Calcineurin Inhibitor CN585 Exhibits Off-Target Effects in the Human Fungal Pathogen Aspergillus fumigatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Culture Conditions and In Vitro Drug Susceptibility Assays
2.2. Microscopy
2.3. Molecular Modeling of Aspergillus fumigatus eIF4E
2.4. Molecular Dynamic Simulations
2.5. Molecular Docking
3. Results
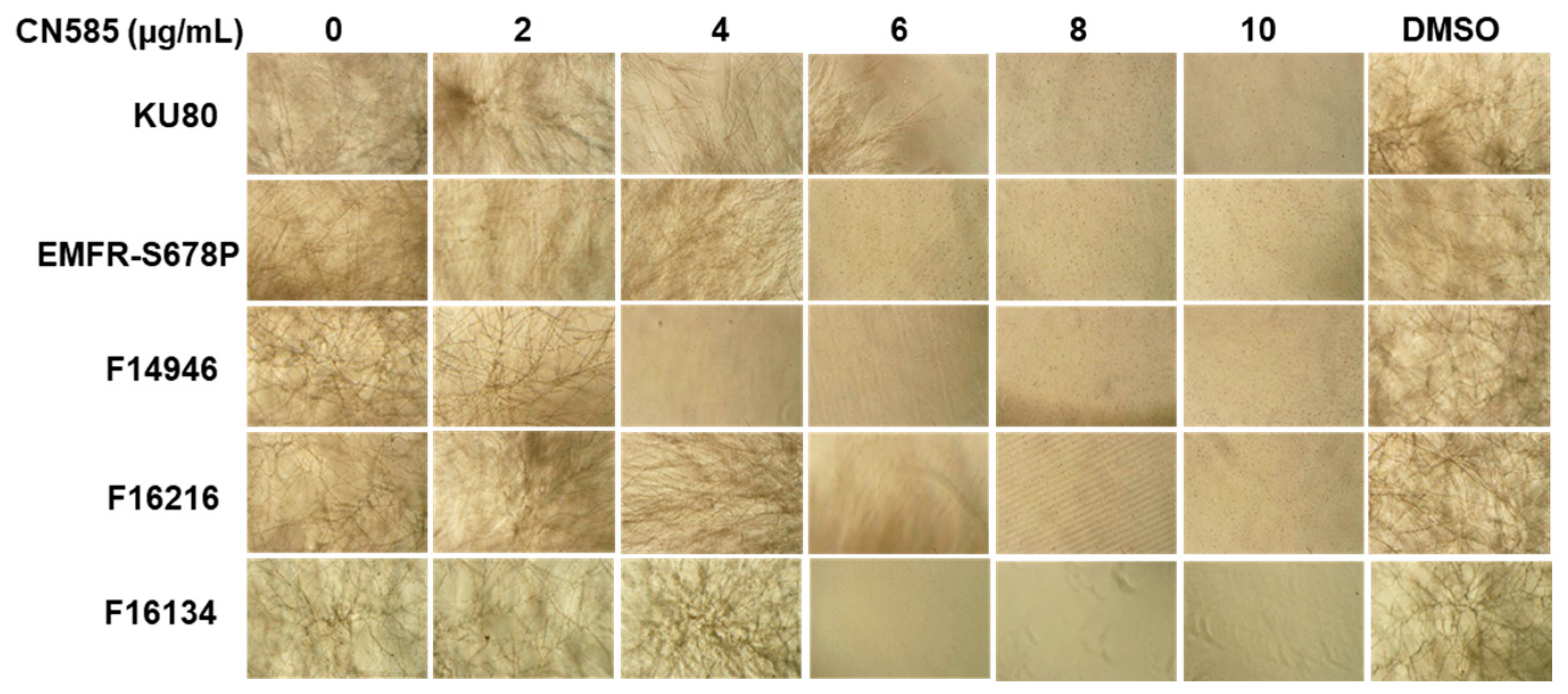
3.1. CN585 Significantly Inhibits Growth of A. fumigatus Wild-Type and the Azole- and Echinocandin-Resistant Strains
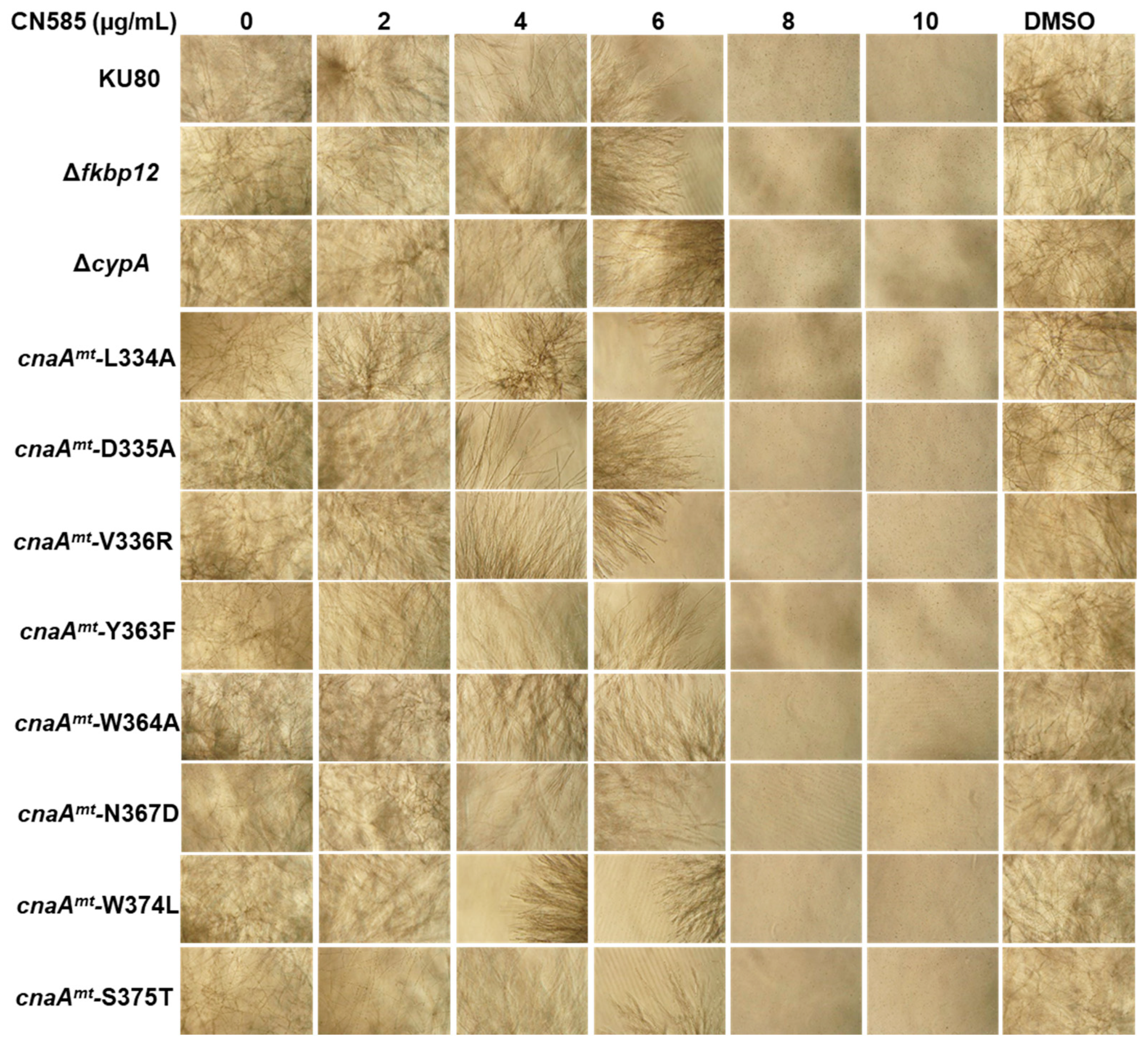
3.2. CN585-Mediated Growth Inhibition Is Independent of Key Residues in the CN-Effector Binding Regions
3.3. Hypersensitivity of the A. fumigatus CN Deletion Mutant to CN585 Indicates Off-Target Effects of CN585
3.4. CN585-Mediated Growth Inhibition Is Independent of the CN-Dependent Transcription Factor CrzA
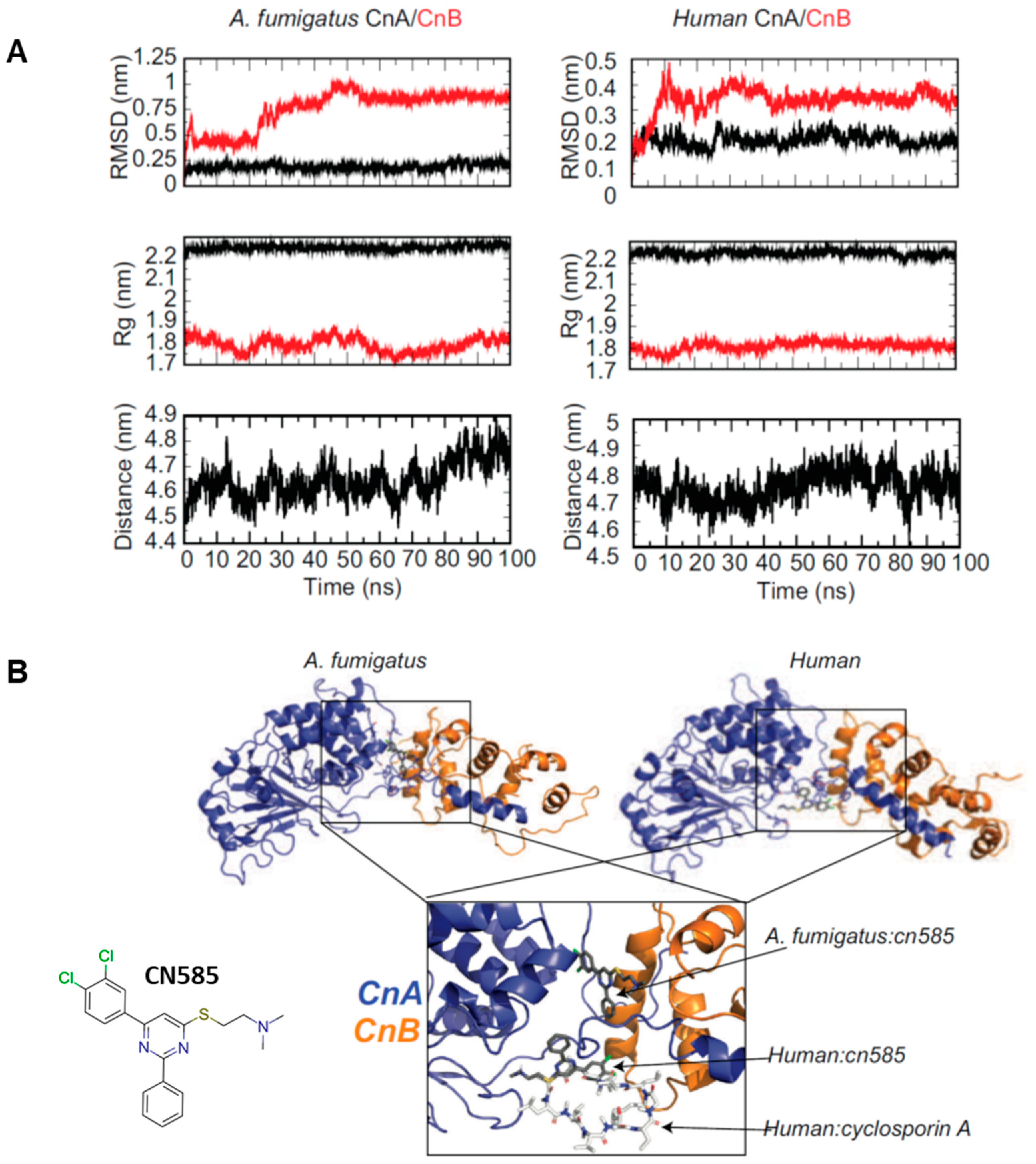
3.5. Molecular Docking of CN585 Reveals Differential Potential Binding Sites between A. fumigatus and Human CN
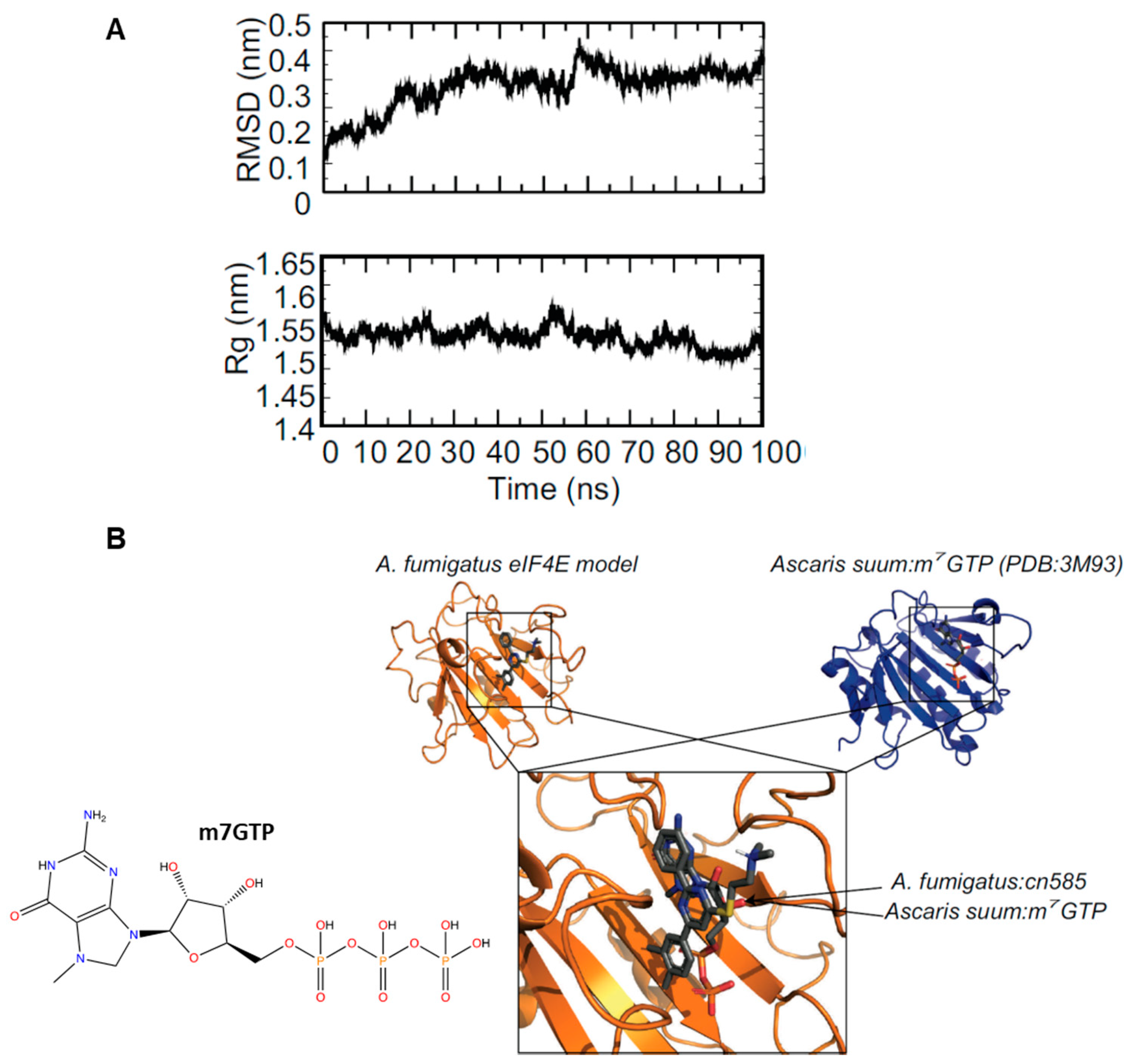
3.6. Molecular Modeling and Docking of CN585 Reveals Similar Potential Binding Sites between A. fumigatus and Human eIF4E:m7GTP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Park, H.S.; Lee, S.C.; Cardenas, M.E.; Heitman, J. Calcium-Calmodulin-Calcineurin Signaling: A Globally Conserved Virulence Cascade in Eukaryotic Microbial Pathogens. Cell Host Microbe 2019, 26, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Hemenway, C.; Heitman, J. Calcineurin. Cell Biochem. Biophys. 1999, 30, 115–151. [Google Scholar] [CrossRef] [PubMed]
- Creamer, T.P. Calcineurin. Cell Commun. Signal. 2020, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, F.; Hallin, U.; Uchino, H. Calcineurin as a multifunctional regulator. J. Biochem. 2002, 131, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pallen, C.J.; Wang, J.H. A multifunctional calmodulin-stimulated phosphatase. Arch. Biochem. Biophys. 1985, 237, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Lee, S.C.; Heitman, J.; Steinbach, W.J. Calcineurin in fungal virulence and drug resistance: Prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach. Virulence 2017, 8, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Rusnak, F.; Mertz, P. Calcineurin: Form and Function. Physiol. Rev. 2000, 80, 1483–1521. [Google Scholar] [CrossRef]
- Hogan, P.G. Calcium-NFAT transcriptional signalling in T cell activation and T cell exhaustion. Cell Calcium 2017, 63, 66–69. [Google Scholar] [CrossRef]
- Grigoriu, S.; Bond, R.; Cossio, P.; Chen, J.A.; Ly, N.; Hummer, G.; Page, R.; Cyert, M.S.; Peti, W. The Molecular Mechanism of Substrate Engagement and Immunosuppressant Inhibition of Calcineurin. PLoS Biol. 2013, 11, e1001492. [Google Scholar] [CrossRef]
- Gwack, Y.; Feske, S.; Srikanth, S.; Hogan, P.G.; Rao, A. Signalling to transcription: Store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium 2007, 42, 145–156. [Google Scholar] [CrossRef]
- Ho, S.; Clipstone, N.; Timmermann, L.; Northrop, J.; Graef, I.; Fiorentino, D.; Nourse, J.; Crabtree, G.R. The Mechanism of Action of Cyclosporin A and FK506. Clin. Immunol. Immunopathol. 1996, 80, S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Su, M.S.-S. Interaction of FKBP12-FK506 with Calcineurin A at the B Subunit-binding Domain. J. Biol. Chem. 1995, 270, 15463–15466. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Farmer, J.D., Jr.; Lane, W.S.; Friedman, J.; Weissman, I.; Schreiber, S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991, 66, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.P.; Kim, J.L.; Kim, E.E.; Sintchak, M.D.; Thomson, J.A.; Fitzgibbon, M.J.; Fleming, M.A.; Caron, P.R.; Hsiao, K.; Navia, M.A. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex. Cell 1995, 82, 507–522. [Google Scholar] [CrossRef]
- Huai, Q.; Kim, H.Y.; Liu, Y.; Zhao, Y.; Mondragon, A.; Liu, J.O.; Ke, H. Crystal structure of calcineurin-cyclophilin-cyclosporin shows common but distinct recognition of immunophilin-drug complexes. Proc. Natl. Acad. Sci. USA 2002, 99, 12037–12042. [Google Scholar] [CrossRef]
- Nambu, M.; Covel, J.A.; Kapoor, M.; Li, X.; Moloney, M.K.; Numa, M.M.; Soltow, Q.A.; Trzoss, M.; Webb, P.; Webb, R.R., 2nd; et al. A calcineurin antifungal strategy with analogs of FK506. Bioorg. Med. Chem. Lett. 2017, 27, 2465–2471. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, K.T.; Lee, S.J.; Beom, J.Y.; Hwangbo, A.; Jung, J.A.; Song, M.C.; Yoo, Y.J.; Kang, S.H.; Averette, A.F.; et al. In Vitro and In Vivo Assessment of FK506 Analogs as Novel Antifungal Drug Candidates. Antimicrob. Agents Chemother. 2018, 62, e01627-18. [Google Scholar] [CrossRef]
- Jung, J.A.; Yoon, Y.J. Development of Non-Immunosuppressive FK506 Derivatives as Antifungal and Neurotrophic Agents. J. Microbiol. Biotechnol. 2020, 30, 1–10. [Google Scholar] [CrossRef]
- Juvvadi, P.R.; Fox, D., 3rd; Bobay, B.G.; Hoy, M.J.; Gobeil, S.M.C.; Venters, R.A.; Chang, Z.; Lin, J.J.; Averette, A.F.; Cole, D.C.; et al. Harnessing calcineurin-FK506-FKBP12 crystal structures from invasive fungal pathogens to develop antifungal agents. Nat. Commun. 2019, 10, 4275. [Google Scholar] [CrossRef]
- Gobeil, S.M.C.; Bobay, B.G.; Juvvadi, P.R.; Cole, D.C.; Heitman, J.; Steinbach, W.J.; Venters, R.A.; Spicer, L.D. Leveraging Fungal and Human Calcineurin-Inhibitor Structures, Biophysical Data and Dynamics to Design Selective and Non-Immunosuppressive FK506 Analogs. mBio 2021, 12, e03000-21. [Google Scholar] [CrossRef]
- Hoy, M.J.; Park, E.; Lee, H.; Lim, W.Y.; Cole, D.C.; DeBouver, N.D.; Bobay, B.G.; Pierce, P.G.; Fox, D., 3rd; Ciofani, M.; et al. Structure-Guided Synthesis of FK506 and FK520 Analogs with Increased Selectivity Exhibit In Vivo Therapeutic Efficacy against Cryptococcus. mBio 2022, 13, e0104922. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, F.; Weiwad, M.; Kilka, S.; Karanik, M.; Pätzel, M.; Baumgrass, R.; Liebscher, J.; Fischer, G. The novel calcineurin inhibitor CN585 has potent immunosuppressive properties in stimulated human T cells. J. Biol. Chem. 2010, 285, 1888–1898. [Google Scholar] [CrossRef]
- Shwab, E.K.; Juvvadi, P.R.; Waitt, G.; Soderblom, E.J.; Barrington, B.C.; Asfaw, Y.G.; Moseley, M.A.; Steinbach, W.J. Calcineurin-dependent dephosphorylation of the transcription factor CrzA at specific sites controls conidiation, stress tolerance, and virulence of Aspergillus fumigatus. Mol. Microbiol. 2019, 112, 62–80. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Liu, W.; Jankowska-Anyszka, M.; Piecyk, K.; Dickson, L.; Wallace, A.; Niedzwiecka, A.; Stepinski, J.; Stolarski, R.; Darzynkiewicz, E.; Kieft, J.; et al. Structural basis for nematode eIF4E binding an m(2,2,7)G-Cap and its implications for translation initiation. Nucleic Acids Res. 2011, 39, 8820–8832. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caselli, E.; Fini, F.; Introvigne, M.L.; Stucchi, M.; Taracila, M.A.; Fish, E.R.; Smolen, K.A.; Rather, P.N.; Powers, R.A.; Wallar, B.J.; et al. 1,2,3-Triazolylmethaneboronate: A Structure Activity Relationship Study of a Class of β-Lactamase Inhibitors against Acinetobacter baumannii Cephalosporinase. ACS Infect. Dis. 2020, 6, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Harrison, S.C. Crystal structure of human calcineurin complexed with cyclosporin A and human cyclophilin. Proc. Natl. Acad. Sci. USA 2002, 99, 13522–13526. [Google Scholar] [CrossRef]
- van Dijk, M.; Wassenaar, T.A.; Bonvin, A.M. A Flexible, Grid-Enabled Web Portal for GROMACS Molecular Dynamics Simulations. J. Chem. Theory Comput. 2012, 8, 3463–3472. [Google Scholar] [CrossRef]
- van Zundert, G.C.P.; Rodrigues, J.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef]
- Schüttelkopf, A.W.; van Aalten, D.M. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 1355–1363. [Google Scholar] [CrossRef]
- Steinbach, W.J.; Cramer, R.A., Jr.; Perfect, B.Z.; Henn, C.; Nielsen, K.; Heitman, J.; Perfect, J.R. Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2007, 51, 2979–2981. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J.; Schell, W.A.; Blankenship, J.R.; Onyewu, C.; Heitman, J.; Perfect, J.R. In Vitro Interactions between Antifungals and Immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2004, 48, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.J.; Cerar, D.; Anderson, M.J.; Albarrag, A.; Fisher, M.C.; Pasqualotto, A.C.; Laverdiere, M.; Arendrup, M.C.; Perlin, D.S.; Denning, D.W. Frequency and evolution of Azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 2009, 15, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; Garcia-Effron, G.; Park, S.; Perlin, D.S. A Ser678Pro substitution in Fks1p confers resistance to echinocandin drugs in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2007, 51, 4174–4176. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Huang, C.; Wang, H.L.; Zhou, K.; Xiao, F.X.; Qun, W. The importance of Loop 7 for the activity of calcineurin. FEBS Lett. 2004, 577, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Pemble, C.W.t.; Ma, Y.; Steinbach, W.J. Novel motif in calcineurin catalytic subunit is required for septal localization of calcineurin in Aspergillus fumigatus. FEBS Lett. 2016, 590, 501–508. [Google Scholar] [CrossRef]
- Falloon, K.; Juvvadi, P.R.; Richards, A.D.; Vargas-Muñiz, J.M.; Renshaw, H.; Steinbach, W.J. Characterization of the FKBP12-Encoding Genes in Aspergillus fumigatus. PLoS ONE 2015, 10, e0137869. [Google Scholar] [CrossRef]
- Juvvadi, P.R.; Fortwendel, J.R.; Rogg, L.E.; Burns, K.A.; Randell, S.H.; Steinbach, W.J. Localization and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus. Mol. Microbiol. 2011, 82, 1235–1259. [Google Scholar]
- Stathopoulos, A.M.; Cyert, M.S. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 1997, 11, 3432–3444. [Google Scholar] [CrossRef]
- Soriani, F.M.; Malavazi, I.; da Silva Ferreira, M.E.; Savoldi, M.; Von Zeska Kress, M.R.; de Souza Goldman, M.H.; Loss, O.; Bignell, E.; Goldman, G.H. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol. Microbiol. 2008, 67, 1274–1291. [Google Scholar] [CrossRef]
- Keiser, M.J.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.J.; Shoichet, B.K. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, F.; Weiwad, M. Calcineurin inhibitors: Status quo and perspectives. Biomol. Concepts 2011, 2, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Cardenas, M.E.; Heitman, J. Calcineurin mutants render T lymphocytes resistant to cyclosporin A. Mol. Pharmacol. 1996, 50, 506–511. [Google Scholar] [PubMed]
- Alberg, D.G.; Schreiber, S.L. Structure-based design of a cyclophilin-calcineurin bridging ligand. Science 1993, 262, 248–250. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juvvadi, P.R.; Bobay, B.G.; Cole, D.C.; Awwa, M.; Steinbach, W.J. Calcineurin Inhibitor CN585 Exhibits Off-Target Effects in the Human Fungal Pathogen Aspergillus fumigatus. J. Fungi 2022, 8, 1281. https://doi.org/10.3390/jof8121281
Juvvadi PR, Bobay BG, Cole DC, Awwa M, Steinbach WJ. Calcineurin Inhibitor CN585 Exhibits Off-Target Effects in the Human Fungal Pathogen Aspergillus fumigatus. Journal of Fungi. 2022; 8(12):1281. https://doi.org/10.3390/jof8121281
Chicago/Turabian StyleJuvvadi, Praveen R., Benjamin G. Bobay, D. Christopher Cole, Monaf Awwa, and William J. Steinbach. 2022. "Calcineurin Inhibitor CN585 Exhibits Off-Target Effects in the Human Fungal Pathogen Aspergillus fumigatus" Journal of Fungi 8, no. 12: 1281. https://doi.org/10.3390/jof8121281
APA StyleJuvvadi, P. R., Bobay, B. G., Cole, D. C., Awwa, M., & Steinbach, W. J. (2022). Calcineurin Inhibitor CN585 Exhibits Off-Target Effects in the Human Fungal Pathogen Aspergillus fumigatus. Journal of Fungi, 8(12), 1281. https://doi.org/10.3390/jof8121281






