food Chem. 2021, 69, 4464–4479. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.M.; Bai, N.; Yang, X.W.; Zhang, K.Q.; Yang, J.K. Transcriptomic analysis reveals that Rho GTPases regulate trap development and lifestyle transition of the nematode-trapping fungus Arthrobotrys oligospora. Microbiol. Spectr. 2022, 10, e0175921. [Google Scholar] [CrossRef]
- Peñalva, M.A. Tracing the endocytic pathway of Aspergillus nidulans with FM4-64. Fungal Genet. Biol. 2005, 42, 963–975. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, M.; Seo, K.; Taruno, A.; Mizoro, Y.; Yamaguchi, Y.; Doi, M.; Nakao, R.; Kori, H.; Abe, T.; Ohmori, H.; et al. A light-induced small G-protein gem limits the circadian clock phase-shift magnitude by inhibiting voltage-dependent calcium channels. Cell Rep. 2022, 39, 110844. [Google Scholar] [CrossRef]
- Chang, Y.T.; Kowalczyk, M.; Fogerson, P.; Lee, Y.J.; Haque, M.; Adams, E.; Wang, D.; Denardo, L.; Tessier-Lavigne, M.; Huguenard, J.; et al. Loss of Rai1 enhances hippocampal excitability and epileptogenesis in mouse models of Smith-Magenis syndrome. Proc. Natl. Acad. Sci. USA 2020, 119, e2210122119. [Google Scholar] [CrossRef] [PubMed]
- Uruno, T.; Liu, J.; Zhang, P.; Fan, Y.; Egile, C.; Li, R.; Mueller, S.C.; Zhan, X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 2001, 3, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, M.; Stradal, T.E.; Rottner, K. Cortactin: Cell functions of a multifaceted actin-binding protein. Trends Cell Biol. 2018, 28, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Raduka, A.; Rezaee, F. Respiratory syncytial virus disrupts the airway epithelial barrier by decreasing cortactin and destabilizing F-actin. J. Cell Sci. 2022, 135, jcs259871. [Google Scholar] [CrossRef] [PubMed]
- Sorribes-Dauden, R.; Peris, D.; Martínez-Pastor, M.T.; Puig, S. Structure and function of the vacuolar Ccc1/VIT1 family of iron transporters and its regulation in fungi. Comput. Struct. Biotechnol. J. 2020, 18, 3712–3722. [Google Scholar] [CrossRef]
- Ram, H.; Sardar, S.; Gandass, N. Vacuolar iron transporter (Like) proteins: Regulators of cellular iron accumulation in plants. Physiol. Plant. 2021, 171, 823–832. [Google Scholar] [CrossRef]
- Slemmon, J.R.; Martzen, M.R. Neuromodulin (GAP-43) can regulate a calmodulin-dependent target in vitro. Biochemistry 1994, 33, 5653–5660. [Google Scholar] [CrossRef]
- Heinisch, J.J.; Rodicio, R. Protein kinase C in fungi—More than just cell wall integrity. FEMS Microbiol. Rev. 2018, 42, fux051. [Google Scholar] [CrossRef] [Green Version]
- Snelders, E.; Moyrand, F.; Sturny-Leclère, A.; Vernel-Pauillac, F.; Volant, S.; Janbon, G.; Alanio, A. The role of glycosylphosphatidylinositol (gpi) anchored proteins in Cryptococcus neoformans. Microbes Infect. 2022, 28, 105016. [Google Scholar] [CrossRef]
- Yamada, K.; Basak, A.K.; Goto-Yamada, S.; Tarnawska-Glatt, K.; Hara-Nishimura, I. Vacuolar processing enzymes in the plant life cycle. New Phytol. 2020, 226, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Stein, R.M.; Kang, H.J.; McCorvy, J.D.; Glatfelter, G.C.; Jones, A.J.; Che, T.; Slocum, S.; Huang, X.P.; Savych, O.; Moroz, Y.S.; et al. Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nature 2020, 579, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, Y.D.; Stoney, P.N.; Shearer, K.D.; Sementilli, A.; Nanescu, S.E.; Sementilli, P.; McCaffery, P. Expression in the human brain of retinoic acid induced 1, a protein associated with neurobehavioural disorders. Brain Struct. Funct. 2015, 220, 1195–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.L.; Ven, T.N.; Crane, M.M.; Brunner, M.L.C.; Pun, A.K.; Helget, K.L.; Brower, K.; Chen, D.E.; Doan, H.; Dillard-Telm, J.D.; et al. Loss of vacuolar acidity results in iron-sulfur cluster defects and divergent homeostatic responses during aging in Saccharomyces cerevisiae. Geroscience 2020, 42, 749–764. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Wu, Q.-F.; Li, S.-H.; Yan, J.-X.; Wu, L.; Cheng, Q.-Y.; He, Z.-Q.; Yue, X.-T.; Zhang, K.-Q.; Zhang, L.-L.; et al. The Multifaceted Gene 275 Embedded in the PKS-PTS Gene Cluster Was Involved in the Regulation of Arthrobotrisin Biosynthesis, TCA Cycle, and Septa Formation in Nematode-Trapping Fungus Arthrobotrys oligospora. J. Fungi 2022, 8, 1261. https://doi.org/10.3390/jof8121261
Zhou J, Wu Q-F, Li S-H, Yan J-X, Wu L, Cheng Q-Y, He Z-Q, Yue X-T, Zhang K-Q, Zhang L-L, et al. The Multifaceted Gene 275 Embedded in the PKS-PTS Gene Cluster Was Involved in the Regulation of Arthrobotrisin Biosynthesis, TCA Cycle, and Septa Formation in Nematode-Trapping Fungus Arthrobotrys oligospora. Journal of Fungi. 2022; 8(12):1261. https://doi.org/10.3390/jof8121261
Chicago/Turabian StyleZhou, Jiao, Qun-Fu Wu, Shu-Hong Li, Jun-Xian Yan, Li Wu, Qian-Yi Cheng, Zhi-Qiang He, Xu-Tong Yue, Ke-Qin Zhang, Long-Long Zhang, and et al. 2022. "The Multifaceted Gene 275 Embedded in the PKS-PTS Gene Cluster Was Involved in the Regulation of Arthrobotrisin Biosynthesis, TCA Cycle, and Septa Formation in Nematode-Trapping Fungus Arthrobotrys oligospora" Journal of Fungi 8, no. 12: 1261. https://doi.org/10.3390/jof8121261
APA StyleZhou, J., Wu, Q.-F., Li, S.-H., Yan, J.-X., Wu, L., Cheng, Q.-Y., He, Z.-Q., Yue, X.-T., Zhang, K.-Q., Zhang, L.-L., & Niu, X.-M. (2022). The Multifaceted Gene 275 Embedded in the PKS-PTS Gene Cluster Was Involved in the Regulation of Arthrobotrisin Biosynthesis, TCA Cycle, and Septa Formation in Nematode-Trapping Fungus Arthrobotrys oligospora. Journal of Fungi, 8(12), 1261. https://doi.org/10.3390/jof8121261






