Soil Fungal Community Composition and Diversity of Culturable Endophytic Fungi from Plant Roots in the Reclaimed Area of the Eastern Coast of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Physicochemical Analysis of the Soil Samples
2.3. Isolation of Endophytic Fungi
2.4. Identification of Isolated Fungi
2.5. High-Throughput Sequencing and Data Processing
2.6. Statistical Analysis
3. Results and Discussion
3.1. Soil Physicochemical Properties
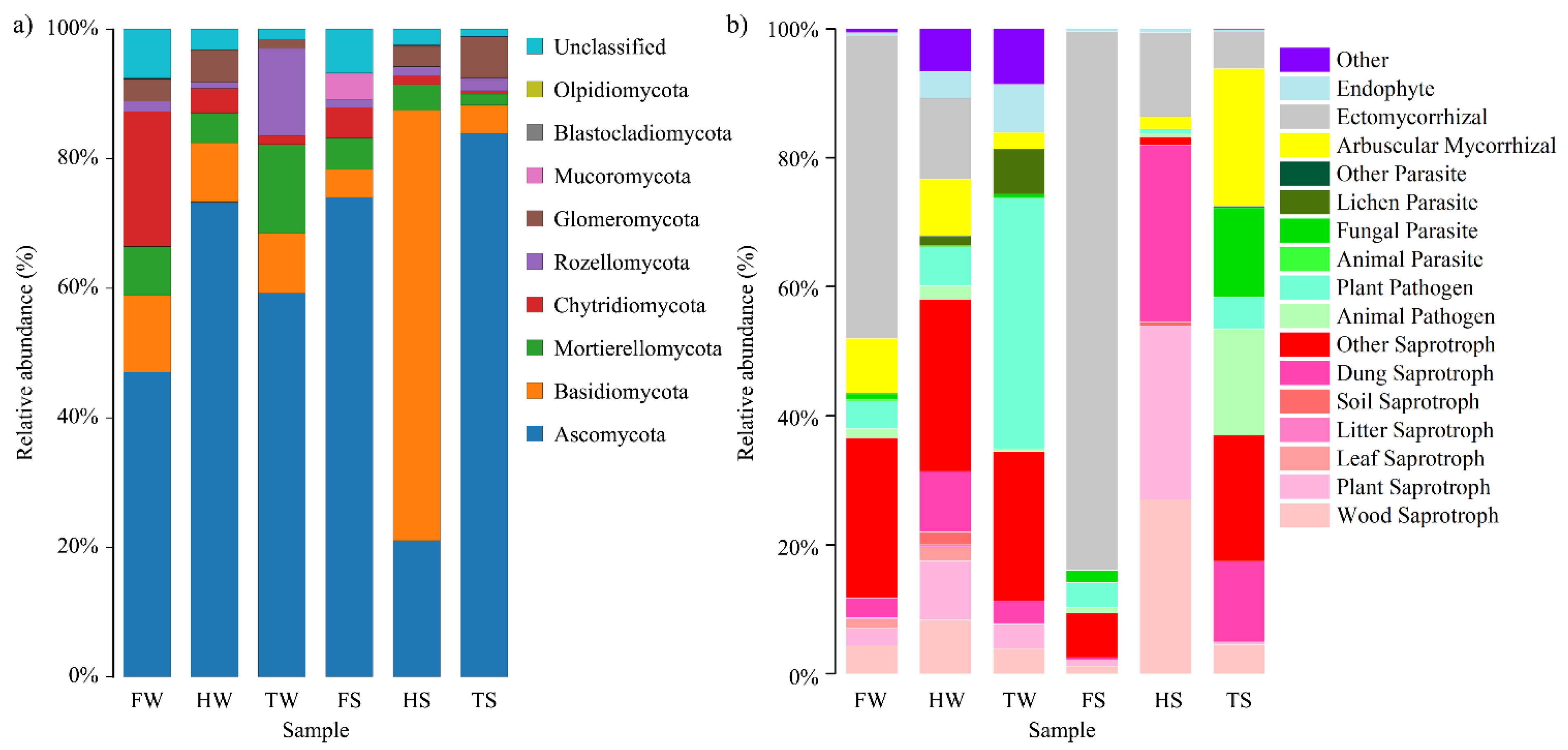
3.2. Soil Fungal Diversity and Community Structure
3.3. Isolation of Endophytic Fungi from Plant Roots
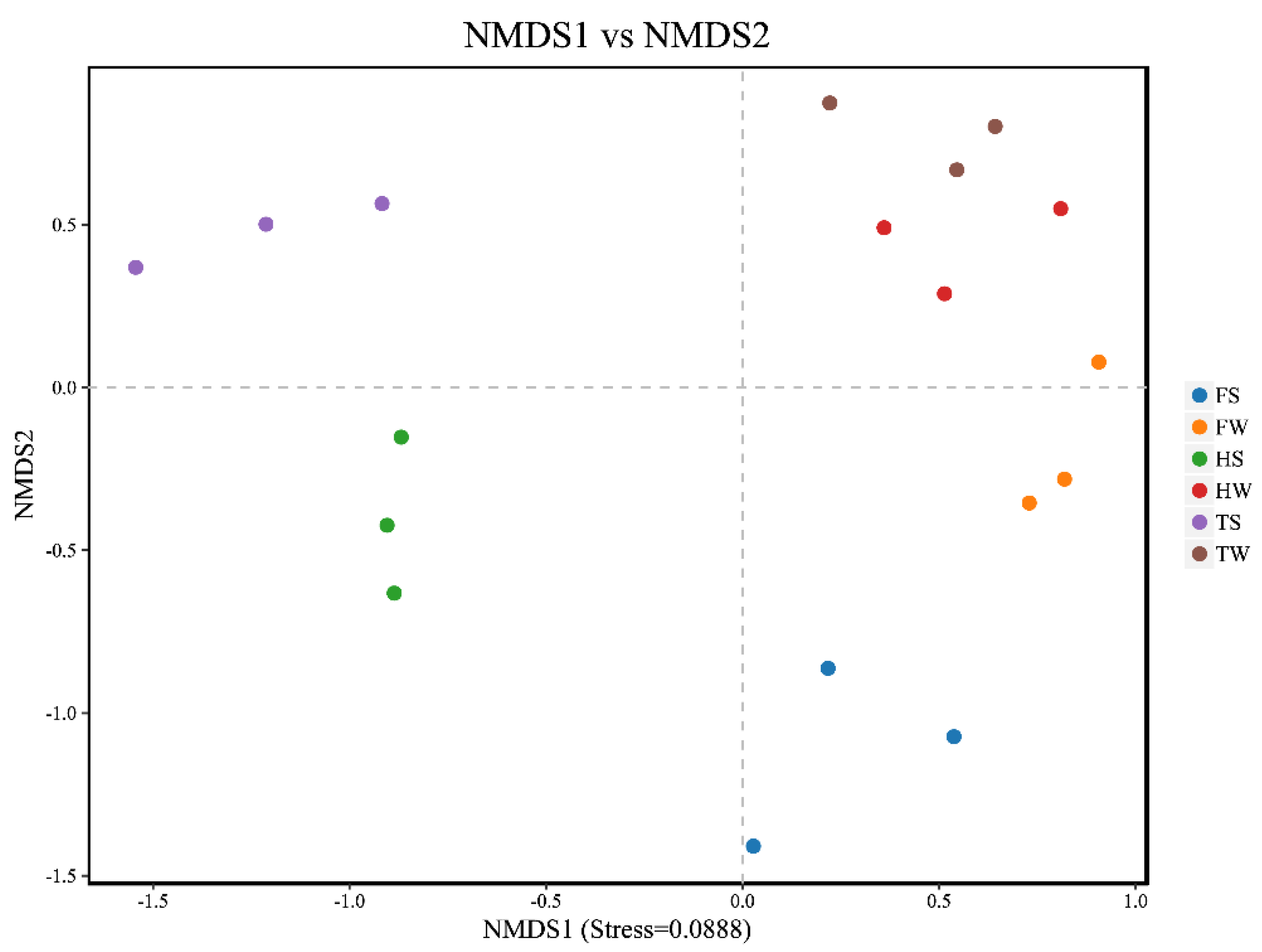
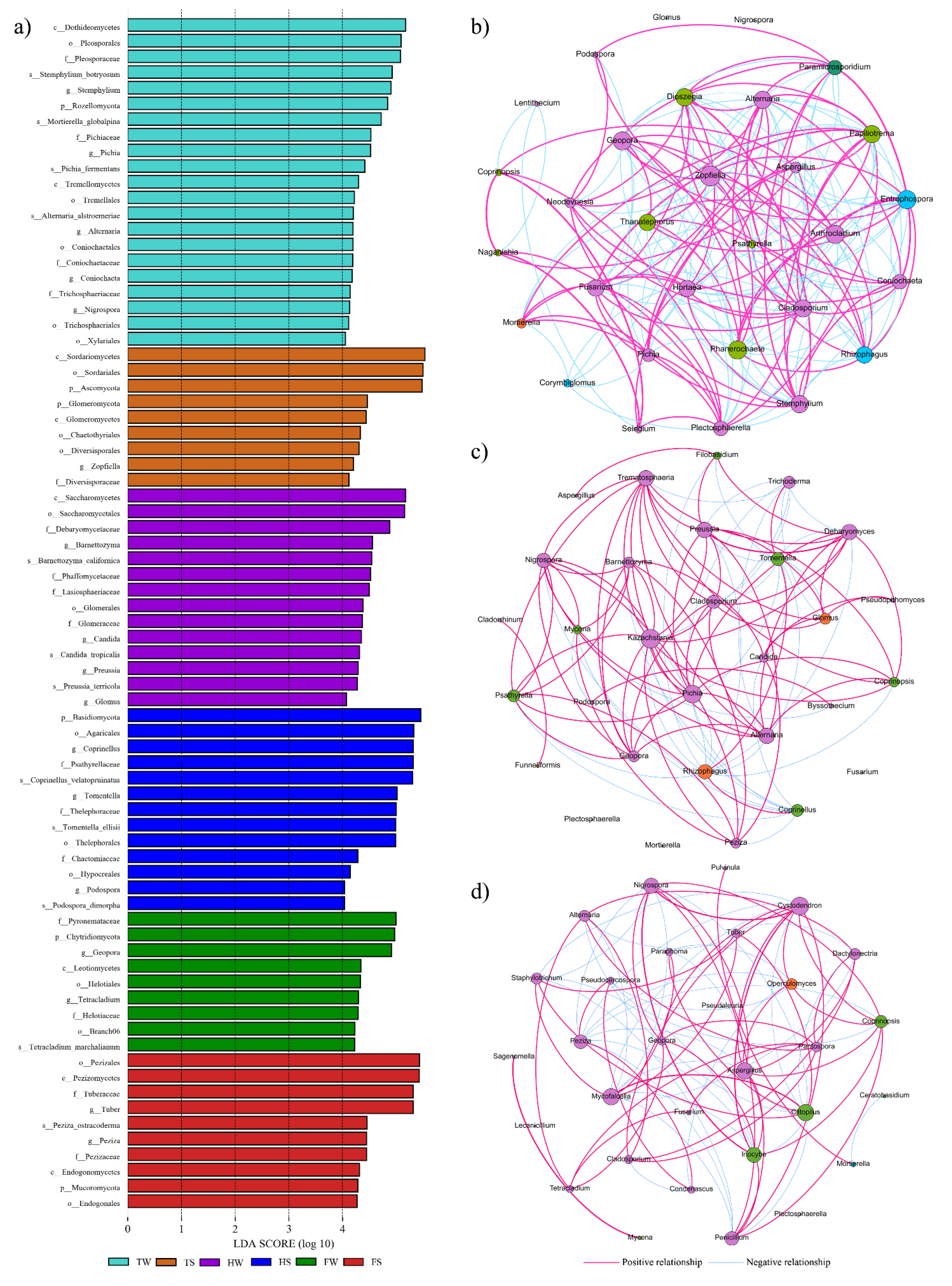
3.4. Spatial and Temporal Variation of Soil Fungal Communities
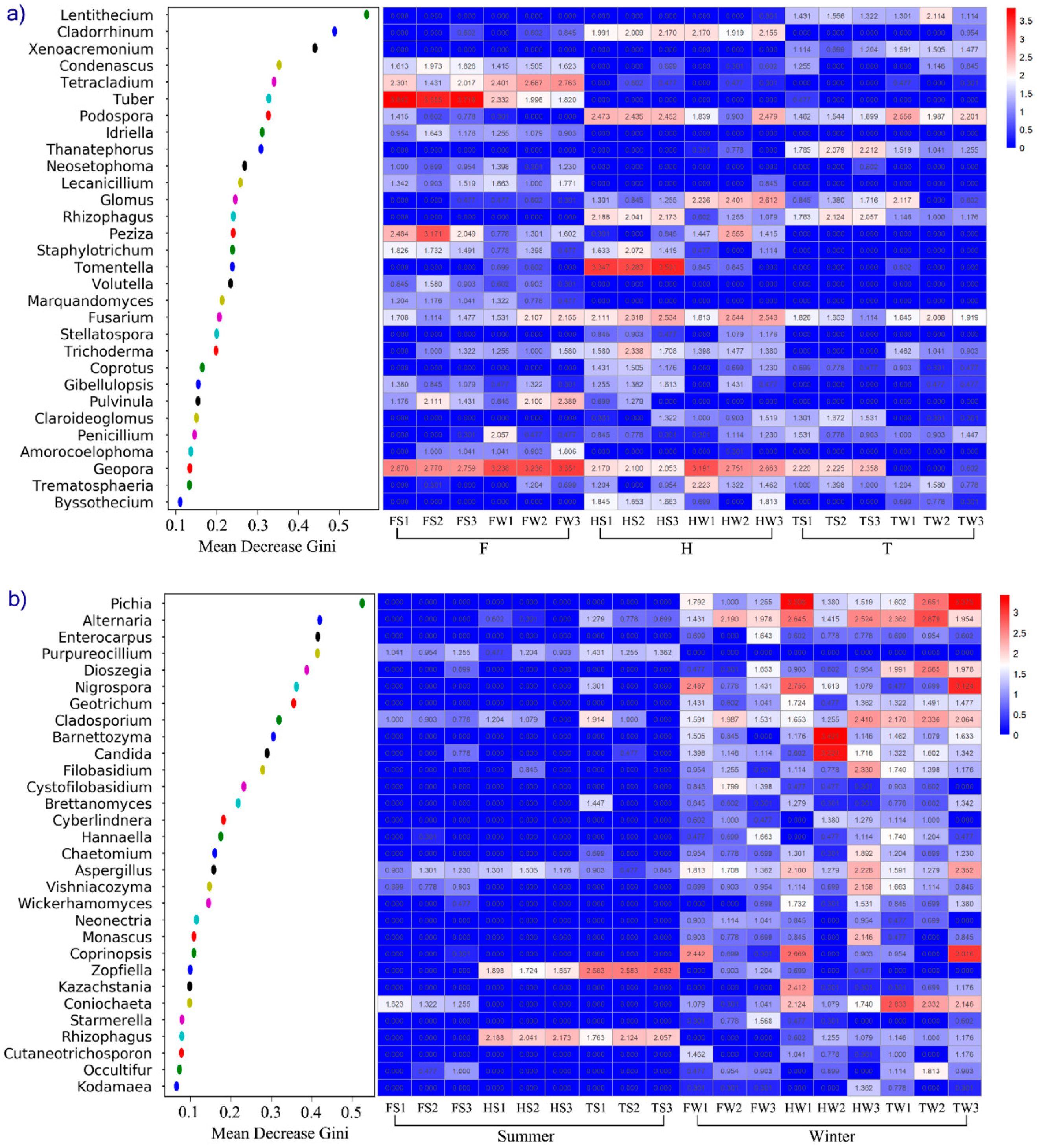
3.5. The Relationship between Fungi Genera and Soil Physical-Chemical Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Liao, X.; Huang, L.; Shan, Q.; Hu, A.; Yan, D.; Zhang, J.; Long, X.E. Soil profile rather than reclamation time drives the mudflat soil microbial community in the wheat-maize rotation system of Nantong, China. J. Soils Sediments 2021, 21, 1672–1687. [Google Scholar] [CrossRef]
- Li, J.; Pu, L.; Zhu, M.; Zhang, J.; Li, P.; Dai, X.; Xu, Y.; Liu, L. Evolution of soil properties following reclamation in coastal areas: A review. Geoderma 2014, 226–227, 130–139. [Google Scholar] [CrossRef]
- Chung, C.H.; Zhuo, R.Z.; Xu, G.W. Creation of Spartina plantations for reclaiming Dongtai, China, tidal flats and offshore sands. Ecol. Eng. 2004, 23, 135–150. [Google Scholar] [CrossRef]
- Cui, X.; Hu, J.; Wang, J.; Yang, J.; Lin, X. Reclamation negatively influences arbuscular mycorrhizal fungal community structure and diversity in coastal saline-alkaline land in Eastern China as revealed by Illumina sequencing. Appl. Soil Ecol. 2016, 98, 140–149. [Google Scholar] [CrossRef]
- Rozema, J.; Flowers, T. Ecology crops for a salinized world. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef]
- Colmer, T.D.; Pedersen, O.; Wetson, A.M.; Flowers, T.J. Oxygen dynamics in a salt-marsh soil and in Suaeda maritima during tidal submergence. Environ. Exp. Bot. 2013, 92, 73–82. [Google Scholar] [CrossRef]
- Li, J.L.; Sun, X.; Zheng, Y.; Lu, P.P.; Wang, Y.L.; Guo, L.D. Diversity and community of culturable endophytic fungi from stems and roots of desert halophytes in northwest China. MycoKeys 2020, 62, 75–95. [Google Scholar] [CrossRef]
- Teste, F.P.; Jones, M.D.; Dickie, I.A. Dual-mycorrhizal plants: Their ecology and relevance. New Phytol. 2020, 225, 1835–1851. [Google Scholar] [CrossRef] [Green Version]
- Wilgan, R. Dual and Tripartite Symbiosis of Invasive Woody Plants. In Symbiotic Soil Microorganisms; Shrivastava, N., Mahajan, S., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 87–97. [Google Scholar]
- Thiem, D.; Golebiewski, M.; Hulisz, P.; Piernik, A.; Hrynkiewicz, K. How does salinity shape bacterial and fungal microbiomes of Alnus glutinosa roots? Front. Microbiol. 2018, 9, 651. [Google Scholar] [CrossRef]
- Xu, F.; Cai, T.; Yang, X.; Sui, W. Soil fungal community variation by large-scale reclamation in Sanjiang plain, China. Ann. Microbiol. 2017, 67, 679–689. [Google Scholar] [CrossRef]
- Fernandes, E.G.; Pereira, O.L.; da Silva, C.C.; Bento, C.B.; de Queiroz, M.V. Diversity of endophytic fungi in Glycine max. Microbiol. Res. 2015, 181, 84–92. [Google Scholar] [CrossRef]
- Gonçalves, D.R.; Pena, R.; Zotz, G.; Albach, D.C. Effects of fungal inoculation on the growth of Salicornia (Amaranthaceae) under different salinity conditions. Symbiosis 2021, 84, 195–208. [Google Scholar] [CrossRef]
- Wang, F.Y.; Liu, R.J.; Lin, X.G.; Zhou, J.M. Arbuscular mycorrhizal status of wild plants in saline-alkaline soils of the Yellow River Delta. Mycorrhiza 2004, 14, 133–137. [Google Scholar]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant. Sci. 2017, 8, 1739. [Google Scholar] [CrossRef]
- Cely, M.V.; de Oliveira, A.G.; de Freitas, V.F.; de Luca, M.B.; Barazetti, A.R.; Dos Santos, I.M.; Gionco, B.; Garcia, G.V.; Prete, C.E.; Andrade, G. Inoculant of arbuscular mycorrhizal fungi (Rhizophagus clarus) increase yield of soybean and cotton under field conditions. Front. Microbiol. 2016, 7, 720. [Google Scholar] [CrossRef]
- Verbruggen, E.; van der Heijden, M.G.; Rillig, M.C.; Kiers, E.T. Mycorrhizal fungal establishment in agricultural soils: Factors determining inoculation success. New Phytol. 2013, 197, 1104–1109. [Google Scholar] [CrossRef] [Green Version]
- Fontana, D.C.; de Paula, S.; Torres, A.G.; de Souza, V.H.M.; Pascholati, S.F.; Schmidt, D.; Neto, D.D. Endophytic fungi: Biological control and induced resistance to phytopathogens and abiotic stresses. Pathogens 2021, 10, 570. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttila, A.M.; Compant, S.; Campisano, A.; Doring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [Green Version]
- Botnen, S.; Kauserud, H.; Carlsen, T.; Blaalid, R.; Høiland, K. Mycorrhizal fungal communities in coastal sand dunes and heaths investigated by pyrosequencing analyses. Mycorrhiza 2015, 25, 447–456. [Google Scholar] [CrossRef]
- Thiem, D.; Piernik, A.; Hrynkiewicz, K. Ectomycorrhizal and endophytic fungi associated with Alnus glutinosa growing in a saline area of central Poland. Symbiosis 2017, 75, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Johansen, R.B.; Johnston, P.; Mieczkowski, P.; Perry, P.L.W.; Robeson, M.S.; Burns, B.R.; Vilgalys, R. A native and an invasive dune grass share similar, patchily distributed, root-associated fungal communities. Fungal Ecol. 2016, 23, 141–155. [Google Scholar] [CrossRef]
- Simoes, M.F.; Antunes, A.; Ottoni, C.A.; Amini, M.S.; Alam, I.; Alzubaidy, H.; Mokhtar, N.A.; Archer, J.A.C.; Bajic, V.B. Soil and rhizosphere associated fungi in gray mangroves (Avicennia marina) from the red sea—A metagenomic approach. Genom. Proteom. Bioinf. 2015, 13, 310–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Qiu, Q.; Yang, Z.; Hu, Z.; Tam, N.F.Y.; Xin, G. Arbuscular mycorrhizal fungi in two mangroves in South China. Plant Soil 2009, 331, 181–191. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Tebbe, C.C.; Huang, L.; Jia, J.; Wang, W.; Wang, X.; Yu, L.; Zhao, Q. Spartina alterniflora invasions reduce soil fungal diversity and simplify co-occurrence networks in a salt marsh ecosystem. Sci. Total Environ. 2021, 758, 143667. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Maunoury, L.; Deveau, A.; Moreno, M.; Todesco, F.; Belmondo, S.; Murat, C.; Courty, P.E.; Jkalski, M.; Selosse, M.A. Two ectomycorrhizal truffles, Tuber melanosporum and T. aestivum, endophytically colonise roots of non-ectomycorrhizal plants in natural environments. New Phytol. 2020, 225, 2542–2556. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Xiao, R.; Zhang, K.; Gao, H.; Cui, B.; Liu, X. Soil organic carbon as affected by land use in young and old reclaimed regions of a coastal estuary wetland, China. Soil Use Manag. 2013, 29, 57–64. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Wulf, M.; Rillig, M.C. Facilitation between woody and herbaceous plants that associate with arbuscular mycorrhizal fungi in temperate European forests. Ecol. Evol. 2017, 7, 1181–1189. [Google Scholar] [CrossRef]
- Puttsepp, U.; Roslingd, A.; Taylor, A.F.S. Ectomycorrhizal fungal communities associated with Salix viminalis L. and S. dasyclados wimm. clones in a short-rotation forestry plantation. Forest Ecol. Manag. 2004, 196, 413–424. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef]
- He, C.; Wang, W.; Hou, J.; Li, X. Dark septate endophytes isolated from wild licorice roots grown in the desert regions of northwest China enhance the growth of host plants under water deficit stress. Front. Microbiol. 2021, 12, 522449. [Google Scholar] [CrossRef] [PubMed]
- Barrow, J.R.; Osuna, P. Phosphorus solubilization and uptake by dark septate fungi in fourwing saltbush, Atriplex canescens (Pursh) Nutt. J. Arid Environ. 2002, 51, 449–459. [Google Scholar] [CrossRef]
- Li, X.; He, C.; He, X.; Su, F.; Hou, L.; Ren, Y.; Hou, Y. Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development. Plant Soil 2019, 439, 259–272. [Google Scholar] [CrossRef]
- He, C.; Wang, W.; Hou, J. Plant performance of enhancing licorice with dual inoculating dark septate endophytes and Trichoderma viride mediated via effects on root development. BMC Plant Biol. 2020, 20, 325. [Google Scholar] [CrossRef] [PubMed]
- Newsham, K.K. Phialophora graminicola, a dark septate fungus, is a beneficial associate of the grass Vulpia ciliata ssp. Ambigua. New Phytol. 1999, 144, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Ghosh, R.; Manda, N.C. Production of bioactive compounds with bactericidal and antioxidant potential by endophytic fungus Alternaria alternata AE1 isolated from Azadirachta indica A. Juss. PLoS ONE 2019, 14, e0214744. [Google Scholar] [CrossRef] [Green Version]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef]
- Smolyanyuk, E.V.; Bilanenko, E.N. Communities of halotolerant micromycetes from the areas of natural salinity. Microbiology 2011, 80, 877–883. [Google Scholar] [CrossRef]
- Yamagiwa, Y.; Inagaki, Y.; Ichinose, Y.; Toyoda, K.; Hyakumachi, M.; Shiraishi, T. Talaromyces wortmannii FS2 emits β-caryphyllene, which promotes plant growth and induces resistance. J. Gen. Plant Pathol. 2011, 77, 336–341. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella Species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- Dean, R.; Kan, J.A.L.V.; Pretorius, Z.A.; Hammond-kosack, K.E.; Pietro, A.D.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crous, P.W.; Hawksworth, D.L.; Wingfield, M.J. Identifying and naming plant-pathogenic fungi: Past, present, and future. Annu. Rev. Phytopathol. 2015, 53, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant. Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef]
- Shen, S.Y.; Fulthorpe, R. Seasonal variation of bacterial endophytes in urban trees. Front. Microbiol. 2015, 6, 427. [Google Scholar] [CrossRef] [PubMed]
- Hrynkiewicz, K.; Szymanska, S.; Piernik, A.; Thiem, D. Ectomycorrhizal community structure of Salix and Betula spp. at a saline site in central Poland in relation to the seasons and soil parameters. Water Air Soil Pollut. 2015, 226, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Shan, X.; Yang, Y.; Wang, Y.; Druzhinina, I.S.; Pan, X.; Jin, W.; He, X.; Wang, X.; Zhang, X.; et al. Facultative symbiosis with a saprotrophic soil fungus promotes potassium uptake in American sweetgum trees. Plant Cell Environ. 2021, 44, 2793–2809. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Y.; Xie, L.; Liu, X.; Wei, Y.; Zhou, X.; Zhang, S. A ribosomal protein AgRPS3aE from halophilic Aspergillus glaucus confers salt tolerance in heterologous organisms. Int. J. Mol. Sci. 2015, 16, 3058–3070. [Google Scholar] [CrossRef] [Green Version]
- Sati, S.C.; Pant, P. Evaluation of phosphate solubilization by root endophytic aquatic Hyphomycete Tetracladium setigerum. Symbiosis 2018, 77, 141–145. [Google Scholar] [CrossRef]
- Házi, J.; Nagy, L.G.; Vágvölgyi, C.; Papp, T. Coprinellus radicellus, a new species with northern distribution. Mycol Prog. 2010, 10, 363–371. [Google Scholar] [CrossRef]
- Massimo, N.C.; Devan, M.M.N.; Arendt, K.R.; Wilch, M.H.; Riddle, J.M.; Furr, S.H.; Steen, C.; U’Ren, J.M.; Sandberg, D.C.; Arnold, A.E. Fungal endophytes in aboveground tissues of desert plants: Infrequent in culture, but highly diverse and distinctive symbionts. Microb. Ecol. 2015, 70, 61–76. [Google Scholar] [CrossRef]
- Paoletti, M.; Saupe, S.J. The genome sequence of Podospora anserina, a classic model fungus. Genome Biol. 2008, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Hernández, M.V.; Arévalo-Galarza, L.; Jaen-Contreras, D.; Escamilla-García, J.L.; Mora-Aguilera, A.; Hernández-Castro, E.; Cibrián-Tovar, J.; Téliz-Ortiz, D. Effect of Glomus mosseae and Entrophospora colombiana on plant growth, production, and fruit quality of ‘Maradol’ papaya (Carica papaya L.). Sci. Hortic. 2011, 128, 255–260. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiori, S.; Scherm, B.; Liu, J.; Farrell, R.; Mannazzu, I.; Budroni, M.; Maserti, B.E.; Wisniewski, M.E.; Migheli, Q. Identification of differentially expressed genes associated with changes in the morphology of Pichia fermentans on apple and peach fruit. FEMS Yeast Res. 2012, 12, 785–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limtong, S.; Yongmanitchai, W.; Tun, M.M.; Kawasaki, H.; Seki, T. Kazachstania siamensis sp. nov., an ascomycetous yeast species from forest soil in Thailand. Int. J. Syst. Evol. Microbiol. 2007, 57, 419–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spadaro, D.; Vola, R.; Piano, S.; Gullino, M.L. Mechanisms of action and efficacy of four isolates of the yeast Metschnikowia pulcherrima active against postharvest pathogens on apples. Postharvest Biol. Technol. 2002, 24, 123–134. [Google Scholar] [CrossRef]
- Amprayn, K.O.; Rose, M.T.; Kecskés, M.; Pereg, L.; Nguyen, H.T.; Kennedy, I.R. Plant growth promoting characteristics of soil yeast (Candida tropicalis HY) and its effectiveness for promoting rice growth. Appl. Soil Ecol. 2012, 61, 295–299. [Google Scholar] [CrossRef]
- Saunders, M.; Kohn, L.M. Host-synthesized secondary compounds influence the in vitro interactions between fungal endophytes of maize. Appl. Environ. Microbiol. 2008, 74, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Geml, J.; Gravendeel, B.; van der Gaag, K.J.; Neilen, M.; Lammers, Y.; Raes, N.; Semenova, T.A.; de Knijff, P.; Noordeloos, M.E. The contribution of DNA metabarcoding to fungal conservation: Diversity assessment, habitat partitioning and mapping red-Listed fungi in protected coastal Salix repens communities in the Netherlands. PLoS ONE 2014, 9, e99852. [Google Scholar] [CrossRef]
- Huang, X.; Liu, L.; Wen, T.; Zhu, R.; Zhang, J.; Cai, Z. Illumina MiSeq investigations on the changes of microbial community in the Fusarium oxysporum f.sp. cubense infected soil during and after reductive soil disinfestation. Microbiol. Res. 2015, 181, 33–42. [Google Scholar]
- Kim, J.C.; Choi, G.J.; Park, J.H.; Kim, H.T.; Cho, K.Y. Activity against plant pathogenic fungi of phomalactone isolated from Nigrospora sphaerica. Pest Manag. Sci. 2001, 57, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Kokaew, J.; Manoch, L.; Worapong, J.; Chamswarng, C.; Strobel, G. Coniochaeta ligniaria an endophytic fungus from Baeckea frutescens and its antagonistic effects against plant pathogenic fungi. Thai J. Agric. Sci. 2011, 44, 123–131. [Google Scholar]
- Franco, M.E.E.; Lopez, S.M.Y.; Medina, R.; Lucentini, C.G.; Troncozo, M.I.; Pastorino, G.N.; Saparrat, M.C.N.; Balatti, P.A. The mitochondrial genome of the plant-pathogenic fungus Stemphylium lycopersici uncovers a dynamic structure due to repetitive and mobile elements. PLoS ONE 2017, 12, e0185545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logrieco, A.; Moretti, A.; Solfrizzo, M. Alternaria toxins and plant diseases: An overview of origin, occurrence and risks. World Mycotoxin J. 2009, 2, 129–140. [Google Scholar] [CrossRef]
- Spadaro, J.T.; Gold, M.H.; Renganathan, V. Degradation of azo dyes by the lignin-degrading fungus Phanerochaete chrysosporium. Appl. Environ. Microbiol. 1992, 58, 2397–2401. [Google Scholar] [CrossRef] [Green Version]
- Nicolás, C.; Bertelsen, T.M.; Floudas, D.; Bentzer, J.; Smits, M.; Johansson, T.; Troein, C.; Persson, P.; Tunlid, A. The soil organic matter decomposition mechanisms in ectomycorrhizal fungi are tuned for liberating soil organic nitrogen. ISME J. 2019, 13, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhou, X.; Jiang, A.; Fan, J.; Lan, T.; Zhang, J.; Cai, Z. Distinct impacts of reductive soil disinfestation and chemical soil disinfestation on soil fungal communities and memberships. Appl. Microbiol. Biotechnol. 2018, 102, 7623–7634. [Google Scholar] [CrossRef]
- Liu, K.; Ding, X.; Wang, H.F.; Zhang, X.; Hozzein, W.N.; Wadaan, M.A.; Lan, A.; Zhang, B.; Li, W. Eukaryotic microbial communities in hypersaline soils and sediments from the alkaline hypersaline Huama Lake as revealed by 454 pyrosequencing. Antonie Leeuwenhoek 2014, 105, 871–880. [Google Scholar] [CrossRef]
- Birkhofer, K.; Schoning, I.; Alt, F.; Herold, N.; Klarner, B.; Maraun, M.; Marhan, S.; Oelmann, Y.; Wubet, T.; Yurkov, A.; et al. General relationships between abiotic soil properties and soil biota across spatial scales and different land-use types. PLoS ONE 2012, 7, e43292. [Google Scholar] [CrossRef]
- Tian, C.Y.; Feng, G.; Zhang, F.S. Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline soil on salinity tolerance of plants. Appl. Soil Ecol. 2004, 26, 143–148. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Aldubise, A.; Egamberdieva, D. Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J. Plant Interact. 2015, 10, 230–242. [Google Scholar] [CrossRef] [Green Version]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. 2012, 32, 181–200. [Google Scholar] [CrossRef] [Green Version]

| Sampling Sites | F | H | T | |||
|---|---|---|---|---|---|---|
| Latitude/Longitude | 32.603° N, 120.880° E | 32.609° N, 120.933° E | 32.155° N, 121.475° E | |||
| Vegetation Information | a dense tree canopy | a sparse tree layer and dense herb and shrub layers | sparse tree, herb and shrub layers | |||
| Soil properties | ||||||
| Seasons | Summer | Winter | Summer | Winter | Summer | Winter |
| pH | 9.58 ± 0.05 | 8.84 ± 0.24 | 9.53 ± 0.27 | 8.92 ± 0.03 | 9.79 ± 0.17 | 8.70 ± 0.17 |
| EC (μS/cm) | 104.3 ± 33.2 | 129.3 ± 26.1 | 619.7 ± 217.9 | 2668.0 ± 321.2 | 384.8 ± 45.4 | 1005.0 ± 136.4 |
| SN (%) | 0.07 ± 0.02 | 0.12 ± 0.02 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.04 ± 0.01 | 0.05 ± 0.01 |
| SC (%) | 1.44 ± 0.19 | 1.80 ± 0.12 | 1.25 ± 0.06 | 1.32 ± 0.09 | 1.23 ± 0.03 | 1.17 ± 0.05 |
| Sampling Sites | Seasons | Diversity Index | Richness Index | ||
|---|---|---|---|---|---|
| Shannon | Simpson | ACE | Chao1 | ||
| F | Summer | 3.901 ± 0.312 | 0.750 ± 0.061 | 192.310 ± 22.377 | 194.241 ± 22.156 |
| Winter | 5.599 ± 0.129 | 0.947 ± 0.010 | 306.042 ± 21.626 | 309.471 ± 23.004 | |
| H | Summer | 3.446 ± 0.030 | 0.747 ± 0.009 | 142.355 ± 5.127 | 144.526 ± 4.278 |
| Winter | 4.959 ± 0.598 | 0.913 ± 0.037 | 269.587 ± 11.640 | 271.892 ± 8.320 | |
| T | Summer | 2.653 ± 0.135 | 0.535 ± 0.028 | 121.253 ± 11.840 | 121.558 ± 12.478 |
| Winter | 4.778 ± 0.401 | 0.906 ± 0.021 | 238.112 ± 33.987 | 240.534 ± 34.843 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, F.; Fan, X.; Ji, W.; Hai, Z.; Hu, N.; Li, X.; Liu, G.; Yu, C.; Chen, Y.; Lian, B.; et al. Soil Fungal Community Composition and Diversity of Culturable Endophytic Fungi from Plant Roots in the Reclaimed Area of the Eastern Coast of China. J. Fungi 2022, 8, 124. https://doi.org/10.3390/jof8020124
Zhong F, Fan X, Ji W, Hai Z, Hu N, Li X, Liu G, Yu C, Chen Y, Lian B, et al. Soil Fungal Community Composition and Diversity of Culturable Endophytic Fungi from Plant Roots in the Reclaimed Area of the Eastern Coast of China. Journal of Fungi. 2022; 8(2):124. https://doi.org/10.3390/jof8020124
Chicago/Turabian StyleZhong, Fei, Xinlei Fan, Wenhui Ji, Zhixing Hai, Naican Hu, Xintong Li, Guoyuan Liu, Chunmei Yu, Yanhong Chen, Bolin Lian, and et al. 2022. "Soil Fungal Community Composition and Diversity of Culturable Endophytic Fungi from Plant Roots in the Reclaimed Area of the Eastern Coast of China" Journal of Fungi 8, no. 2: 124. https://doi.org/10.3390/jof8020124
APA StyleZhong, F., Fan, X., Ji, W., Hai, Z., Hu, N., Li, X., Liu, G., Yu, C., Chen, Y., Lian, B., Wei, H., & Zhang, J. (2022). Soil Fungal Community Composition and Diversity of Culturable Endophytic Fungi from Plant Roots in the Reclaimed Area of the Eastern Coast of China. Journal of Fungi, 8(2), 124. https://doi.org/10.3390/jof8020124







