Increasing Incidence and Shifting Epidemiology of Candidemia in Greece: Results from the First Nationwide 10-Year Survey
Abstract
:1. Introduction
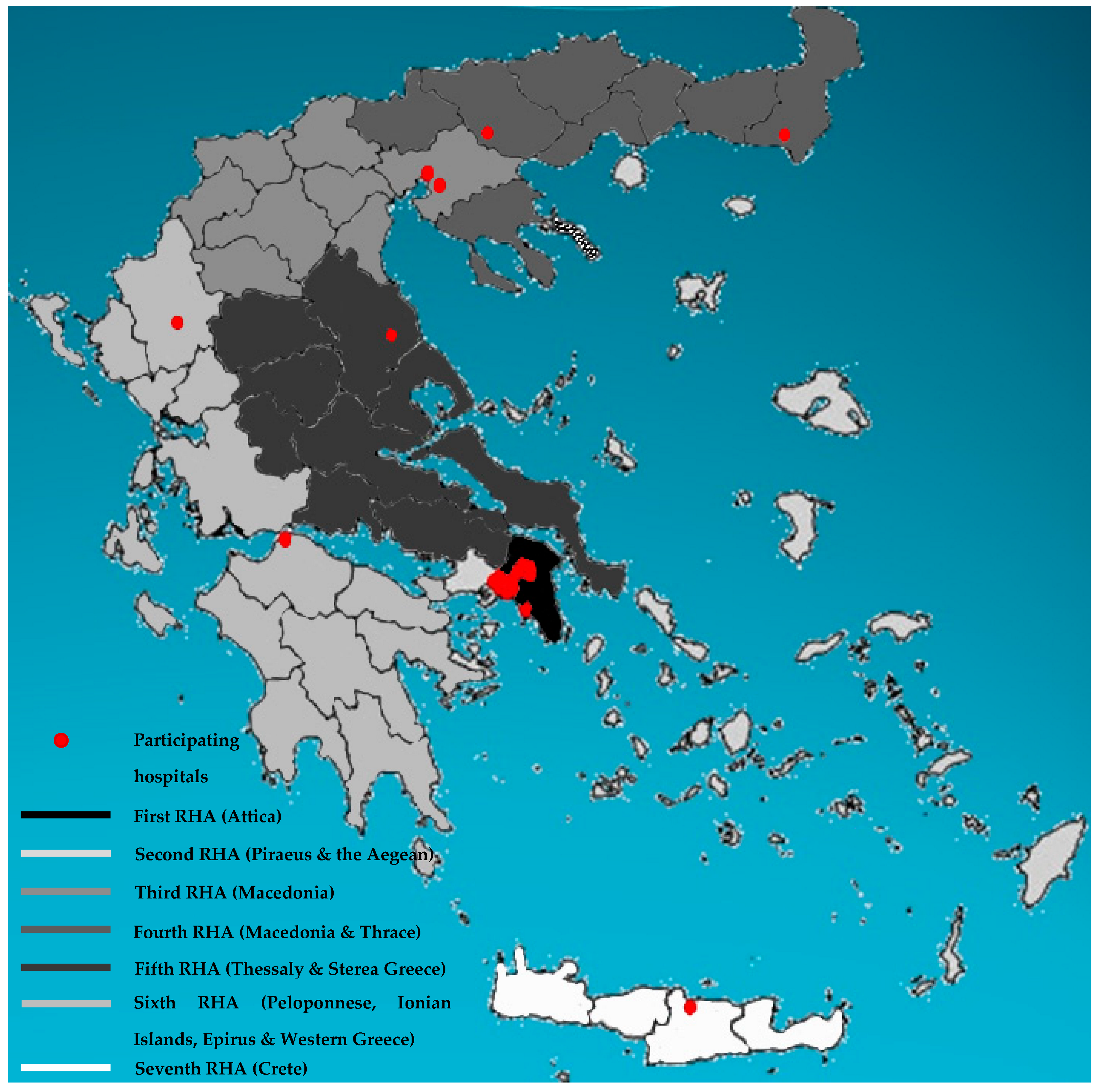
2. Materials and Methods
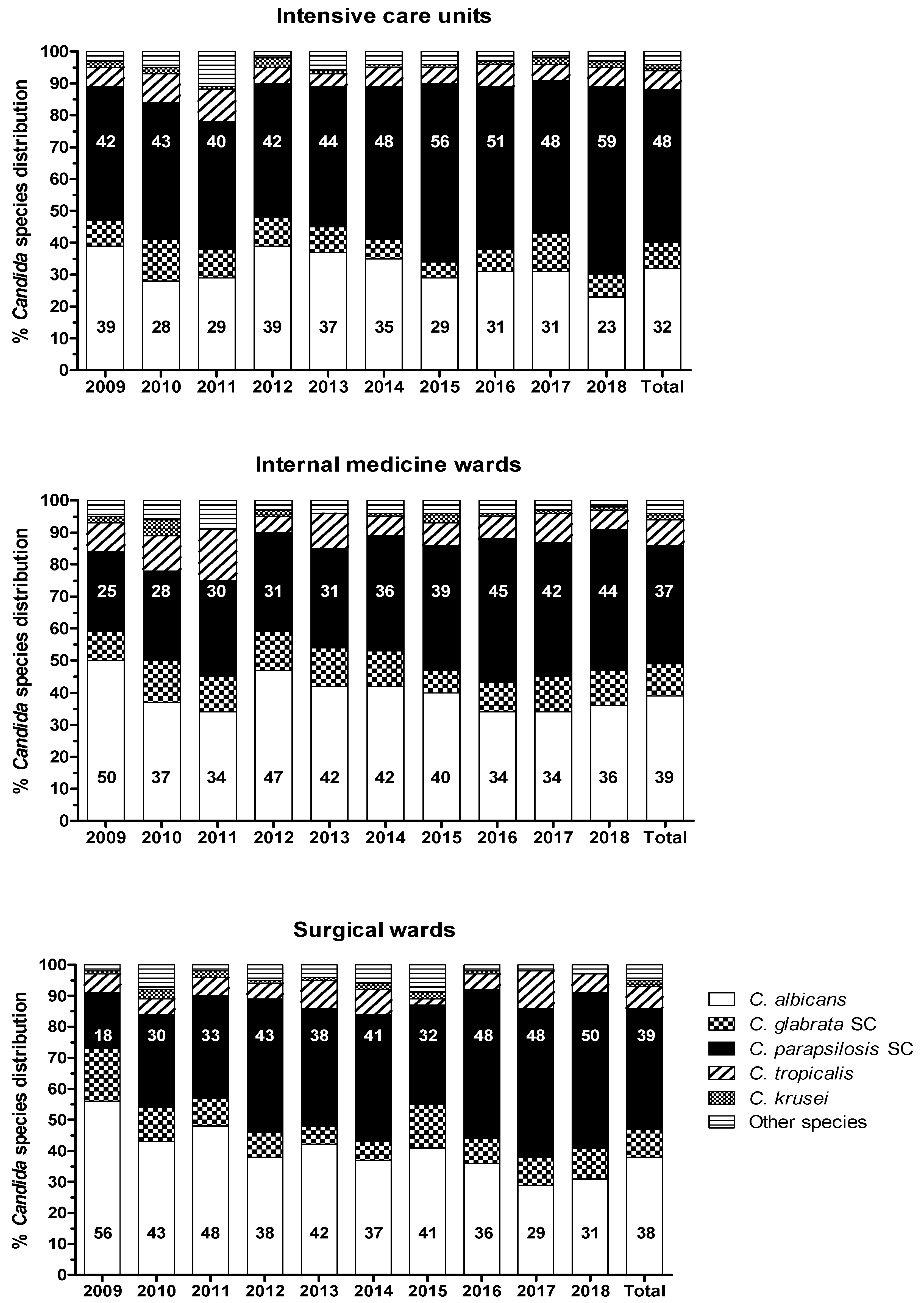
3. Results
- (i).
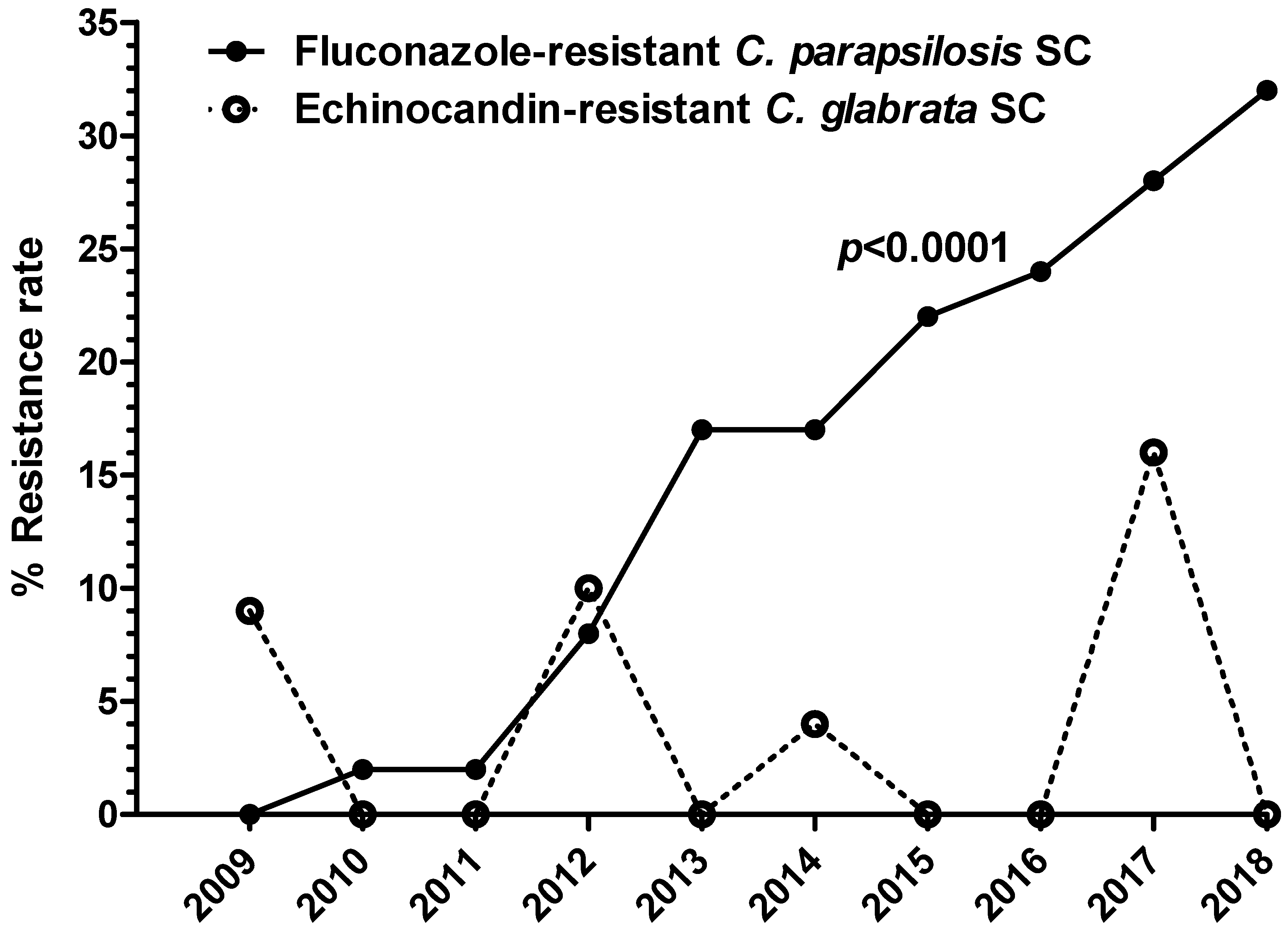
- Azoles. For ITC and POS, resistant/non-WT strains were observed among C. albicans (4% and 3%, respectively), C. parapsilosis SC (7% and 5%, respectively), and C. glabrata SC (7% and 12%, respectively). Interestingly, a significant proportion of the ITC resistant/non-WT isolates, all recovered from IMWs and SWs patients were pan-azole-resistant/non-WT (54%, 41%, and 47% of C. albicans, C. parapsilosis SC, and C. glabrata SC, respectively). VRC-resistant/non-WT phenotypes were identified among strains of C. albicans (3%), C. parapsilosis SC (1%), C. glabrata SC (6%), and C. tropicalis (1%), while 7% and 10% of C. parapsilosis SC and C. tropicalis isolates, respectively, displayed elevated VRC MICs (0.25–0.5 mg/L), categorizing them as intermediate. Worryingly, reduced susceptibility to FLC was mostly seen. In particular, 3% of C. albicans (18% pan-azole-resistant/non-WT), 20% of C. parapsilosis SC, 5% of C. glabrata SC (3% pan-azole-resistant/non-WT), and 6% of C. tropicalis isolates were FLC-resistant, whereas 2% of C. albicans as well as C. parapsilosis SC and 8% of C. tropicalis isolates were categorized as intermediate. The FLC-resistant C. parapsilosis SC isolates were found in all units (48% in ICUs, 34% in IMWs and 18% in SWs) of the participating hospitals, presenting the 32%, 21%, and 20% of C. parapsilosis SC isolates recovered from candidemic patients admitted to ICUs, IMWs, and SWs, respectively. Alarmingly, their isolation rate was steadily rising throughout the study period: from 1% during 2009–2011 to 14% between 2012–2014 and further to 27% during 2015–2018 (p < 0.0001) (Figure 4). Moreover, they have shown different susceptibility profiles to other azoles; 3% were pan-azole-resistant/non-WT isolates, whilst those with the highest MICs for FLC (≥32 mg/L) were also VRC-resistant (20%).
- (ii).
- Echinocandins. All three echinocandins exhibited very good activity against most Candida spp., including C. parapsilosis SC isolates (100% susceptibility). Non-susceptible to AFG and MFG C. albicans, C. tropicalis, and C. krusei isolates remained below 2%. Of note, echinocandin resistance was found in 3% of C. glabrata SC isolates, whereof 70% demonstrated elevated MIC values for all echinocandins (AFG, CAS, and MFG MIC 0.5–1, 0.5–2, and 0.5 mg/L, respectively) but not to azoles. These strains were isolated from ICU patients hospitalized in different and far from each other medical centres and were distributed equally through the years of the study period (0–3 isolates annually; p = 0.80) (Figure 4).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Koehler, P.; Stecher, M.; Cornely, O.A.; Koehler, D.; Vehreschild, M.J.G.T.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J.J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Lockhart, S.; Berkow, E.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nor Ain Wan Ismail, W.; Jasmi, N.; Mehmood Khan, T.; Hoi Hong, Y.; Fen Neoh, C. Systematic Literature Review The Economic Burden of Candidemia and Invasive Candidiasis: A Systematic Review. Value Health Reg. Issues 2020, 21, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Montero, J.; Díaz-Martín, A.; García-Cabrera, E.; de Pipaón, M.R.P.; Hernández-Caballero, C.; Lepe-Jimánez, J.A. Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp. bloodstream infections. J. Antimicrob. Chemother. 2013, 68, 206–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrosky-Zeichner, L.; Kullberg, B.J.; Bow, E.J.; Hadley, S.; León, C.; Nucci, M.; Patterson, T.F.; Perfect, J.R. Early treatment of candidemia in adults: A review. Med. Mycol. 2011, 49, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef]
- Tóth, R.; Nosek, J.; Mora-Montes, H.M.; Gabaldon, T.; Bliss, J.M.; Nosanchuk, J.D.; Turner, S.A.; Butler, G.; Vágvölgyi, C.; Gácser, A. Candida parapsilosis: From Genes to the Bedside. Clin. Microbiol. Rev. 2019, 32, e00111-18. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, J.; Fisher, M.C. Global epidemiology of emerging Candida auris. Curr. Opin. Microbiol. 2019, 52, 84–89. [Google Scholar] [CrossRef]
- Siopi, M.; Tarpatzi, A.; Kalogeropoulou, E.; Damianidou, S.; Vasilakopoulou, A.; Vourli, S.; Pournaras, S.; Meletiadis, J. Epidemiological trends of fungemia in Greece with a focus on candidemia during the recent financial crisis: A 10-year survey in a tertiary care academic hospital and review of literature. Antimicrob. Agents Chemother. 2020, 64, e01516-19. [Google Scholar] [CrossRef]
- Ministry of Health: Supervised Bodies and Legal Entities. Available online: https://www.moh.gov.gr/articles/ministry/organogramma/129-foreis(webpage in Greek); (accessed on 25 October 2021).
- Bassetti, M.; Giacobbe, D.R.; Vena, A.; Trucchi, C.; Ansaldi, F.; Antonelli, M.; Adamkova, V.; Alicino, C.; Almyroudi, M.P.; Atchade, E.; et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: Results of the EUCANDICU project. Crit. Care 2019, 23. [Google Scholar] [CrossRef] [Green Version]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST) Method for the Determination of Broth Dilution Minimum Inhibitory Concntrations of Antifungal Agents for Yeasts. EUCAST E.DEF 7.3.1. 2017. Available online: http://www.eucast.org (accessed on 25 September 2021).
- The European Committee on Antimicrobial Susceptibility Testing. Overview of Antifungal ECOFFs and Clinical Breakpoints for Yeasts, Moulds and Dermatophytes Using the EUCAST E.Def 7.3, E.Def 9.3 and E.Def 11.0 Procedures. Version 2. 2020. Available online: http://www.eucast.org (accessed on 25 September 2021).
- Pfaller, M.A.; Diekema, D.J.; Procop, G.W.; Wiederhold, N.P. Multicenter Evaluation of the New Vitek 2 Yeast Susceptibility Test Using New CLSI Clinical Breakpoints for Fluconazole. J. Clin. Microbiol. 2014, 52, 2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astvad, K.M.; Perlin, D.S.; Johansen, H.K.; Jensen, R.H.; Arendrup, M.C. Evaluation of Caspofungin Susceptibility Testing by the New Vitek 2 AST-YS06 Yeast Card Using a Unique Collection of FKS Wild-Type and Hot Spot Mutant Isolates, Including the Five Most Common Candida Species. Antimicrob. Agents Chemother. 2013, 57, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuenca-Estrella, M.; Gomez-Lopez, A.; Alastruey-Izquierdo, A.; Bernal-Martinez, L.; Cuesta, I.; Buitrago, M.J.; Rodriguez-Tudela, J.L. Comparison of the Vitek 2 antifungal susceptibility system with the clinical and laboratory standards institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) Broth Microdilution Reference Methods and with the Sensititre YeastOne and Etest techniques for in vitro detection of antifungal resistance in yeast isolates. J. Clin. Microbiol. 2010, 48, 1782–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dannaoui, E.; Espinel-Ingroff, A. Antifungal Susceptibly Testing by Concentration Gradient Strip Etest Method for Fungal Isolates: A Review. J. Fungi 2019, 5, 108. [Google Scholar] [CrossRef] [Green Version]
- Eschenauer, G.A.; Nguyen, M.H.; Shoham, S.; Vazquez, J.a.; Morris, A.J.; Pasculle, W.a.; Kubin, C.J.; Klinker, K.P.; Carver, P.L.; Hanson, K.E.; et al. Real-world experience with echinocandin MICs against Candida species in a multicenter study of hospitals that routinely perform susceptibility testing of bloodstream isolates. Antimicrob. Agents Chemother. 2014, 58, 1897–1906. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 1st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- CLSI. Epidemiological Cutoff Values for Antifungal Susceptibility Testing, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Salsé, M.; Gangneux, J.-P.; Cassaing, S.; Delhaes, L.; Fekkar, A.; Dupont, D.; Botterel, F.; Costa, D.; Bourgeois, N.; Bouteille, B.; et al. Multicentre study to determine the Etest epidemiological cut-off values of antifungal drugs in Candida spp. and Aspergillus fumigatus species complex. Clin. Microbiol. Infect. 2019, 25, 1546–1552. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Turnidge, J.; Alastruey-Izquierdo, A.; Botterel, F.; Canton, E.; Castro, C.; Chen, Y.C.; Chen, Y.; Chryssanthou, E.; Dannaoui, E.; et al. Method-dependent epidemiological cutoff values for detection of triazole resistance in Candida and Aspergillus species for the Sensititre Yeastone colorimetric broth and etest agar diffusion methods. Antimicrob. Agents Chemother. 2019, 63, e01651-18. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Ingroff, A.; Arendrup, M.; Canton, E.; Cordob, S.; Dannaoui, E.; Garcia-Rodriguez, J.; Gonzalez, G.M.; Govender, N.P.; Martin-Mazuelos, E.; Lackner, M.; et al. Multicenter study of method-dependent epidemiological cutoff values for detection of resistance in candida spp. and aspergillus spp. to amphotericin B and echinocandins for the etest agar diffusion method. Antimicrob. Agents Chemother. 2017, 61, 1792–1808. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Ingroff, A.; Alvarez-Fernandez, M.; Cantón, E.; Carver, P.L.; Chen, S.C.-A.; Eschenauer, G.; Getsinger, D.L.; Gonzalez, G.M.; Govender, N.P.; Grancini, A.; et al. Multicenter study of epidemiological cutoff values and detection of resistance in Candida spp. to anidulafungin, caspofungin, and micafungin using the Sensititre YeastOne colorimetric method. Antimicrob. Agents Chemother. 2015, 59, 6725–6732. [Google Scholar] [CrossRef] [Green Version]
- Cantón, E.; Pemán, J.; Hervás, D.; Iñiguez, C.; Navarro, D.; Echeverría, J.; Martínez-Alarcón, J.; Fontanals, D.; Gomila-Sard, B.; Buendía, B.; et al. Comparison of three statistical methods for establishing tentative wild-type population and epidemiological cutoff values for echinocandins, amphotericin B, flucytosine, and six Candida species as determined by the colorimetric Sensititre YeastOne method. J. Clin. Microbiol. 2012, 50, 3921–3926. [Google Scholar] [CrossRef] [Green Version]
- Population Estimates and Projections|DataBank. Available online: https://databank.worldbank.org/source/population-estimates-and-projections# (accessed on 25 September 2021).
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Sood, P.; Rudramurthy, S.M.; Chen, S.; Kaur, H.; Capoor, M.; Chhina, D.; Rao, R.; Eshwara, V.K.; Xess, I.; et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2014, 41, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Merelli, M.; Righi, E.; Diaz-Martin, A.; Rosello, E.M.; Luzzati, R.; Parra, A.; Trecarichi, E.M.; Sanguinetti, M.; Posteraro, B.; et al. Epidemiology, Species Distribution, Antifungal Susceptibility, and Outcome of Candidemia across Five Sites in Italy and Spain. J. Clin. Microbiol. 2013, 51, 4167–4172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamaletsou, M.N.; Drogari-Apiranthitou, M.; Denning, D.W.; Sipsas, N.V. An estimate of the burden of serious fungal diseases in Greece. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1115–1120. [Google Scholar] [CrossRef]
- Dorgan, E.; Denning, D.W.; McMullan, R. Burden of fungal disease in Ireland. J. Med. Microbiol. 2015, 64, 423–426. [Google Scholar] [CrossRef] [Green Version]
- Alobaid, K.; Ahmad, S.; Asadzadeh, M.; Mokaddas, E.; Al-Sweih, N.; Albenwan, K.; Alfouzan, W.; Al-Obaid, I.; Jeragh, A.; Al-Roomi, E.; et al. Epidemiology of Candidemia in Kuwait: A Nationwide, Population-Based Study. J. Fungi 2021, 7, 673. [Google Scholar] [CrossRef]
- Corzo-León, D.E.; Armstrong-James, D.; Denning, D.W. Burden of serious fungal infections in Mexico. Mycoses 2015, 58 (Suppl 5), 34–44. [Google Scholar] [CrossRef] [Green Version]
- Özenci, V.; Klingspor, L.; Ullberg, M.; Chryssanthou, E.; Denning, D.W.; Kondori, N. Estimated burden of fungal infections in Sweden. Mycoses 2019, 62, 1043–1048. [Google Scholar] [CrossRef]
- Chayakulkeeree, M.; Denning, D.W. Serious fungal infections in Thailand. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 931–935. [Google Scholar] [CrossRef] [Green Version]
- Sinkó, J.; Sulyok, M.; Denning, D.W. Burden of serious fungal diseases in Hungary. Mycoses 2015, 58 (Suppl 5), 29–33. [Google Scholar] [CrossRef] [Green Version]
- Risum, M.; Astvad, K.; Johansen, H.K.; Schønheyder, H.C.; Rosenvinge, F.; Knudsen, J.D.; Hare, R.K.; Datcu, R.; Røder, B.L.; Antsupova, V.S.; et al. Update 2016–2018 of the Nationwide Danish Fungaemia Surveillance Study: Epidemiologic Changes in a 15-Year Perspective. J. Fungi 2021, 7, 491. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Tudela, J.L.; Alastruey-Izquierdo, A.; Gago, S.; Cuenca-Estrella, M.; León, C.; Miro, J.M.; Nuñez Boluda, A.; Ruiz Camps, I.; Sole, A.; Denning, D.W. Burden of serious fungal infections in Spain. Clin. Microbiol. Infect. 2015, 21, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufresne, S.F.; Cole, D.C.; Denning, D.W.; Sheppard, D.C. Serious fungal infections in Canada. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 987–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, B.; Slavin, M.; Marriott, D.; Halliday, C.; Kidd, S.; Arthur, I.; Bak, N.; Heath, C.H.; Kennedy, K.; Morrissey, C.O.; et al. Changing epidemiology of candidaemia in Australia. J. Antimicrob. Chemother. 2017, 72, 1103–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabino, R.; Verissímo, C.; Brandão, J.; Martins, C.; Alves, D.; Pais, C.; Denning, D.W. Serious fungal infections in Portugal. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Batac, M.C.R.; Denning, D. Serious fungal infections in the Philippines. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 937–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Castanheira, M. Nosocomial Candidiasis: Antifungal Stewardship and the Importance of Rapid Diagnosis. Med. Mycol. 2016, 54, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Adam, K.-M.; Osthoff, M.; Lamoth, F.; Conen, A.; Erard, V.; Boggian, K.; Schreiber, P.W.; Zimmerli, S.; Bochud, P.-Y.; Neofytos, D.; et al. Trends of the Epidemiology of Candidemia in Switzerland: A 15-Year FUNGINOS Survey. Open Forum Infect. Dis. 2021, 8, ofab471. [Google Scholar] [CrossRef]
- Hesstvedt, L.; Gaustad, P.; Andersen, C.T.; Haarr, E.; Hannula, R.; Haukland, H.H.; Hermansen, N.-O.; Larssen, K.W.; Mylvaganam, H.; Ranheim, T.E.; et al. Twenty-two years of candidaemia surveillance: results from a Norwegian national study. Clin. Microbiol. Infect. 2015, 21, 938–945. [Google Scholar] [CrossRef] [Green Version]
- OECD/European Union Health at a Glance: Europe 2020: State of Health in the EU Cycle. Available online: https://ec.europa.eu/health/sites/default/files/state/docs/2020_healthatglance_rep_en.pdf (accessed on 23 October 2021).
- Björnberg, A. Euro Health Consumer Index 2017. PharmacoEcon. Outcomes News 2018, 796, 31. [Google Scholar]
- Mesini, A.; Mikulska, M.; Giacobbe, D.R.; Del Puente, F.; Gandolfo, N.; Codda, G.; Orsi, A.; Tassinari, F.; Beltramini, S.; Marchese, A.; et al. Changing epidemiology of candidaemia: Increase in fluconazole-resistant Candida parapsilosis. Mycoses 2020, 63, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Prigitano, A.; Cavanna, C.; Passera, M.; Ossi, C.; Sala, E.; Lombardi, G.; Grancini, A.; De Luca, C.; Bramati, S.; Gelmi, M.; et al. CAND-LO 2014-15 study: Changing epidemiology of candidemia in Lombardy (Italy). Infection 2016, 44, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Hospitals in Greece 2000–2018|Statista. Available online: https://www.statista.com/statistics/557023/hospitals-in-greece/ (accessed on 23 October 2021).
- Karanikolos, M.; Heino, P.; McKee, M.; Stuckler, D.; Legido-Quigley, H. Effects of the global financial crisis on health in high-income OECD countries: A narrative review. Int. J. Health Serv. 2016, 46, 208–240. [Google Scholar] [CrossRef] [PubMed]
- Economou, C.; Kaitelidou, D.; Kentikelenis, A.; Sissouras, A.; Maresso, A. The impact of the financial crisis on the health system and health in Greece. In In Economic Crisis, Health Systems and Health in Europe: Country Experience; Maresso, A., Mladovsky, P., Thomson, S., Sagan, A., Karanikolos, M., Richardson, E., Cylus, J., Evetovits, T., Jowett, M., Figueras, J., et al., Eds.; Observatory Studies Series, No. 41. European Observatory on Health Systems and Policies: Copenhagen, Denmark, 2015. [Google Scholar]
- Papadimitriou-Olivgeris, M.; Spiliopoulou, A.; Kolonitsiou, F.; Bartzavali, C.; Lambropoulou, A.; Xaplanteri, P.; Anastassiou, E.D.; Marangos, M.; Spiliopoulou, I.; Christofidou, M. Increasing incidence of candidaemia and shifting epidemiology in favor of Candida non-albicans in a 9-year period (2009–2017) in a university Greek hospital. Infection 2018, 47, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngamchokwathana, C.; Chongtrakool, P.; Waesamaae, A.; Chayakulkeeree, M. Risk Factors and Outcomes of Non- albicans Candida Bloodstream Infection in Patients with Candidemia at Siriraj Hospital-Thailand’s Largest National Tertiary Referral Hospital. J. Fungi 2021, 7, 269. [Google Scholar] [CrossRef] [PubMed]
- Govender, N.P.; Patel, J.; Magobo, R.E.; Naicker, S.; Wadula, J.; Whitelaw, A.; Coovadia, Y.; Kularatne, R.; Govind, C.; Lockhart, S.R.; et al. Emergence of azole-resistant Candida parapsilosis causing bloodstream infection: results from laboratory-based sentinel surveillance in South Africa. J. Antimicrob. Chemother. 2016, 71, 1994–2004. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.K.B.; Bonfietti, L.X.; Garcia, R.A.; Araujo, M.R.; Rodrigues, J.S.; Gimenes, V.M.F.; Melhem, M.S.C. Antifungal susceptibility profile of Candida clinical isolates from 22 hospitals of São Paulo State, Brazil. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. Biol. 2021, 54, e10928. [Google Scholar] [CrossRef]
- Bustamante, B.; Martins, M.A.; Bonfietti, L.X.; Szeszs, M.W.; Jacobs, J.; Garcia, C.; Melhem, M.S.C. Species distribution and antifungal susceptibility profile of Candida isolates from bloodstream infections in Lima, Peru. J. Med. Microbiol. 2014, 63, 855–860. [Google Scholar] [CrossRef] [Green Version]
- Nucci, M.; Queiroz-Telles, F.; Alvarado-Matute, T.; Tiraboschi, I.N.; Cortes, J.; Zurita, J.; Guzman-Blanco, M.; Santolaya, M.E.; Thompson, L.; Sifuentes-Osornio, J.; et al. Epidemiology of candidemia in Latin America: a laboratory-based survey. PLoS ONE 2013, 8, e59373. [Google Scholar] [CrossRef] [Green Version]
- Arsić Arsenijević, V.; Otašević, S.; Janić, D.; Minić, P.; Matijašević, J.; Medić, D.; Savić, I.; Delić, S.; Nestorović Laban, S.; Vasiljević, Z.; et al. Candida bloodstream infections in Serbia: First multicentre report of a national prospective observational survey in intensive care units. Mycoses 2018, 61, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Baley, E.D.; Hossain, J.; Di Pentima, M.C. Candida species bloodstream infections in hospitalised children: A 10-year experience. J. Paediatr. Child Health 2015, 51, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou-Olivgeris, M.; Andrianaki, A.M.; Marangos, M.; Sipsas, N.; Apostolidi, E.A.; Maltezos, E.; Panagopoulos, P.; Karapiperis, D.; Arvaniti, K.; Perdikouri, E.-I.; et al. Hospital-wide antifungal prescription in Greek hospitals: a multicenter repeated point-prevalence study. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 39, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Bailly, S.; Maubon, D.; Fournier, P.; Pelloux, H.; Schwebel, C.; Chapuis, C.; Foroni, L.; Cornet, M.; Timsit, J.-F. Impact of antifungal prescription on relative distribution and susceptibility of Candida spp.—Trends over 10 years. J. Infect. 2016, 72, 103–111. [Google Scholar] [CrossRef]
- Forrest, G.N.; Weekes, E.; Johnson, J.K. Increasing incidence of Candida parapsilosis candidemia with caspofungin usage. J. Infect. 2008, 56, 126–129. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Lau, A. Matrix-Assisted Laser Desorption Ionization Time-of-Flight for Fungal Identification. Clin. Lab. Med. 2021, 41, 267–283. [Google Scholar] [CrossRef]
- Sipsas, N.V.; Pagoni, M.N.; Kofteridis, D.P.; Meletiadis, J.; Vrioni, G.; Papaioannou, M.; Antoniadou, A.; Petrikkos, G.; Samonis, G. Management of Invasive Fungal Infections in Adult Patients with Hematological Malignancies in Greece during the Financial Crisis: Challenges and Recommendations. J. Fungi 2018, 4, 94. [Google Scholar] [CrossRef] [Green Version]
- Brilhante, R.S.N.; Sales, J.A.; da Silva, M.L.Q.; de Oliveira, J.S.; Pereira, L.A.; Pereira-Neto, W.A.; Cordeiro, R.A.; Sidrim, J.J.C.; Castelo-Branco, D.S.C.M.; Rocha, M.F.G. Antifungal susceptibility and virulence of Candida parapsilosis species complex: an overview of their pathogenic potential. J. Med. Microbiol. 2018, 67, 903–914. [Google Scholar] [CrossRef]
- Hou, X.; Xiao, M.; Chen, S.C.-A.; Wang, H.; Yu, S.-Y.; Fan, X.; Kong, F.; Xu, Y.-C. Identification and Antifungal Susceptibility Profiles of Candida nivariensis and Candida bracarensis in a Multi-Center Chinese Collection of Yeasts. Front. Microbiol. 2017, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Borman, A.M.; Szekely, A.; Linton, C.J.; Palmer, M.D.; Brown, P.; Johnson, E.M. Epidemiology, antifungal susceptibility, and pathogenicity of Candida africana isolates from the United Kingdom. J. Clin. Microbiol. 2013, 51, 967–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gülmez, D.; Alp, S.; Gursoy, G.; Ayaz, C.M.; Dogan, O.; Arikan-Akdagli, S.; Akova, M. Mixed fungaemia: an 18-year report from a tertiary-care university hospital and a systematic review. Clin. Microbiol. Infect. 2020, 26, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Muñoz, P.; Guinea, J.; Rodríguez-Créixems, M.; Peláez, T.; Bouza, E. Mixed fungemia: Incidence, risk factors, and mortality in a general hospital. Clin. Infect. Dis. 2007, 44, e109-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-García, J.; Mesquida, A.; Sánchez-Carrillo, C.; Reigadas, E.; Muñoz, P.; Escribano, P.; Guinea, J. Monitoring the Epidemiology and Antifungal Resistance of Yeasts Causing Fungemia in a Tertiary Care Hospital in Madrid, Spain: Any Relevant Changes in the Last 13 Years? Antimicrob. Agents Chemother. 2021, 65, e01827-20. [Google Scholar] [CrossRef] [PubMed]
- Mete, B.; Zerdali, E.Y.; Aygun, G.; Saltoglu, N.; Balkan, I.I.; Karaali, R.; Kaya, S.Y.; Karaismailoglu, B.; Kaya, A.; Urkmez, S.; et al. Change in species distribution and antifungal susceptibility of candidemias in an intensive care unit of a university hospital (10-year experience). Eur. J. Clin. Microbiol. Infect. Dis. 2020, 40, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Iqbal, N.; Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Bolden, C.B.; Baughman, W.; Stein, B.; Hollick, R.; Park, B.J.; et al. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J. Clin. Microbiol. 2012, 50, 3435–3442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lortholary, O.; Desnos-Ollivier, M.; Sitbon, K.; Fontanet, A.; Bretagne, S.; Dromer, F.; Bouges-Michel, C.; Poilane, I.; Dunan, J.; Galeazzi, G.; et al. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: A prospective multicenter study involving 2,441 patients. Antimicrob. Agents Chemother. 2011, 55, 532–538. [Google Scholar] [CrossRef] [Green Version]
- Arendrup, M.C.; Bruun, B.; Christensen, J.J.; Fuursted, K.; Johansen, H.K.; Kjældgaard, P.; Knudsen, J.D.; Kristensen, L.; Møller, J.; Nielsen, L.; et al. National surveillance of fungemia in Denmark (2004 to 2009). J. Clin. Microbiol. 2011, 49, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.H.; Peacock, J.E.; Morris, A.J.; Tanner, D.C.; Nguyen, M.L.; Snydman, D.R.; Wagener, M.M.; Rinaldi, M.G.; Yu, V.L. The changing face of candidemia: Emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 1996, 100, 617–623. [Google Scholar] [CrossRef]
- Biggest Threats and Data|Antibiotic/Antimicrobial Resistance|CDC. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fdrugresistance%2Fbiggest_threats.html#candida (accessed on 28 October 2021).
- Meletiadis, J.; Curfs-Breuker, I.; Meis, J.F.; Mouton, J.W. In Vitro Antifungal Susceptibility Testing of Candida Isolates with the EUCAST Methodology, a New Method for ECOFF Determination. Antimicrob. Agents Chemother. 2017, 61, e02372-16. [Google Scholar] [CrossRef] [Green Version]
- Cantón, E.; Pemán, J.; Quindós, G.; Eraso, E.; Miranda-Zapico, I.; Álvarez, M.; Merino, P.; Campos-Herrero, I.; Marco, F.; de la Pedrosa, E.G.G.; et al. Prospective Multicenter Study of the Epidemiology, Molecular Identification, and Antifungal Susceptibility of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis Isolated from Patients with Candidemia. Antimicrob. Agents Chemother. 2011, 55, 5590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castanheira, M.; Deshpande, L.M.; Messer, S.A.; Rhomberg, P.R.; Pfaller, M.A. Analysis of global antifungal surveillance results reveals predominance of Erg11 Y132F alteration among azole-resistant Candida parapsilosis and Candida tropicalis and country-specific isolate dissemination. Int. J. Antimicrob. Agents 2020, 55, 105799. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, D.Y.; de Almeida, J.N.J.; Lima, G.M.E.; Nunes, M.d.O.; Camargo, C.H.; Grenfell, R.d.C.; Benard, G.; Del Negro, G.M.B. An Azole-Resistant Candida parapsilosis Outbreak: Clonal Persistence in the Intensive Care Unit of a Brazilian Teaching Hospital. Front. Microbiol. 2018, 9, 2997. [Google Scholar] [CrossRef] [PubMed]
- Pinhati, H.M.S.; Casulari, L.A.; Souza, A.C.R.; Siqueira, R.A.; Damasceno, C.M.G.; Colombo, A.L. Outbreak of candidemia caused by fluconazole resistant Candida parapsilosis strains in an intensive care unit. BMC Infect. Dis. 2016, 16, 433. [Google Scholar] [CrossRef]
- Fekkar, A.; Blaize, M.; Bouglé, A.; Normand, A.-C.; Raoelina, A.; Kornblum, D.; Kamus, L.; Piarroux, R.; Imbert, S. Hospital Outbreak of Fluconazole-Resistant Candida parapsilosis: Arguments for Clonal Transmission and Long-Term Persistence. Antimicrob. Agents Chemother. 2021, 65, e02036-20. [Google Scholar] [CrossRef]
- Singh, A.; Singh, P.K.; de Groot, T.; Kumar, A.; Mathur, P.; Tarai, B.; Sachdeva, N.; Upadhyaya, G.; Sarma, S.; Meis, J.F.; et al. Emergence of clonal fluconazole-resistant Candida parapsilosis clinical isolates in a multicentre laboratory-based surveillance study in India. J. Antimicrob. Chemother. 2019, 74, 1260–1268. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Suh, J.W.; Kim, M.J. Epidemiological Trends of Candidemia and the Impact of Adherence to the Candidemia Guideline: Six-Year Single-Center Experience. J. Fungi 2021, 7, 275. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, Y.-J.; Yong, D.; Byun, J.-H.; Kim, T.S.; Chang, Y.S.; Choi, M.J.; Byeon, S.A.; Won, E.J.; Kim, S.H.; et al. Fluconazole-Resistant Candida parapsilosis Bloodstream Isolates with Y132F Mutation in ERG11 Gene, South Korea. Emerg. Infect. Dis. 2018, 24, 1768–1770. [Google Scholar] [CrossRef] [Green Version]
- Shuping, L.; Mpembe, R.; Mhlanga, M.; Naicker, S.D.; Maphanga, T.G.; Tsotetsi, E.; Wadula, J.; Velaphi, S.; Nakwa, F.; Chibabhai, V.; et al. Epidemiology of Culture-confirmed Candidemia among Hospitalized Children in South Africa, 2012-2017. Pediatr. Infect. Dis. J. 2021, 40, 730–737. [Google Scholar] [CrossRef]
- Martini, C.; Torelli, R.; Groot, T.d.; De Carolis, E.; Morandotti, G.A.; De Angelis, G.; Posteraro, B.; Meis, J.F.; Sanguinetti, M. Prevalence and Clonal Distribution of Azole-Resistant Candida parapsilosis Isolates Causing Bloodstream Infections in a Large Italian Hospital. Front. Cell. Infect. Microbiol. 2020, 10, 232. [Google Scholar] [CrossRef]
- Demirci-Duarte, S.; Arikan-Akdagli, S.; Gülmez, D. Species distribution, azole resistance and related molecular mechanisms in invasive Candida parapsilosis complex isolates: Increase in fluconazole resistance in 21 years. Mycoses 2021, 64, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Hilmioğlu-Polat, S.; Daneshnia, F.; Pan, W.; Hafez, A.; Fang, W.; Liao, W.; Şahbudak-Bal, Z.; Metin, D.Y.; de Almeida Júnior, J.N.; et al. Clonal Candidemia Outbreak by Candida parapsilosis Carrying Y132F in Turkey: Evolution of a Persisting Challenge. Front. Cell. Infect. Microbiol. 2021, 22, 676177. [Google Scholar] [CrossRef] [PubMed]
- Arikan-Akdagli, S.; Gülmez, D.; Doğan, Ö.; Çerikçioğlu, N.; Doluca Dereli, M.; Birinci, A.; Yıldıran, Ş.T.; Ener, B.; Öz, Y.; Metin, D.Y.; et al. First multicentre report of in vitro resistance rates in candidaemia isolates in Turkey. J. Glob. Antimicrob. Resist. 2019, 18, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Olshtain-Pops, K.; Krieger, M.; Oren, I.; Bishara, J.; Dan, M.; Wiener-Well, Y.; Weinberger, M.; Zimhony, O.; Chowers, M.; et al. Antibiotic exposure as a risk factor for fluconazole-resistant Candida bloodstream infection. Antimicrob. Agents Chemother. 2012, 56, 2518–2523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebert, C.; Villaran, R.; Tolentino, J.; Best, L.; Boonlayangoor, S.; Pitrak, D.; Lin, M.; Weber, S.G. Prior antimicrobial exposure and the risk for bloodstream infection with fluconazole-non-susceptible Candida strains. Scand. J. Infect. Dis. 2010, 42, 506–509. [Google Scholar] [CrossRef]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18 (Suppl 7), 19–37. [Google Scholar] [CrossRef] [Green Version]
- Country Overview of Antimicrobial Consumption: Greece. 2019. Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/database/country-overview (accessed on 28 October 2021).
- Astvad, K.M.T.; Johansen, H.K.; Røder, B.L.; Rosenvinge, F.S.; Knudsen, J.D.; Lemming, L.; Schønheyder, H.C.; Hare, R.K.; Kristensen, L.; Nielsen, L.; et al. Update from a 12-Year Nationwide Fungemia Surveillance: Increasing Intrinsic and Acquired Resistance Causes Concern. J. Clin. Microbiol. 2018, 56, e01564-17. [Google Scholar] [CrossRef] [Green Version]
- Healey, K.R.; Perlin, D.S. Fungal Resistance to Echinocandins and the MDR Phenomenon in Candida glabrata. J. Fungi 2018, 4, 105. [Google Scholar] [CrossRef]
- Rivero-Menendez, O.; Navarro-Rodriguez, P.; Bernal-Martinez, L.; Martin-Cano, G.; Lopez-Perez, L.; Sanchez-Romero, I.; Perez-Ayala, A.; Capilla, J.; Zaragoza, O.; Alastruey-Izquierdo, A. Clinical and Laboratory Development of Echinocandin Resistance in Candida glabrata: Molecular Characterization. Front. Microbiol. 2019, 10, 1585. [Google Scholar] [CrossRef] [Green Version]
- Perlin, D.S. Mechanisms of echinocandin antifungal drug resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 1–11. [Google Scholar] [CrossRef]
- Farmakiotis, D.; Tarrand, J.J.; Kontoyiannis, D.P. Drug-resistant Candida glabrata infection in cancer patients. Emerg. Infect. Dis. 2014, 20, 1833–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jiménez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing Echinocandin Resistance in Candida glabrata: Clinical Failure Correlates With Presence of FKS Mutations and Elevated Minimum Inhibitory Concentrations. Clin. Infect. Dis. An Off. Publ. Infect. Dis. Soc. Am. 2013, 56, 1724–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; McGuire, T.M.; Hollingworth, S.A.; Dong, Y.; Driel, M.L. Van Antifungal agents for invasive candidiasis in non-neutropenic critically ill adults: What do the guidelines recommend? Int. J. Infect. Dis. 2019, 89, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klepser, M. The value of amphotericin B in the treatment of invasive fungal infections. J. Crit. Care 2011, 26, 225-e1. [Google Scholar] [CrossRef]
- Kuse, E.R.; Chetchotisakd, P.; da Cunha, C.A.; Ruhnke, M.; Barrios, C.; Raghunadharao, D.; Sekhon, J.S.; Freire, A.; Ramasubramanian, V.; Demeyer, I.; et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007, 369, 1519–1527. [Google Scholar] [CrossRef]

| Candida spp. and Antifungal Agent | No of Isolates | Clinical Breakpoints * | ECVs/ECOFFs * | |||
|---|---|---|---|---|---|---|
| S | I/SDD | R | WT | Non-WT | ||
| No (%) | No (%) | No (%) | No (%) | No (%) | ||
| C. albicans | ||||||
| Anidulafungin | 724 | 724 (100) | 0 (0) | 0 (0) | - | - |
| Caspofungin | 1883 | 1794 (95) | 24 (1) | 50 (3) | 15 (1) | - |
| Micafungin | 1883 | 1846 (98) | 25 (1) | 12 (1) | - | - |
| Flucytosine | 1868 | - | - | - | 1868 (100) | 0 (0) |
| Fluconazole | 1883 | 1793 (95) | 41 (2) | 49 (3) | - | - |
| Itraconazole | 709 | - | - | - | 681 (96) | 28 (4) |
| Posaconazole | 724 | 15 (2) | - | - | 690 (95) | 19 (3) |
| Voriconazole | 1883 | 1732 (92) | 94 (5) | 57 (3) | - | - |
| Amphotericin B | 1883 | - | - | - | 1883 (100) | 0 (0) |
| C. parapsilosis SC | ||||||
| Anidulafungin | 396 | 396 (100) | 0 (0) | 0 (0) | - | - |
| Caspofungin | 2216 | 2216 (100) | 0 (0) | 0 (0) | - | - |
| Micafungin | 2216 | 2216 (100) | 0 (0) | 0 (0) | - | - |
| Flucytosine | 2189 | - | - | - | 2189 (100) | 0 (0) |
| Fluconazole | 2216 | 1717 (78) | 58 (2) | 441 (20) | - | - |
| Itraconazole | 369 | - | - | - | 342 (93) | 27 (7) |
| Posaconazole | 369 | - | - | - | 376 (95) | 20 (5) |
| Voriconazole | 2216 | 2027 (92) | 163 (7) | 26 (1) | - | - |
| Amphotericin B | 2216 | - | - | - | 2216 (100) | 0 (0) |
| C. glabrata SC | ||||||
| Anidulafungin | 203 | 196 (97) | 0 (0) | 7 (3) | - | - |
| Caspofungin | 500 | 486 (97) | 5 (1) | 9 (2) | - | - |
| Micafungin | 500 | 485 (97) | 3 (1) | 12 (2) | - | - |
| Flucytosine | 500 | - | - | - | 500 (100) | 0 (0) |
| Fluconazole | 500 | 477 (95) | 23 (5) | - | - | |
| Itraconazole | 203 | - | - | - | 188 (93) | 15 (7) |
| Posaconazole | 203 | - | - | - | 179 (88) | 24 (12) |
| Voriconazole | 500 | - | - | - | 470 (94) | 30 (6) |
| Amphotericin B | 500 | - | - | - | 500 (100) | 0 (0) |
| C. tropicalis | ||||||
| Anidulafungin | 75 | 75 (100) | 0 (0) | 0 (0) | - | - |
| Caspofungin | 373 | 366 (98) | 0 (0) | 7 (2) | - | - |
| Micafungin | 373 | 370 (99) | 0 (0) | 3 (1) | - | - |
| Flucytosine | 373 | - | - | - | 373 (100) | 0 (0) |
| Fluconazole | 373 | 322 (86) | 28 (8) | 23 (6) | - | - |
| Itraconazole | 75 | - | - | - | 75 (100) | 0 (0) |
| Posaconazole | 75 | - | - | - | 75 (100) | 0 (0) |
| Voriconazole | 373 | 330 (88) | 38 (10) | 5 (1) | - | - |
| Amphotericin B | 373 | - | - | - | 373 (100) | 0 (0) |
| C. krusei | ||||||
| Anidulafungin | 33 | 33(100) | 0 (0) | 0 (0) | - | - |
| Caspofungin | 77 | 74 (96) | 1 (1) | 2 (3) | - | - |
| Micafungin | 77 | 77 (100) | 0 (0) | 0 (0) | - | - |
| Flucytosine | 77 | - | - | - | 77 (100) | 0 (0) |
| Fluconazole | 77 | - | - | - | 77 (100) | 0 (0) |
| Itraconazole | 33 | - | - | - | 33 (100) | 0 (0) |
| Posaconazole | 33 | - | - | - | 33 (100) | 0 (0) |
| Voriconazole | 77 | 77 (100) | 0 (0) | 0 (0) | - | - |
| Amphotericin B | 77 | - | - | - | 77 (100) | 0 (0) |
| Total | ||||||
| Anidulafungin | 1431 | 1424 (99.5) | 0 (0) | 7 (0.5) | ||
| Caspofungin | 5049 | 4928 (97.6) | 38 (0.8) | 68 (1.3) | 15 (0.3) | |
| Micafungin | 5049 | 4981 (98.7) | 41 (0.8) | 27 (0.5) | ||
| Flucytosine | 5007 | 5007 (100) | 0 (0) | |||
| Fluconazole | 5049 | 3832 (75.9) | 604 (12) | 536 (10.6) | 77 (1.5) | |
| Itraconazole | 1389 | 1319 (95) | 70 (5) | |||
| Posaconazole | 1431 | 15 (1) | 1353 (94.6) | 63 (4.4) | ||
| Voriconazole | 5049 | 4166 (82.6) | 295 (5.8) | 88 (1.7) | 470 (9.3) | 30 (0.6) |
| Amphotericin B | 5049 | 5049 (100) | 0 (0) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamali, V.; Siopi, M.; Charpantidis, S.; Samonis, G.; Tsakris, A.; Vrioni, G.; on behalf of the Candi-Candi Network. Increasing Incidence and Shifting Epidemiology of Candidemia in Greece: Results from the First Nationwide 10-Year Survey. J. Fungi 2022, 8, 116. https://doi.org/10.3390/jof8020116
Mamali V, Siopi M, Charpantidis S, Samonis G, Tsakris A, Vrioni G, on behalf of the Candi-Candi Network. Increasing Incidence and Shifting Epidemiology of Candidemia in Greece: Results from the First Nationwide 10-Year Survey. Journal of Fungi. 2022; 8(2):116. https://doi.org/10.3390/jof8020116
Chicago/Turabian StyleMamali, Vasiliki, Maria Siopi, Stefanos Charpantidis, George Samonis, Athanasios Tsakris, Georgia Vrioni, and on behalf of the Candi-Candi Network. 2022. "Increasing Incidence and Shifting Epidemiology of Candidemia in Greece: Results from the First Nationwide 10-Year Survey" Journal of Fungi 8, no. 2: 116. https://doi.org/10.3390/jof8020116







