Toxic Indoor Air Is a Potential Risk of Causing Immuno Suppression and Morbidity—A Pilot Study
Abstract
:1. Introduction
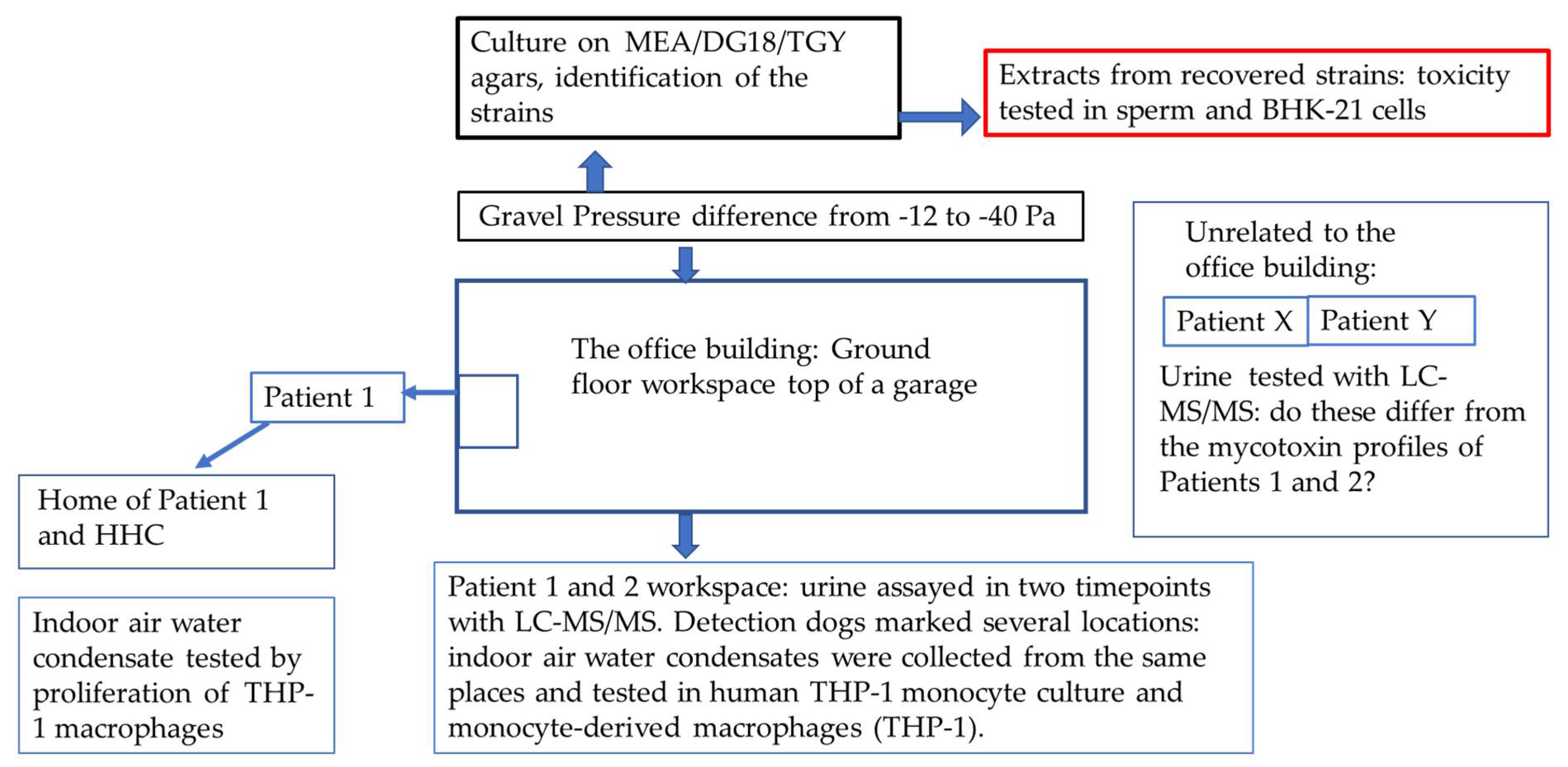
2. Materials and Methods
2.1. The Patients
2.2. Detection of Mycotoxins in the Patients’ Urine Samples
2.3. The Office Building
2.4. Sampling of the Indoor Air Condensate Water
2.5. Indoor Air Toxicity Studies
2.6. Detection of Mycotoxins from the Condensed Indoor Air Sample
2.7. Indoor Air Relative Humidity Measurements and Estimation of Respiratory Mycotoxin Exposure
2.8. Collection of a Sample for Microbiological Analysis
2.9. Detection of Toxicity from the Microbial Growth
2.10. Ethical Considerations
3. Results
3.1. Clinical Picture in Persons Exposed to Toxic Indoor Air
3.2. Detection of Mycotoxins from the Urine Samples
3.3. Evaluation of the Office before Our Investigations
3.4. Detection of Mycotoxins from the Water Condensate
3.5. Toxicity Studies from the Water Condensates
3.6. Microbiological Analysis and the Toxicity of the Cultured Microbial Colonies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salin, J.T.; Salkinoja-Salonen, M.; Salin, P.J.; Nelo, K.; Holma, T.; Ohtonen, P.; Syrjala, H. Building-related symptoms are linked to the in vitro toxicity of indoor dust and airborne microbial propagules in schools: A cross-sectional study. Environ. Res. 2017, 154, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Tuuminen, T.; Valtonen, V.; Vaali, K. Dampness and Mold Hypersensitivity Syndrome as an Umbrella for Many Chronic Diseases—The Clinician’s Point of View, 2nd ed.; Science Direct: Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, Netherlands, 2019. [Google Scholar]
- Valtonen, V. Clinical Diagnosis of the Dampness and Mold Hypersensitivity Syndrome: Review of the Literature and Suggested Diagnostic Criteria. Front. Immunol. 2017, 8, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyvonen, S.; Lohi, J.; Tuuminen, T. Moist and Mold Exposure is Associated with High Prevalence of Neurological Symptoms and MCS in a Finnish Hospital Workers Cohort. Saf. Health Work 2020, 11, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Hyvönen, S.; Poussa, T.; Lohi, J.; Tuuminen, T. High prevalence of neurological sequelae and multiple chemical sensitivity among occupants. Arch. Environ. Occup. Health 2020, 76, 145–151. [Google Scholar] [CrossRef]
- Vornanen-Winqvist, C.; Jarvi, K.; Andersson, M.A.; Duchaine, C.; Letourneau, V.; Kedves, O.; Kredics, L.; Mikkola, R.; Kurnitski, J.; Salonen, H. Exposure to indoor air contaminants in school buildings with and without reported indoor air quality problems. Environ. Int. 2020, 141, 105781. [Google Scholar] [CrossRef]
- Hyvönen, S.M.; Lohi, J.J.; Räsänen, L.A.; Heinonen, T.; Mannerström, M.; Vaali, K.K.; Tuuminen, T. Association of toxic indoor air with multi-organ symptoms in pupils attending a moisture-damaged school in Finland. Am. J. Clin. Exp. Immunol. 2020; in press. [Google Scholar]
- Salin, J.; Ohtonen, P.; Syrjala, H. Teachers’ work-related non-literature-known building-related symptoms are also connected to indoor toxicity: A cross-sectional study. Indoor Air 2021. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/ina.12822 (accessed on 21 November 2021). [CrossRef]
- Tuuminen, T. The Roles of Autoimmunity and Biotoxicosis in Sick Building Syndrome as a "Starting Point" for Irreversible Dampness and Mold Hypersensitivity Syndrome. Antibodies 2020, 9, 26. [Google Scholar] [CrossRef]
- Tuuminen, T.; Rinne, K.S. Severe Sequelae to Mold-Related Illness as Demonstrated in Two Finnish Cohorts. Front Immunol. 2017, 8, 382. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Pørneki, A.D.; Lindgreen, J.N.; Li, Y.; Madsen, A.M. Species of Fungi and Pollen in the PM1 and the Inhalable Fraction of Indoor Air in Homes. Atmosphere 2021, 12, 404. [Google Scholar] [CrossRef]
- Castagnoli, E.; Marik, T.; Mikkola, R.; Kredics, L.; Andersson, M.A.; Salonen, H.; Kurnitski, J. Indoor Trichoderma strains emitting peptaibols in guttation droplets. J. Appl. Microbiol. 2018, 125, 1408–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salo, M.J.; Marik, T.; Mikkola, R.; Andersson, M.A.; Kredics, L.; Salonen, H.; Kurnitski, J. Penicillium expansum strain isolated from indoor building material was able to grow on gypsum board and emitted guttation droplets containing chaetoglobosins and communesins A, B and D. J. Appl. Microbiol. 2019, 127, 1135–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, M.A.; Salo, J.; Kedves, O.; Kredics, L.; Druzhinina, I.; Kurnitski, J.; Salonen, H. Bioreactivity, Guttation and Agents Influencing Surface Tension of Water Emitted by Actively Growing Indoor Mould Isolates. Microorganisms 2020, 8, 1940. [Google Scholar] [CrossRef]
- Gareis, M.; Gareis, E.M. Guttation droplets of Penicillium nordicum and Penicillium verrucosum contain high concentrations of the mycotoxins ochratoxin A and B. Mycopathologia 2007, 163, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Gareis, M.; Gottschalk, C. Stachybotrys spp. and the guttation phenomenon. Mycotoxin Res. 2014, 30, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Jinap, S.; Soleimany, F. Qualitative and Quantitative Analysis of Mycotoxins. Compr. Rev. Food Sci. Food Saf. 2009, 8, 202–251. [Google Scholar] [CrossRef] [PubMed]
- Salo, J. Development of Analytical Methods for Assaying Metabolites of Molds in Buildings; Aalto University: Espoo, Finland, 2014. [Google Scholar]
- Mannerström, M.; Toimela, T.; Ahoniemi, J.; Makiou, A.S.; Heinonen, T. Cytotoxicity of Water Samples Condensed from Indoor Air: An Indicator of Poor Indoor Air Quality. Appl. Vitr. Toxicol. 2020, 6, 120–130. [Google Scholar] [CrossRef]
- Andersson, M.A.; Mikkola, R.; Rasimus, S.; Hoornstra, D.; Salin, P.; Rahkila, R.; Heikkinen, M.; Mattila, S.; Peltola, J.; Kalso, S.; et al. Boar spermatozoa as a biosensor for detecting toxic substances in indoor dust and aerosols. Toxicol. Vitr. 2010, 24, 2041–2052. [Google Scholar] [CrossRef]
- Castagnoli, E.; Salo, J.; Toivonen, M.S.; Marik, T.; Mikkola, R.; Kredics, L.; Vicente-Carrillo, A.; Nagy, S.; Andersson, M.T.; Andersson, M.A.; et al. An Evaluation of Boar Spermatozoa as a Biosensor for the Detection of Sublethal and Lethal Toxicity. Toxins 2018, 10, 463. [Google Scholar] [CrossRef] [Green Version]
- Mikkola, R.; Andersson, M.A.; Hautaniemi, M.; Salkinoja-Salonen, M.S. Toxic indole alkaloids avrainvillamide and stephacidin B produced by a biocide tolerant indoor mold Aspergillus westerdijkiae. Toxicon 2015, 99, 58–67. [Google Scholar] [CrossRef]
- Salo, J.M.; Kedves, O.; Mikkola, R.; Kredics, L.; Andersson, M.A.; Kurnitski, J.; Salonen, H. Detection of Chaetomium globosum, Ch. cochliodes and Ch. rectangulare during the Diversity Tracking of Mycotoxin-Producing Chaetomium-Like Isolates Obtained in Buildings in Finland. Toxins 2020, 12, 443. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.A.; Mikkola, R.; Raulio, M.; Kredics, L.; Maijala, P.; Salkinoja-Salonen, M.S. Acrebol, a novel toxic peptaibol produced by an Acremonium exuviarum indoor isolate. J. Appl. Microbiol. 2009, 106, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Muth, W.L.; Nash, C.H., 3rd. Biosynthesis of mycophenolic acid: Purification and characterization of S-adenosyl-L-methionine: Demethylmycophenolic acid O-methyltransferase. Antimicrob. Agents Chemother. 1975, 8, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puel, O.; Tadrist, S.; Galtier, P.; Oswald, I.P.; Delaforge, M. Byssochlamys nivea as a source of mycophenolic acid. Appl. Environ. Microbiol. 2005, 71, 550–553. [Google Scholar] [CrossRef] [Green Version]
- Vinokurova, N.G.; Ivanushkina, N.E.; Kochkina, G.A.; Rinbasarov, M.U.; Ozerskaia, S.M. Production of mycophenolic acid by fungi of the genus Penicillium link. Prikl. Biokhim. Mikrobiol. 2005, 41, 95–98. [Google Scholar] [CrossRef]
- Bentley, R. Mycophenolic Acid: A one hundred year odyssey from antibiotic to immunosuppressant. Chem. Rev. 2000, 100, 3801–3826. [Google Scholar] [CrossRef]
- Regueira, T.B.; Kildegaard, K.R.; Hansen, B.G.; Mortensen, U.H.; Hertweck, C.; Nielsen, J. Molecular basis for mycophenolic acid biosynthesis in Penicillium brevicompactum. Appl. Environ. Microbiol. 2011, 77, 3035–3043. [Google Scholar] [CrossRef] [Green Version]
- Ismaiel, A.A.; Ahmed, A.S.; El-Sayed el, S.R. Optimization of submerged fermentation conditions for immunosuppressant mycophenolic acid production by Penicillium roqueforti isolated from blue-molded cheeses: Enhanced production by ultraviolet and gamma irradiation. World J. Microbiol. Biotechnol. 2014, 30, 2625–2638. [Google Scholar] [CrossRef]
- Lafont, P.; Debeaupuis, J.P.; Gaillardin, M.; Payen, J. Production of mycophenolic acid by Penicillium roqueforti strains. Appl. Environ. Microbiol. 1979, 37, 365–368. [Google Scholar] [CrossRef] [Green Version]
- van Oss, C.J.; Giese, R.F.; Docoslis, A. Hyperhydrophobicity of the Water-Air Interface. J. Dispers. Sci. Technol. 2005, 26, 585–590. [Google Scholar] [CrossRef]
- Salonen, H.; Heinonen, T.; Mannerström, M.; Jackson, M.; Andesson, M.; Mikkola, R.; Kurnitski, J.; Khurshid, S.; Novoselac, A.; Corsi, R. Assessing indoor air toxicity with condensate collected from air using the mitochondrial activity of human BJ fibroblasts and THP-1 monocytes. In Proceedings of the Conference of International Society of Indoor Air Quality and Climate, Philadelphia, PA, USA, 22–27 July 2018. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ost, M.; Keipert, S.; Klaus, S. Targeted mitochondrial uncoupling beyond UCP1—The fine line between death and metabolic health. Biochimie 2017, 134, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO Standard. Indoor Air—Part 21: Detection and Enumeration of Moulds. Sampling from Materials; ISO 16000-21; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- American Public Health Association; American Water Works Association. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 1980; Volume 15. [Google Scholar]
- Valvira, Asumisterveysasetuksen soveltamisohje, Osa IV. In Asumisterveysasetus §20; Updated 2020; National Supervisory Authority for Welfare and Health, Valvira: Helsinki, Finland, 2016; Volume Ohje 8/2016, Available online: https://www.valvira.fi/documents/14444/261239/Asumisterveysasetuksen+soveltamisohje+osa+IV.pdf/cdfaaa39-d2e5-4bd6-b9e9-6d9c0f60bff6 (accessed on 21 November 2021).
- Pessi, A.-M.; Jalkanen, K. Laboratorio-Opas, 1st ed.; Suomen Ympäristö-ja Terveysalan Kustannus Oy: Pori, Finland, 2018. [Google Scholar]
- Gravesen, S.; Frisvad, J.C.; Samson, R.A. Microfungi; Special-Trykkeriet Viborg a/s: Copenhagen, Denmark, 1994. [Google Scholar]
- Institut Scientifique de Sante Publique & BCCM. Moulds in the Indoor Environment and Outdoor; Institut Scientifique de Sante Publique & BCCM: Bruxelles, Belgium, 2017. [Google Scholar]
- Bequin, H. Moisiss ures de L’Environnement Intérieur et Extérieur; Section of Mycology & Aerobiology, Ed.; Institut Scientifique de Sante Publique: Bruxelles, Belgium, 2004. [Google Scholar]
- Larone, D.H. Medically Important Fungi. A Guide to Identification, 2nd ed.; American Society for Microbiology: Washington, DC, USA, 1993. [Google Scholar]
- Klich, M.A. Identification of Common Aspergillus Species; CAB Direct: Wageningen, The Netherlands, 2002. [Google Scholar]
- Rasimus, S.; Mikkola, R.; Andersson, M.A.; Teplova, V.V.; Venediktova, N.; Ek-Kommonen, C.; Salkinoja-Salonen, M. Psychrotolerant Paenibacillus tundrae isolates from barley grains produce new cereulide-like depsipeptides (paenilide and homopaenilide) that are highly toxic to mammalian cells. Appl. Environ. Microbiol. 2012, 78, 3732–3743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bencsik, O.; Papp, T.; Berta, M.; Zana, A.; Forgo, P.; Dombi, G.; Andersson, M.A.; Salkinoja-Salonen, M.; Vagvolgyi, C.; Szekeres, A. Ophiobolin A from Bipolaris oryzae perturbs motility and membrane integrities of porcine sperm and induces cell death on mammalian somatic cell lines. Toxins 2014, 6, 2857–2871. [Google Scholar] [CrossRef] [Green Version]
- Morawska, L.; Allen, J.; Bahnfleth, W.; Bluyssen, P.M.; Boerstra, A.; Buonanno, G.; Cao, J.; Dancer, S.J.; Floto, A.; Franchimon, F.; et al. A paradigm shift to combat indoor respiratory infection. Science 2021, 372, 689–691. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Frisvad, J.C. Mycotoxins on Building Materials; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011. [Google Scholar]
- Seguin, V.; Gente, S.; Heutte, N.; Verite, P.; Kientz-Bouchart, V.; Sage, L.; Goux, D.; Garon, D. First report of mycophenolic acid production by Eurotium repens isolated from agricultural and indoor environments. World Mycotoxin J. 2014, 7, 321–328. [Google Scholar] [CrossRef]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2019; Volume 2, p. 481. [Google Scholar]
- Warensjo Lemming, E.; Montano Montes, A.; Schmidt, J.; Cramer, B.; Humpf, H.U.; Moraeus, L.; Olsen, M. Mycotoxins in blood and urine of Swedish adolescents-possible associations to food intake and other background characteristics. Mycotoxin Res. 2020, 36, 193–206. [Google Scholar] [CrossRef] [Green Version]
- van Gelder, T.; Hesselink, D.A. Mycophenolate revisited. Transpl. Int. 2015, 28, 508–515. [Google Scholar] [CrossRef]
- Mok, C.C. Mycophenolate mofetil for lupus nephritis: An update. Expert Rev. Clin. Immunol. 2015, 11, 1353–1364. [Google Scholar] [CrossRef]
- Johannessen, L.N.; Nilsen, A.M.; Lovik, M. The mycotoxins citrinin and gliotoxin differentially affect production of the pro-inflammatory cytokines tumour necrosis factor-alpha and interleukin-6, and the anti-inflammatory cytokine interleukin-10. Clin. Exp. Allergy 2005, 35, 782–789. [Google Scholar] [CrossRef]
- Genuis, S.J.; Kyrillos, E. The chemical disruption of human metabolism. Toxicol. Mech. Methods 2017, 27, 477–500. [Google Scholar] [CrossRef] [PubMed]



| Inhaled Air Vol (L) | Number of Inhalations | Inhaled m3 of Air/8 h | RH (%) | Temp (°C) | Humid Ratio | Inhaled Water/8 h/mL/g Water | |
|---|---|---|---|---|---|---|---|
| Space 1 (Pat 1) | 0.5 | 13 | 3.12 | 30.8 | 21.4 | 4.877 | 15.22 |
| Space 2 | 0.5 | 13 | 3.12 | 30.2 | 21.4 | 4.781 | 14.92 |
| Space 3 | 0.5 | 13 | 3.12 | 30.6 | 21.6 | 4.905 | 15.30 |
| Space 4 (Pat 2) | 0.5 | 13 | 3.12 | 29.1 | 21.6 | 4.663 | 14.55 |
| Patient’s History | Symptoms | Findings during the Visit | |
|---|---|---|---|
| No 1 | No previous exposure to dampness microbiota | * Irritation of eyes | * Symptoms became worse when entering the problematic building |
| No change in the diet | * Very severe headache | * Symptoms did not relieve during weekends | |
| *Recurrent sinusitis | Status uneventful | ||
| * Multiple courses of antibiotics | The lesions were indurated, watery and had been scratched except for dermatitis on hands, legs, stomach, knees and back | ||
| * Thermoregulation problems | |||
| * Itching of the skin | |||
| * Exanthema | |||
| * Sore throat | |||
| * Gastrointestinal reflux for months | |||
| * “Brain fog” | |||
| * A flu-like feeling | |||
| No 2 | Lived in a town house with his/her family | * Dyspnoea | * Symptoms had started to ease in 2 weeks if the person was absence from work |
| All the other family members were asymptomatic | * Migraine | * Large psoriatic lesions especially on the elbows, back, scalp and chest, lips were cracked open | |
| * Phlegm | * The blood pressure was elevated | ||
| * Muscle pain | |||
| * Fatigue | |||
| * Concentration problems | |||
| * Prolonged cough | |||
| * Sleeping problems | |||
| No 3 | Previously suffered from gastrointestinal problems and allergy | * Sore throat | *Redness of eye conjunctiva, unrelated to season |
| * Blisters on the oral mucosa | Exanthema in the lower neck region | ||
| * A flu-like feeling | |||
| * Blurred vision | |||
| * Severe fatigue | |||
| * Dyspnea | |||
| * Joint pain | |||
| Irregular peristaltic action and | |||
| menorrhagia | |||
| No 4 | Gastrointestinal dysbiosis, leaky gut | * Thermoregulation problems | Otherwise, the status uneventful |
| * Two flu-like episodes | Axillary temperature 36.8 °C, exanthema in the lower neck region | ||
| * Increased sputum production | |||
| No 5 | Previously healthy, the individual had not been exposed to dampness and mold | * Two courses of antibiotics for | Status uneventful |
| tonsillitis | |||
| * Sore throat | |||
| * Evenings: eyes dry and voice hoarse | |||
| * Occasional instances of fatigue | |||
| * Memory problems | |||
| No 6 | Did not attend the doctor’s office | Was interviewed by telephone |
| Mycotoxins Reference Values in ng/g Creatinine | Patient 1 First Test | Patient 1 Follow-Up Test | HHC to Patient 1 (Time-Point as for Pat 1 Follow-Up) | Patient 2 First Test | Patient 2 Follow-Up Test | Patient X | Patient Y |
|---|---|---|---|---|---|---|---|
| Aflatoxin M1 (3.5–20) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ochratoxin A (4–20) | 11.23 | 9.82 | * 63.52 | 7.01 | 10.96 | 8.58 | 18.36 |
| Gliotoxin (200–2000) | 0 | 0 | 0 | 0 | 0 | 0 | * 910.98 |
| Sterigmatocystin (0.2–1.75) | 1.14 | 0 | 0 | 0.74 | 0 | 0 | 0 |
| Mycophenolic acid (5–50) | * 284.64 | 30.45 | 0 | * 50 | * 130.98 | 17.79 | 7.11 |
| Roridin E (1–6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Verrucarin A (1–10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Enniantin B (0.07–1) | <0.07 | 0 | 0 | 0 | 0 | 0.43 | 0 |
| Zearalenone (0.5–10) | 0 | 0 | 0 | 4.36 | * 15.54 | 5.55 | 0 |
| Chaetoglobosin A (20–80) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Citrinin (10–50) | <10 | <10 | 14.9 | 17.47 | 21.56 | 0 | 0 |
| Sample | RH % | T °C | Change in Cell Viability %, Mean ± stdev, p | |
|---|---|---|---|---|
| THP-1 Monocytes | THP-1 Macrophages | |||
| Working room 1 | 30.8 | 21.4 | 22.26 ± 20.81 * | −9.54 ± 1.25 *** |
| Sample 2 | 30.2 | 21.4 | 18.12 ± 16.6 | −2.87 ± 2.06 * |
| Sample 3 | 30.6 | 21.6 | 7.66 ± 10.70 | −6.26 ± 2.40 *** |
| Sample 4 | 29.1 | 21.7 | 28.40 ± 12.59 *** | −6.62 ± 1.26 *** |
| Sample | RH % | T °C | Change in THP-1 Macrophage Viability %, Mean ± Stdev, p (Significance) | |
|---|---|---|---|---|
| 10% Condensate | 25% Condensate | |||
| Dormitory 1st floor (Patient 1 and HHC) | 35.6 | 22.4 | −2.13 ± 4.55 | −13.35 ± 5.84 *** |
| Bedroom (son) 2nd floor | 37.8 | 22.2 | 1.78 ± 3.02 | 1.56 ± 3.72 |
| Bedroom (daughter) 2nd floor | 38.7 | 22.0 | 6.33 ± 10.30 | 1.07 ± 8.56 |
| Living room 2nd floor (HHC worked in this space) | 39.6 | 22.0 | 2.83 ± 6.17 | 4.50 ± 4.65 * |
| Sample | Fungal Genus or Group | Culture Plate | Toxicity in Sperm Test | Toxicity in BHK-Test |
|---|---|---|---|---|
| Sample 2 | Penicillium | MEA | Toxic | No |
| Sample 4 | Penicillium | MEA | Toxic | No |
| Sample 9 | Acremonium sensu lato | MEA | Toxic | Toxic |
| Sample 16 | Acremonium sensu lato | MEA | Toxic | Toxic |
| Sample 18 | Penicillium | DG18 | Toxic | No |
| Sample 19 | Penicillium | DG18 | Toxic | No |
| Sample 21 | Aspergillus ochraceus-group | DG18 | Toxic | No |
| Sample 27 | Aspergillus ochraceus-group | DG18 | Toxic | No |
| Sample 28 | Aspergillus section Aspergillus (Eurotium) | DG18, direct cultivation | Toxic | Toxic |
| Sample 29 | Aspergillus section Aspergillus (Eurotium) | DG18, direct cultivation | Toxic | Toxic |
| Sample 32 | Bacterium | TGY | Toxic | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaali, K.; Tuomela, M.; Mannerström, M.; Heinonen, T.; Tuuminen, T. Toxic Indoor Air Is a Potential Risk of Causing Immuno Suppression and Morbidity—A Pilot Study. J. Fungi 2022, 8, 104. https://doi.org/10.3390/jof8020104
Vaali K, Tuomela M, Mannerström M, Heinonen T, Tuuminen T. Toxic Indoor Air Is a Potential Risk of Causing Immuno Suppression and Morbidity—A Pilot Study. Journal of Fungi. 2022; 8(2):104. https://doi.org/10.3390/jof8020104
Chicago/Turabian StyleVaali, Kirsi, Marja Tuomela, Marika Mannerström, Tuula Heinonen, and Tamara Tuuminen. 2022. "Toxic Indoor Air Is a Potential Risk of Causing Immuno Suppression and Morbidity—A Pilot Study" Journal of Fungi 8, no. 2: 104. https://doi.org/10.3390/jof8020104
APA StyleVaali, K., Tuomela, M., Mannerström, M., Heinonen, T., & Tuuminen, T. (2022). Toxic Indoor Air Is a Potential Risk of Causing Immuno Suppression and Morbidity—A Pilot Study. Journal of Fungi, 8(2), 104. https://doi.org/10.3390/jof8020104






