Pneumocystis Pneumonia: Pitfalls and Hindrances to Establishing a Reliable Animal Model
Abstract
:1. Introduction
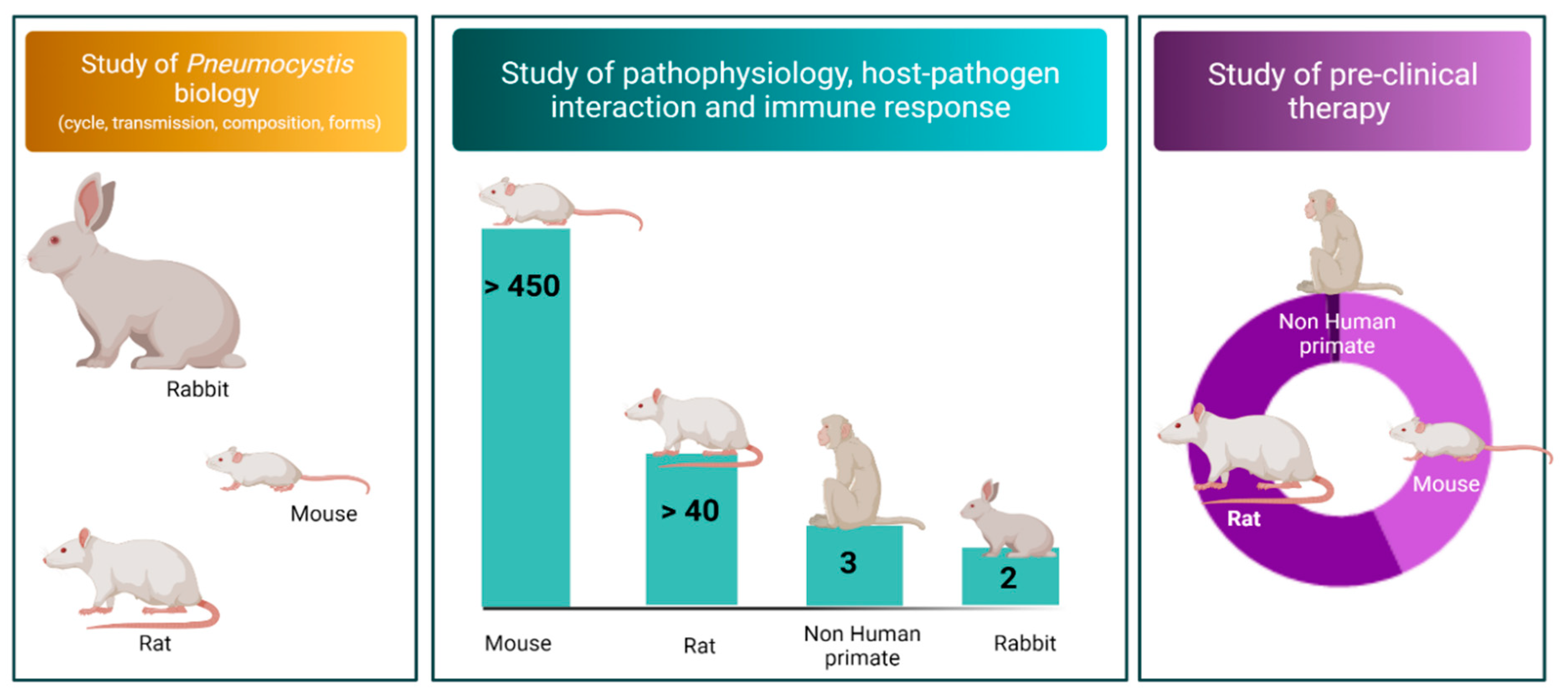
2. General Description of the Various Animal Models: Host Species and Strains, Sex, Weight, and Age
3. Selection of the Regimen Inducing Susceptibility to Pneumocystis Pneumonia
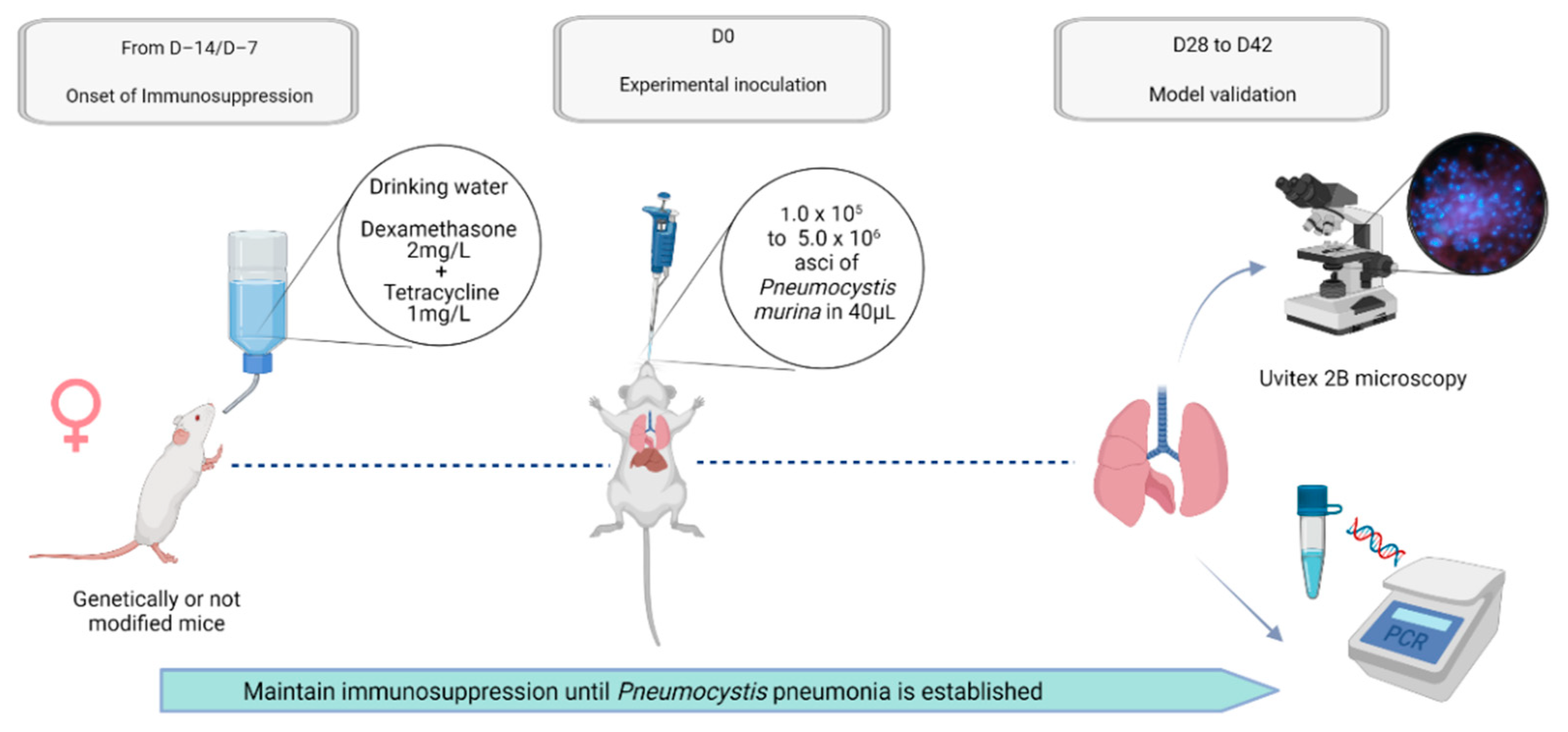
4. Implementation of the Experimental Infection
5. Validation of the Model and Outcome Parameters to Follow Up
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salzer, H.J.F.; Schäfer, G.; Hoenigl, M.; Günther, G.; Hoffmann, C.; Kalsdorf, B.; Alanio, A.; Lange, C. Clinical, Diagnostic, and Treatment Disparities between HIV-Infected and Non-HIV-Infected Immunocompromised Patients with Pneumocystis Jirovecii Pneumonia. Respiration 2018, 96, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Gajdusek, D.C. Pneumocystis Carinii; Etiologic Agent of Interstitial Plasma Cell Pneumonia of Premature and Young Infants. Pediatrics 1957, 19, 543–565. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control (CDC). A Cluster of Kaposi’s Sarcoma and Pneumocystis Carinii Pneumonia among Homosexual Male Residents of Los Angeles and Orange Counties, California. MMWR Morb. Mortal. Wkly. Rep. 1982, 31, 305–307. [Google Scholar]
- Fillatre, P.; Decaux, O.; Jouneau, S.; Revest, M.; Gacouin, A.; Robert-Gangneux, F.; Fresnel, A.; Guiguen, C.; Le Tulzo, Y.; Jégo, P.; et al. Incidence of Pneumocystis Jiroveci Pneumonia among Groups at Risk in HIV-Negative Patients. Am. J. Med. 2014, 127, 1242.e11–1242.e17. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.F.; Limper, A.H. Pneumocystis Pneumonia. N. Engl. J. Med. 2004, 350, 2487–2498. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Asai, N.; Motojima, S.; Ohkuni, Y.; Matsunuma, R.; Nakashima, K.; Iwasaki, T.; Nakashita, T.; Otsuka, Y.; Kaneko, N. Early Diagnosis and Treatment Are Crucial for the Survival of Pneumocystis Pneumonia Patients without Human Immunodeficiency Virus Infection. J. Infect. Chemother. 2012, 18, 898–905. [Google Scholar] [CrossRef]
- Li, M.-C.; Lee, N.-Y.; Lee, C.-C.; Lee, H.-C.; Chang, C.-M.; Ko, W.-C. Pneumocystis Jiroveci Pneumonia in Immunocompromised Patients: Delayed Diagnosis and Poor Outcomes in Non-HIV-Infected Individuals. J. Microbiol. Immunol. Infect. 2014, 47, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Cushion, M.T.; Linke, M.J.; Ashbaugh, A.; Sesterhenn, T.; Collins, M.S.; Lynch, K.; Brubaker, R.; Walzer, P.D. Echinocandin Treatment of Pneumocystis Pneumonia in Rodent Models Depletes Cysts Leaving Trophic Burdens That Cannot Transmit the Infection. PLoS ONE 2010, 5, e8524. [Google Scholar] [CrossRef]
- Martinez, A.; Halliez, M.C.M.; Aliouat, E.M.; Chabé, M.; Standaert-Vitse, A.; Fréalle, E.; Gantois, N.; Pottier, M.; Pinon, A.; Dei-Cas, E.; et al. Growth and Airborne Transmission of Cell-Sorted Life Cycle Stages of Pneumocystis Carinii. PLoS ONE 2013, 8, e79958. [Google Scholar] [CrossRef]
- Limper, A.H.; Martin, W.J. Pneumocystis Carinii: Inhibition of Lung Cell Growth Mediated by Parasite Attachment. J. Clin. Investig. 1990, 85, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.L.; Yajko, D.M.; Hadley, W.K. Extrapulmonary Pneumocystosis. Clin. Microbiol. Rev. 1997, 10, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Desoubeaux, G.; Franck-Martel, C.; Caille, A.; Drillaud, N.; Lestrade Carluer de Kyvon, M.-A.; Bailly, É.; Chandenier, J. Use of Calcofluor-Blue Brightener for the Diagnosis of Pneumocystis Jirovecii Pneumonia in Bronchial-Alveolar Lavage Fluids: A Single-Center Prospective Study. Med. Mycol. 2017, 55, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desoubeaux, G.; Chesnay, A.; Mercier, V.; Bras-Cachinho, J.; Moshiri, P.; Eymieux, S.; De Kyvon, M.-A.; Lemaignen, A.; Goudeau, A.; Bailly, É. Combination of β-(1, 3)-D-Glucan Testing in Serum and QPCR in Nasopharyngeal Aspirate for Facilitated Diagnosis of Pneumocystis Jirovecii Pneumonia. Mycoses 2019, 62, 1015–1022. [Google Scholar] [CrossRef]
- Atzori, C.; Aliouat, E.M.; Bartlett, M.S.; Dujardin, L.; Cargnel, A.; Dei-Cas, E. Current in Vitro Culture Systems for Pneumocystis. FEMS Immunol. Med. Microbiol. 1998, 22, 169–172. [Google Scholar] [CrossRef]
- Schildgen, V.; Mai, S.; Khalfaoui, S.; Lüsebrink, J.; Pieper, M.; Tillmann, R.L.; Brockmann, M.; Schildgen, O. Pneumocystis Jirovecii Can Be Productively Cultured in Differentiated CuFi-8 Airway Cells. mBio 2014, 5, e01186-14. [Google Scholar] [CrossRef] [Green Version]
- Walzer, P.D.; Young, L.S. Clinical Relevance of Animal Models of Pneumocystis Carinii Pneumonia. Diagn. Microbiol. Infect. Dis. 1984, 2, 1–6. [Google Scholar] [CrossRef]
- Ceré, N.; Polack, B. Animal Pneumocystosis: A Model for Man. Vet. Res. 1999, 30, 1–26. [Google Scholar]
- Elsegeiny, W.; Zheng, M.; Eddens, T.; Gallo, R.L.; Dai, G.; Trevejo-Nunez, G.; Castillo, P.; Kracinovsky, K.; Cleveland, H.; Horne, W.; et al. Murine Models of Pneumocystis Infection Recapitulate Human Primary Immune Disorders. JCI Insight 2018, 3, e91894. [Google Scholar] [CrossRef]
- Eddens, T.; Elsegeiny, W.; Ricks, D.; Goodwin, M.; Horne, W.T.; Zheng, M.; Kolls, J.K. Transcriptomic and Proteomic Approaches to Finding Novel Diagnostic and Immunogenic Candidates in Pneumocystis. mSphere 2019, 4, e00488-19. [Google Scholar] [CrossRef] [Green Version]
- Hoy, Z.; Wright, T.W.; Elliott, M.; Malone, J.; Bhagwat, S.; Wang, J.; Gigliotti, F. Combination Immunotherapy with Passive Antibody and Sulfasalazine Accelerates Fungal Clearance and Promotes the Resolution of Pneumocystis-Associated Immunopathogenesis. Infect. Immun. 2020, 88, e00640-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, S.; Samuelson, D.R.; Assouline, B.; Morre, M.; Shellito, J.E. Treatment with Interleukin-7 Restores Host Defense against Pneumocystis in CD4+ T-Lymphocyte-Depleted Mice. Infect. Immun. 2016, 84, 108–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, G.-S.; Zhang, C.; Shao, S.; Jung, H.-W.; Durant, P.J.; Lee, C.-H. All-Trans Retinoic Acid in Combination with Primaquine Clears Pneumocystis Infection. PLoS ONE 2013, 8, e53479. [Google Scholar] [CrossRef] [PubMed]
- Cosio, T.; Gaziano, R.; Zuccari, G.; Costanza, G.; Grelli, S.; Di Francesco, P.; Bianchi, L.; Campione, E. Retinoids in Fungal Infections: From Bench to Bedside. Pharmaceuticals 2021, 14, 962. [Google Scholar] [CrossRef]
- Mouse Genome Sequencing Consortium; Waterston, R.H.; Lindblad-Toh, K.; Birney, E.; Rogers, J.; Abril, J.F.; Agarwal, P.; Agarwala, R.; Ainscough, R.; Alexandersson, M.; et al. Initial Sequencing and Comparative Analysis of the Mouse Genome. Nature 2002, 420, 520–562. [Google Scholar] [CrossRef]
- Furuta, T.; Fujita, M.; Mukai, R.; Sakakibara, I.; Sata, T.; Miki, K.; Hayami, M.; Kojima, S.; Yoshikawa, Y. Severe Pulmonary Pneumocystosis in Simian Acquired Immunodeficiency Syndrome Induced by Simian Immunodeficiency Virus: Its Characterization by the Polymerase-Chain-Reaction Method and Failure of Experimental Transmission to Immunodeficient Animals. Parasitol. Res. 1993, 79, 624–628. [Google Scholar] [CrossRef]
- Patil, S.P.; Board, K.F.; Lebedeva, I.P.; Norris, K.A. Immune Responses to Pneumocystis Colonization and Infection in a Simian Model of AIDS. J. Eukaryot. Microbiol. 2003, 50, 661–662. [Google Scholar] [CrossRef]
- Board, K.F.; Patil, S.; Lebedeva, I.; Capuano, S.; Trichel, A.M.; Murphey-Corb, M.; Rajakumar, P.A.; Flynn, J.L.; Haidaris, C.G.; Norris, K.A. Experimental Pneumocystis Carinii Pneumonia in Simian Immunodeficiency Virus-Infected Rhesus Macaques. J. Infect. Dis. 2003, 187, 576–588. [Google Scholar] [CrossRef] [Green Version]
- Kling, H.M.; Shipley, T.W.; Patil, S.; Morris, A.; Norris, K.A. Pneumocystis Colonization in Immunocompetent and Simian Immunodeficiency Virus-Infected Cynomolgus Macaques. J. Infect. Dis. 2009, 199, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Kling, H.M.; Shipley, T.W.; Patil, S.P.; Kristoff, J.; Bryan, M.; Montelaro, R.C.; Morris, A.; Norris, K.A. Relationship of Pneumocystis Jiroveci Humoral Immunity to Prevention of Colonization and Chronic Obstructive Pulmonary Disease in a Primate Model of HIV Infection. Infect. Immun. 2010, 78, 4320–4330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kling, H.M.; Shipley, T.W.; Guyach, S.; Tarantelli, R.; Morris, A.; Norris, K.A. Trimethoprim-Sulfamethoxazole Treatment Does Not Reverse Obstructive Pulmonary Changes in Pneumocystis-Colonized Nonhuman Primates with SHIV Infection. J. Acquir. Immune Defic. Syndr. 2014, 65, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kling, H.M.; Norris, K.A. Vaccine-Induced Immunogenicity and Protection Against Pneumocystis Pneumonia in a Nonhuman Primate Model of HIV and Pneumocystis Coinfection. J. Infect. Dis. 2016, 213, 1586–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobos Jiménez, V.; Rabacal, W.; Rayens, E.; Norris, K.A. Immunization with Pneumocystis Recombinant KEX1 Induces Robust and Durable Humoral Responses in Immunocompromised Non-Human Primates. Hum. Vaccin. Immunother. 2019, 15, 2075–2080. [Google Scholar] [CrossRef]
- Stokes, D.C.; Gigliotti, F.; Rehg, J.E.; Snellgrove, R.L.; Hughes, W.T. Experimental Pneumocystis Carinii Pneumonia in the Ferret. Br. J. Exp. Pathol. 1987, 68, 267–276. [Google Scholar] [PubMed]
- Gigliotti, F.; Harmsen, A.G.; Haidaris, C.G.; Haidaris, P.J. Pneumocystis Carinii Is Not Universally Transmissible between Mammalian Species. Infect. Immun. 1993, 61, 2886–2890. [Google Scholar] [CrossRef] [Green Version]
- Simpson-Haidaris, P.J.; Courtney, M.A.; Wright, T.W.; Goss, R.; Harmsen, A.; Gigliotti, F. Induction of Fibrinogen Expression in the Lung Epithelium during Pneumocystis Carinii Pneumonia. Infect. Immun. 1998, 66, 4431–4439. [Google Scholar] [CrossRef]
- Hong, S.; Park, K.; Lee, S. Susceptibility of Various Animals to Pneumocystis Carinii Infection. Korean J. Parasitol. 1992, 30, 277–281. [Google Scholar] [CrossRef]
- Nielsen, J.; Bille-Hansen, V.; Settnes, O.P. Experimental Corticosteroid Induction of Pneumocystis Carinii Pneumonia in Piglets. APMIS 1999, 107, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Settnes, O.P.; Henriksen, S.A.; Jorsal, S.E.; Bille-Hansen, V. Pneumocystis Carinii Pneumonia—Pig Provocation Model. J. Eukaryot. Microbiol. 1997, 44, 31S. [Google Scholar] [CrossRef]
- Evans, S.E.; Leventakos, K.; Ben-Ami, R.; You, D.; Thakkar, S.G.; Lewis, R.E.; Kontoyiannis, D.P. Toll-Deficient Drosophila Are Resistant to Infection by Pneumocystis Spp.: Additional Evidence of Specificity to Mammalian Hosts. Virulence 2010, 1, 523–525. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, B.B.; Bishop, L.R.; Kovacs, J.A.; Mylonakis, E. Galleria Mellonella Are Resistant to Pneumocystis Murina Infection. Mycopathologia 2011, 171, 273–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cere, N.; Drouet-Viard, F.; Dei-Cas, E.; Chanteloup, N.; Coudert, P. In Utero Transmission of Pneumocystis Carinii Sp. f. Oryctolagi. Parasite 1997, 4, 325–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, C.A.; Chabé, M.; Aliouat, E.M.; Durand-Joly, I.; Gantois, N.; Conseil, V.; López, C.; Duriez, T.; Dei-Cas, E.; Vargas, S.L. Exploring Transplacental Transmission of Pneumocystis Oryctolagi in First-Time Pregnant and Multiparous Rabbit Does. Med. Mycol. 2007, 45, 701–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swain, S.D.; Meissner, N.N.; Siemsen, D.W.; McInnerney, K.; Harmsen, A.G. Pneumocystis Elicits a STAT6-Dependent, Strain-Specific Innate Immune Response and Airway Hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2012, 46, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Tisdale, N.L.; Ashbaugh, A.; Hendrix, K.; Collins, M.S.; Porollo, A.P.; Cushion, M.T. The Effects of Sex and Strain on Pneumocystis Murina Fungal Burdens in Mice. bioRxiv 2019, 781245. [Google Scholar] [CrossRef] [Green Version]
- Boylan, C.; Current, W. Improved Rat Model of Pneumocystis Carinii Pneumonia: Induced Laboratory Infections in Pneumocystis-Free Animals. Infect. Immun. 1992, 60, 1589–1597. [Google Scholar] [CrossRef] [Green Version]
- Bitar, D.; Lortholary, O.; Dromer, F.; Coignard, B.; Che, D. Mycoses Invasives En France Métropolitaine, PMSI 2001–2010: Incidence, Létalité et Tendances. Bull. Epidémiologique Hebd. 2013, 12–13, 109–114. [Google Scholar]
- Wickramasekaran, R.N.; Jewell, M.P.; Sorvillo, F.; Kuo, T. The Changing Trends and Profile of Pneumocystosis Mortality in the United States, 1999–2014. Mycoses 2017, 60, 607–615. [Google Scholar] [CrossRef]
- Halabieh, N.A.E.; Petrillo, E.; Laviano, A.; Delfino, M.; Fanelli, F.R. A Case of Pneumocystis Jirovecii Pneumonia in a Severely Malnourished, HIV-Negative Patient. J. Parenter. Enter. Nutr. 2016, 40, 722–724. [Google Scholar] [CrossRef]
- Hanachi, M.; Bohem, V.; Bemer, P.; Kayser, N.; de Truchis, P.; Melchior, J.-C. Negative Role of Malnutrition in Cell-Mediated Immune Response: Pneumocystis Jirovecii Pneumonia (PCP) in a Severely Malnourished, HIV-Negative Patient with Anorexia Nervosa. Clin. Nutr. ESPEN 2018, 25, 163–165. [Google Scholar] [CrossRef]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the Global Challenges of Ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Garvy, B.A.; Harmsen, A.G. Susceptibility to Pneumocystis Carinii Infection: Host Responses of Neonatal Mice from Immune or Naive Mothers and of Immune or Naive Adults. Infect. Immun. 1996, 64, 3987–3992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garvy, B.A.; Qureshi, M.H. Delayed Inflammatory Response to Pneumocystis Carinii Infection in Neonatal Mice Is Due to an Inadequate Lung Environment. J. Immunol. 2000, 165, 6480–6486. [Google Scholar] [CrossRef] [PubMed]
- Kurkjian, C.; Hollifield, M.; Lines, J.L.; Rogosky, A.; Empey, K.M.; Qureshi, M.; Brown, S.A.; Garvy, B.A. Alveolar Macrophages in Neonatal Mice Are Inherently Unresponsive to Pneumocystis Murina Infection. Infect. Immun. 2012, 80, 2835–2846. [Google Scholar] [CrossRef] [Green Version]
- Weisbroth, S.H.; Geistfeld, J.; Weisbroth, S.P.; Williams, B.; Feldman, S.H.; Linke, M.J.; Orr, S.; Cushion, M.T. Latent Pneumocystis Carinii Infection in Commercial Rat Colonies: Comparison of Inductive Immunosuppressants plus Histopathology, PCR, and Serology as Detection Methods. J. Clin. Microbiol. 1999, 37, 1441–1446. [Google Scholar] [CrossRef] [Green Version]
- Desoubeaux, G.; Cray, C. Rodent Models of Invasive Aspergillosis Due to Aspergillus Fumigatus: Still a Long Path toward Standardization. Front. Microbiol. 2017, 8, 841. [Google Scholar] [CrossRef]
- Yale, S.H.; Limper, A.H. Pneumocystis Carinii Pneumonia in Patients without Acquired Immunodeficiency Syndrome: Associated Illness and Prior Corticosteroid Therapy. Mayo Clin. Proc. 1996, 71, 5–13. [Google Scholar] [CrossRef]
- Bienvenu, A.-L.; Traore, K.; Plekhanova, I.; Bouchrik, M.; Bossard, C.; Picot, S. Pneumocystis Pneumonia Suspected Cases in 604 Non-HIV and HIV Patients. Int. J. Infect. Dis. 2016, 46, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-J.; Lee, T.-F.; Ruan, S.-Y.; Yu, C.-J.; Chien, J.-Y.; Hsueh, P.-R. Clinical Characteristics, Treatment Outcomes, and Prognostic Factors of Pneumocystis Pneumonia in Non-HIV-Infected Patients. Infect. Drug Resist. 2019, 12, 1457–1467. [Google Scholar] [CrossRef] [Green Version]
- Shellito, J.E.; Tate, C.; Ruan, S.; Kolls, J. Murine CD4+ T Lymphocyte Subsets and Host Defense against Pneumocystis Carinii. J. Infect. Dis. 2000, 181, 2011–2017. [Google Scholar] [CrossRef]
- Swain, S.D.; Wright, T.W.; Degel, P.M.; Gigliotti, F.; Harmsen, A.G. Neither Neutrophils nor Reactive Oxygen Species Contribute to Tissue Damage during Pneumocystis Pneumonia in Mice. Infect. Immun. 2004, 72, 5722–5732. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.-Y.; Müller, N.; Herold, M.J.; van den Brandt, J.; Reichardt, H.M. Glucocorticoids Exert Opposing Effects on Macrophage Function Dependent on Their Concentration. Immunology 2007, 122, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Eddens, T.; Elsegeiny, W.; Nelson, M.P.; Horne, W.; Campfield, B.T.; Steele, C.; Kolls, J.K. Eosinophils Contribute to Early Clearance of Pneumocystis Murina Infection. J. Immunol. 2015, 195, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandakumar, V.; Hebrink, D.; Jenson, P.; Kottom, T.; Limper, A.H. Differential Macrophage Polarization from Pneumocystis in Immunocompetent and Immunosuppressed Hosts: Potential Adjunctive Therapy during Pneumonia. Infect. Immun. 2017, 85, e00939-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cain, D.W.; Cidlowski, J.A. Immune Regulation by Glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Bhagwat, S.P.; Gigliotti, F.; Wang, J.; Wang, Z.; Notter, R.H.; Murphy, P.S.; Rivera-Escalera, F.; Malone, J.; Jordan, M.B.; Elliott, M.R.; et al. Intrinsic Programming of Alveolar Macrophages for Protective Antifungal Innate Immunity Against Pneumocystis Infection. Front. Immunol. 2018, 9, 2131. [Google Scholar] [CrossRef] [Green Version]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Masur, H.; Ognibene, F.P.; Yarchoan, R.; Shelhamer, J.H.; Baird, B.F.; Travis, W.; Suffredini, A.F.; Deyton, L.; Kovacs, J.A.; Falloon, J. CD4 Counts as Predictors of Opportunistic Pneumonias in Human Immunodeficiency Virus (HIV) Infection. Ann. Intern. Med. 1989, 111, 223–231. [Google Scholar] [CrossRef]
- Wolff, L.; Horch, S.; Gemsa, D. The Development of Pneumocystis Carinii Pneumonia in Germ-Free Rats Requires Immunosuppression and Exposure to the Pneumocystis Carinii Organism. Comp. Immunol. Microbiol. Infect. Dis. 1993, 16, 73–76. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Tsai, M.M.; Wright, C.F.; Cushion, M.T. Use of Semiquantitative PCR to Assess Onset and Treatment of Pneumocystis Carinii Infection in Rat Model. J. Clin. Microbiol. 1995, 33, 718–724. [Google Scholar] [CrossRef] [Green Version]
- Limper, A.H.; Hoyte, J.S.; Standing, J.E. The Role of Alveolar Macrophages in Pneumocystis Carinii Degradation and Clearance from the Lung. J. Clin. Investig. 1997, 99, 2110–2117. [Google Scholar] [CrossRef] [PubMed]
- Lasbury, M.E.; Durant, P.J.; Ray, C.A.; Tschang, D.; Schwendener, R.; Lee, C.-H. Suppression of Alveolar Macrophage Apoptosis Prolongs Survival of Rats and Mice with Pneumocystis Pneumonia. J. Immunol. 2006, 176, 6443–6453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, W.T.; Smith, B. Provocation of Infection Due to Pneumocystis Carinii by Cyclosporin A. J. Infect. Dis. 1982, 145, 767. [Google Scholar] [CrossRef]
- Oz, H.S.; Hughes, W.T. Novel Anti-Pneumocystis Carinii Effects of the Immunosuppressant Mycophenolate Mofetil in Contrast to Provocative Effects of Tacrolimus, Sirolimus, and Dexamethasone. J. Infect. Dis. 1997, 175, 901–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsegeiny, W.; Eddens, T.; Chen, K.; Kolls, J.K. Anti-CD20 Antibody Therapy and Susceptibility to Pneumocystis Pneumonia. Infect. Immun. 2015, 83, 2043–2052. [Google Scholar] [CrossRef] [Green Version]
- Opata, M.M.; Hollifield, M.L.; Lund, F.E.; Randall, T.D.; Dunn, R.; Garvy, B.A.; Feola, D.J. B Lymphocytes Are Required during the Early Priming of CD4+ T Cells for Clearance of Pneumocystis Infection in Mice. J. Immunol. 2015, 195, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The Safety and Side Effects of Monoclonal Antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef]
- Akarsu, A.; Soyer, O.; Sekerel, B.E. Hypersensitivity Reactions to Biologicals: From Bench to Bedside. Curr. Treat. Options Allergy 2020, 7, 71–83. [Google Scholar] [CrossRef]
- Chen, W.; Gigliotti, F.; Harmsen, A.G. Latency Is Not an Inevitable Outcome of Infection with Pneumocystis Carinii. Infect. Immun. 1993, 61, 5406–5409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linke, M.J.; Harris, C.E.; Korfhagen, T.R.; McCormack, F.X.; Ashbaugh, A.D.; Steele, P.; Whitsett, J.A.; Walzer, P.D. Immunosuppressed Surfactant Protein A-Deficient Mice Have Increased Susceptibility to Pneumocystis Carinii Infection. J. Infect. Dis. 2001, 183, 943–952. [Google Scholar] [CrossRef] [Green Version]
- Atochina, E.N.; Beck, J.M.; Preston, A.M.; Haczku, A.; Tomer, Y.; Scanlon, S.T.; Fusaro, T.; Casey, J.; Hawgood, S.; Gow, A.J.; et al. Enhanced Lung Injury and Delayed Clearance of Pneumocystis Carinii in Surfactant Protein A-Deficient Mice: Attenuation of Cytokine Responses and Reactive Oxygen-Nitrogen Species. Infect. Immun. 2004, 72, 6002–6011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atochina, E.N.; Gow, A.J.; Beck, J.M.; Haczku, A.; Inch, A.; Kadire, H.; Tomer, Y.; Davis, C.; Preston, A.M.; Poulain, F.; et al. Delayed Clearance of Pneumocystis Carinii Infection, Increased Inflammation, and Altered Nitric Oxide Metabolism in Lungs of Surfactant Protein-D Knockout Mice. J. Infect. Dis. 2004, 189, 1528–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linke, M.; Ashbaugh, A.; Koch, J.; Tanaka, R.; Walzer, P. Surfactant Protein A Limits Pneumocystis Murina Infection in Immunosuppressed C3H/HeN Mice and Modulates Host Response during Infection. Microbes Infect. 2005, 7, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Linke, M.; Ashbaugh, A.; Koch, J.; Tanaka, R.; Walzer, P. Efficient Resolution of Pneumocystis Murina Infection in Surfactant Protein A-Deficient Mice Following Withdrawal of Corticosteroid-Induced Immunosuppression. J. Med. Microbiol. 2006, 55, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linke, M.J.; Ashbaugh, A.A.; Koch, J.V.; Levin, L.; Tanaka, R.; Walzer, P.D. Effects of Surfactant Protein-A on the Interaction of Pneumocystis Murina with Its Host at Different Stages of the Infection in Mice. J. Eukaryot. Microbiol. 2009, 56, 58–65. [Google Scholar] [CrossRef]
- Linke, M.J.; Ashbaugh, A.D.; Demland, J.A.; Walzer, P.D. Pneumocystis Murina Colonization in Immunocompetent Surfactant Protein A Deficient Mice Following Environmental Exposure. Respir. Res. 2009, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Rudmann, D.G.; Preston, A.M.; Moore, M.W.; Beck, J.M. Susceptibility to Pneumocystis Carinii in Mice Is Dependent on Simultaneous Deletion of IFN-Gamma and Type 1 and 2 TNF Receptor Genes. J. Immunol. 1998, 161, 360–366. [Google Scholar]
- Enriquez, J.; Mims, B.M.D.; Trasti, S.; Furr, K.L.; Grisham, M.B. Genomic, Microbial and Environmental Standardization in Animal Experimentation Limiting Immunological Discovery. BMC Immunol. 2020, 21, 50. [Google Scholar] [CrossRef]
- Walzer, P.D.; LaBine, M.; Redington, T.J.; Cushion, M.T. Predisposing Factors in Pneumocystis Carinii Pneumonia: Effects of Tetracycline, Protein Malnutrition, and Corticosteroids on Hosts. Infect. Immun. 1984, 46, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, W.H. Experimental Pulmonary Pneumocystis Carinii Infection in Rabbits. J. Exp. Med. 1959, 110, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Walzer, P.D.; Runck, J.; Orr, S.; Foy, J.; Steele, P.; White, M. Clinically Used Antimicrobial Drugs against Experimental Pneumocystosis, Singly and in Combination: Analysis of Drug Interactions and Efficacies. Antimicrob. Agents Chemother. 1997, 41, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Carlson, M.L.; Craig, I.D.; Lannigan, R. Efficacy of Tetroxoprim/Sulphadiazine in the Treatment of Pneumocystis Carinii Pneumonitis in Rats. J. Antimicrob. Chemother. 1985, 15, 575–578. [Google Scholar] [CrossRef]
- Hughes, W.T. Natural Mode of Acquisition for de Novo Infection with Pneumocystis Carinii. J. Infect. Dis. 1982, 145, 842–848. [Google Scholar] [CrossRef]
- An, C.L.; Gigliotti, F.; Harmsen, A.G. Exposure of Immunocompetent Adult Mice to Pneumocystis Carinii f. Sp. Muris by Cohousing: Growth of P. Carinii f. Sp. Muris and Host Immune Response. Infect. Immun. 2003, 71, 2065–2070. [Google Scholar] [CrossRef] [Green Version]
- Roths, J.B.; Sidman, C.L. Both Immunity and Hyperresponsiveness to Pneumocystis Carinii Result from Transfer of CD4+ but Not CD8+ T Cells into Severe Combined Immunodeficiency Mice. J. Clin. Investig. 1992, 90, 673–678. [Google Scholar] [CrossRef] [Green Version]
- Yasuoka, A.; Oka, S.; Komuro, K.; Shimizu, H.; Kitada, K.; Nakamura, Y.; Shibahara, S.; Takeuchi, T.; Kondo, S.; Shimada, K. Successful Treatment of Pneumocystis Carinii Pneumonia in Mice with Benanomicin A (ME1451). Antimicrob Agents Chemother. 1995, 39, 720–724. [Google Scholar] [CrossRef] [Green Version]
- Gigliotti, F.; Garvy, B.A.; Harmsen, A.G. Antibody-Mediated Shift in the Profile of Glycoprotein A Phenotypes Observed in a Mouse Model of Pneumocystis Carinii Pneumonia. Infect. Immun. 1996, 64, 1892–1899. [Google Scholar] [CrossRef] [Green Version]
- Wright, T.W.; Notter, R.H.; Wang, Z.; Harmsen, A.G.; Gigliotti, F. Pulmonary Inflammation Disrupts Surfactant Function during Pneumocystis Carinii Pneumonia. Infect. Immun. 2001, 69, 758–764. [Google Scholar] [CrossRef] [Green Version]
- Dumoulin, A.; Mazars, E.; Seguy, N.; Gargallo-Viola, D.; Vargas, S.; Cailliez, J.C.; Aliouat, E.M.; Wakefield, A.E.; Dei-Cas, E. Transmission of Pneumocystis Carinii Disease from Immunocompetent Contacts of Infected Hosts to Susceptible Hosts. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 671–678. [Google Scholar] [CrossRef]
- Chabé, M.; Dei-Cas, E.; Creusy, C.; Fleurisse, L.; Respaldiza, N.; Camus, D.; Durand-Joly, I. Immunocompetent Hosts as a Reservoir of Pneumocystis Organisms: Histological and Rt-PCR Data Demonstrate Active Replication. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 89–97. [Google Scholar] [CrossRef]
- Gigliotti, F.; Harmsen, A.G.; Wright, T.W. Characterization of Transmission of Pneumocystis Carinii f. Sp. Muris through Immunocompetent BALB/c Mice. Infect. Immun. 2003, 71, 3852–3856. [Google Scholar] [CrossRef] [Green Version]
- Southam, D.S.; Dolovich, M.; O’Byrne, P.M.; Inman, M.D. Distribution of Intranasal Instillations in Mice: Effects of Volume, Time, Body Position, and Anesthesia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L833–L839. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, N.B.; Mandujano, J.F.; Nelson, S.; Summer, W.R.; Shellito, J.E. Alcohol Ingestion Impairs Host Defenses Predisposing Otherwise Healthy Mice to Pneumocystis Carinii Infection. Alcohol. Clin. Exp. Res. 1995, 19, 1219–1225. [Google Scholar] [CrossRef]
- Harmsen, A.G.; Chen, W.; Gigliotti, F. Active Immunity to Pneumocystis Carinii Reinfection in T-Cell-Depleted Mice. Infect. Immun. 1995, 63, 2391–2395. [Google Scholar] [CrossRef] [Green Version]
- Cushion, M.T.; Orr, S.; Keely, S.P.; Stringer, J.R. Time between Inoculations and Karyotype Forms of Pneumocystis Carinii f. Sp. Carinii Influence Outcome of Experimental Coinfections in Rats. Infect. Immun. 2001, 69, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Vuk-Pavlovic, Z.; Mo, E.K.; Icenhour, C.R.; Standing, J.E.; Fisher, J.H.; Limper, A.H. Surfactant Protein D Enhances Pneumocystis Infection in Immune-Suppressed Mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L442–L449. [Google Scholar] [CrossRef] [Green Version]
- Walzer, P.D.; Kim, C.K.; Foy, J.M.; Linke, M.J.; Cushion, M.T. Inhibitors of Folic Acid Synthesis in the Treatment of Experimental Pneumocystis Carinii Pneumonia. Antimicrob Agents Chemother. 1988, 32, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Sukura, A.; Soveri, T.; Lindberg, L.A. Superiority of Methylprednisolone over Dexamethasone for Induction of Pneumocystis Carinii Infection in Rats. J. Clin. Microbiol. 1991, 29, 2331–2332. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Zhang, P.; Sempowski, G.D.; Shellito, J.E. Thymopoietic and Bone Marrow Response to Murine Pneumocystis Pneumonia. Infect. Immun. 2011, 79, 2031–2042. [Google Scholar] [CrossRef] [Green Version]
- Walzer, P.D.; Schnelle, V.; Armstrong, D.; Rosen, P.P. Nude Mouse: A New Experimental Model for Pneumocystis Carinii Infection. Science 1977, 197, 177–179. [Google Scholar] [CrossRef]
- Durand-Joly, I.; Aliouat, E.M.; Recourt, C.; Guyot, K.; François, N.; Wauquier, M.; Camus, D.; Dei-Cas, E. Pneumocystis Carinii f. Sp. Hominis Is Not Infectious for SCID Mice. J. Clin. Microbiol. 2002, 40, 1862–1865. [Google Scholar] [CrossRef] [Green Version]
- Furuta, T.; Ueda, K.; Fujiwara, K.; Yamanouchi, K. Cellular and Humoral Immune Responses of Mice Subclinically Infected with Pneumocystis Carinii. Infect. Immun. 1985, 47, 544–548. [Google Scholar] [CrossRef] [Green Version]
- Evans, H.M.; Garvy, B.A. The Trophic Life Cycle Stage of Pneumocystis Species Induces Protective Adaptive Responses without Inflammation-Mediated Progression to Pneumonia. Med. Mycol. 2018, 56, 994–1005. [Google Scholar] [CrossRef]
- Cushion, M.T.; Ashbaugh, A.; Hendrix, K.; Linke, M.J.; Tisdale, N.; Sayson, S.G.; Porollo, A. Gene Expression of Pneumocystis Murina after Treatment with Anidulafungin Results in Strong Signals for Sexual Reproduction, Cell Wall Integrity, and Cell Cycle Arrest, Indicating a Requirement for Ascus Formation for Proliferation. Antimicrob. Agents Chemother. 2018, 62, e02513-17. [Google Scholar] [CrossRef] [Green Version]
- Evans, H.M.; Bryant, G.L.; Garvy, B.A. The Life Cycle Stages of Pneumocystis Murina Have Opposing Effects on the Immune Response to This Opportunistic, Fungal Pathogen. Infect. Immun. 2016, 84, 3195–3205. [Google Scholar] [CrossRef] [Green Version]
- Cushion, M.T. Are Members of the Fungal Genus Pneumocystis (a) Commensals; (b) Opportunists; (c) Pathogens; or (d) All of the Above? PLoS Pathog. 2010, 6, e1001009. [Google Scholar] [CrossRef] [Green Version]
- Linke, M.J.; Ashbaugh, A.; Collins, M.S.; Lynch, K.; Cushion, M.T. Characterization of a Distinct Host Response Profile to Pneumocystis Murina Asci during Clearance of Pneumocystis Pneumonia. Infect. Immun. 2013, 81, 984–995. [Google Scholar] [CrossRef] [Green Version]
- Prigge, J.R.; Hoyt, T.R.; Dobrinen, E.; Capecchi, M.R.; Schmidt, E.E.; Meissner, N. Type I IFNs Act upon Hematopoietic Progenitors to Protect and Maintain Hematopoiesis during Pneumocystis Lung Infection in Mice. J. Immunol. 2015, 195, 5347–5357. [Google Scholar] [CrossRef] [Green Version]
- Évaluation des Actes de Diagnostic Biologique de la Pneumocystose (Pneumocystis jirovecii). Available online: https://www.has-sante.fr/jcms/c_2680246/fr/evaluation-des-actes-de-diagnostic-biologique-de-la-pneumocystose-pneumocystis-jirovecii (accessed on 3 May 2021).
- Cissé, O.H.; Ma, L.; Wei Huang, D.; Khil, P.P.; Dekker, J.P.; Kutty, G.; Bishop, L.; Liu, Y.; Deng, X.; Hauser, P.M.; et al. Comparative Population Genomics Analysis of the Mammalian Fungal Pathogen Pneumocystis. mBio 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Chen, Z.; Huang, D.W.; Kutty, G.; Ishihara, M.; Wang, H.; Abouelleil, A.; Bishop, L.; Davey, E.; Deng, R.; et al. Genome Analysis of Three Pneumocystis Species Reveals Adaptation Mechanisms to Life Exclusively in Mammalian Hosts. Nat. Commun. 2016, 7, 10740. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L.; Universities Federation for Animal Welfare. The Principles of Humane Experimental Technique; Universities Federation for Animal Welfare: Wheathampstead, UK, 1992; ISBN 978-0-900767-78-4. [Google Scholar]

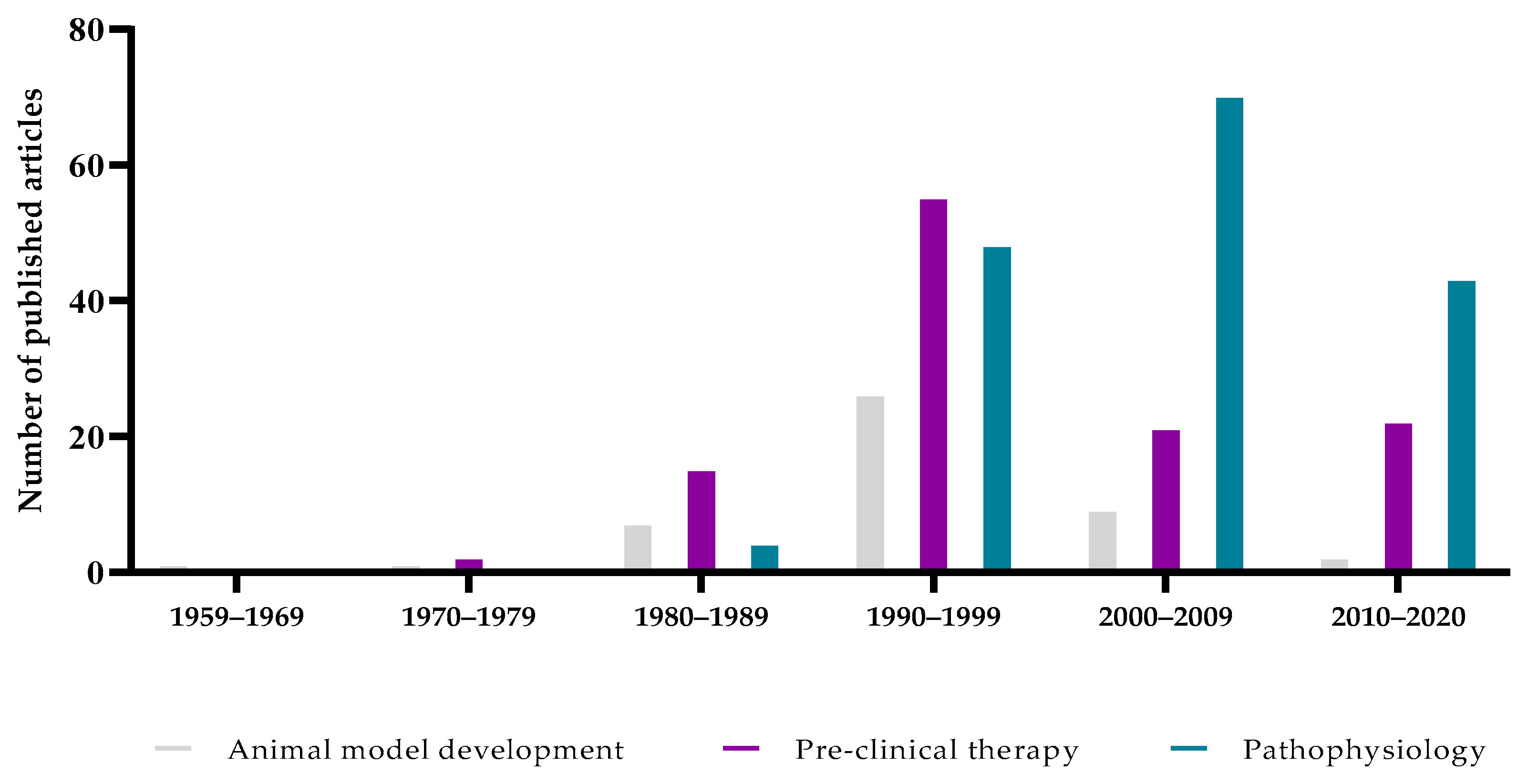
| Mean (Unit ± Standard Deviation) or Number (%); 95% Confidence Interval | |||||
|---|---|---|---|---|---|
| Mouse N = 560 (74.8%) | Rat N = 156 (20.8%) | Rabbit N = 10 (1.3%) | Non-Human Primate N = 10 (1.3%) | Other Animal N = 13 (1.7%) | |
| Weight | 21.0 (±4.5); (18.9–23.3 g) | 189.4 g (±48.4); (181.8–197 g) | - | - | - |
| Sex | |||||
| 36 (6.4%) | 59 (37.8%) | - | 2 (20%) | 2 (15.4%) |
| 28 (5%) | 6 (3.8%) | 1 (10%) | 3 (30%) | - |
| 431 (77%) | 30 (19.2%) | 9 (90%) | 5 (50%) | 9 (69.2%) |
| Animal strains, including: | |||||
| 14 (2.5%) | 138 (88.5%) | 10 (100%) | 10 (100%) | 13 (100%) |
| 546 (95.5%) | 18 (11.5%) | - | ||
| Immunosuppressive regimens, including φ: | |||||
| 67 (12%) | 150 (96.2%) | 2 (20%) | 1 (10%) | 11 (84.6%) |
| 162 (28.9%) | 2 (1.2%) | - | - | - |
| - | 5 (3.2%) | - | 1 (10%) | - |
| 330 (58.9%) | - | - | - | - |
| - | - | - | 8 (80%) | - |
| Exposition, including: | N = 325 (58%) | N = 71 (45.5.%) | N = 5 (50%) | N = 4 (40%) | N = 2 (15.4%) |
| 24 (4.3%) | 32 (20.5%) | 3 (30%) | 2 (20%) | 1 (7.7%) |
| 301 (53.8%) | 39 (25%) | 2 (20%) | 2 (20%) | 1 (7.7%) |
| Nutritionnal regimen | N = 323 (57.7%) | N = 87 (55.8%) | N = 3 (30%) | N = 7 (70%) | N = 2 (15.4%) |
| 297 (53%) | 54 (34.6%) | 3 (30%) | 7 (70%) | 2 (15.4%) |
| 26 (4.6%) | 33 (21.2%) | - | - | - |
| Route of experimental infection inoculum size | |||||
| 104 (18.6%) | 17 (10.9%) | - | 5 (50%) | - |
| 28 (5%) | - | - | - | - |
| 2 × 105 | - | - | - | ||
| 38 (6.8%) | 2 (1.3%) | - | - | - |
| 6.0 × 106 (±7.5 × 106); (3.5–8.5 × 106) | 1.107 (±1.4 × 107); (0.0–3 × 107) | - | - | ||
| 42 (7.5%) | 29 (18.6%) | - | - | - |
| 4.8 × 106 (±1.1 × 107); (1.1–8.5 × 106) | 1.3 × 107 (±3.1 × 106); (0.1–2.5 × 107) | - | - | ||
| 306 (54.6%) | 17 (10.9%) | - | 1 (3.3%) | 1 (7.7%) |
| 6.3 × 106 (±1.5 × 107); (4.5–8.1 × 106) | 1.4.107 (±2.7 × 107); (0.1–2.6 × 107) | 5.106 | 2.105 | ||
| 42 (7.5%) | 91 (58.3%) | 3 (100%) | 4 (40%) | 12 (92.3%) |
| Validation of the model and parameters to follow, including φ: | |||||
| 439 (78.7%) | 146 (94.2%) | 3 (100%) | 6 (60%) | 12 (92.3%) |
| 40 (7.2%) | 7 (4.5%) | - | 4 (40%) | 1 (7.7%) |
| 205 (36.7%) | 19 (12.3%) | 1 (33.3%) | 8 (80%) | 2 (15.4%) |
| 10 (1.8%) | 1 (0.6%) | - | - | - |
 |  |  |  | |
| Relative cost | ✓ | ✓ | ✓ | x |
| Easy to breed | ✓ | ✓ | ✓ | x |
| Simplicity of maintenance and handling | ✓ | ✓ | ✓ | x |
| Study tools available | ✓ | ✓ | x | x |
| Tissue quantity available | ✓ | ✓ | ✓ | ✓ |
| Ethical restrictions | ✓ | ✓ | ✓ | x |
| Inbred strains and transgenic lines available | ✓ | ✓ | x | x |
| Immune response similarity to humans | x | x | x | ✓ |
| Anatomical, physiological, and genetic similarities to humans | ✓ | ✓ | ✓ | ✓ |
| Natural acquisition of Pneumocystis pneumonia | x | x | ✓ | x |
| Experimental acquisition of Pneumocystis pneumonia under virus-induced immunodepression | x | x | x | ✓ |
| Experimental acquisition Pneumocystis pneumonia under steroid-induced immunodepression | ✓ | ✓ | ✓ | ✓ |
| Model Type | Pros | Cons |
|---|---|---|
| Strategies to render animal susceptible to Pneumocystis pneumonia | ||
| Steroids | Targeting T-cells and macrophages, largely involved in immune response against Pneumocystis spp. Major risk factor for the development of Pneumocystis pneumonia in humans Administrable in drinking water for some molecules (convenient, safe, compatible with refinement of experimental procedures) | Start 1–2 weeks prior to experimental inoculation or co-housing Need to be continuously pursued until the infection had been established Anti-inflammatory effects that can interfere with the immune response (confounding bias) Not representative of the viral induced-immunosuppression |
| Immunotherapy | Selective depletion of different cell types to evaluate their impact in the Pneumocystis pneumonia development Avoiding confounding bias seen with steroids | Administrable by injection (no refinement of experimental procedure) Start 1–2 weeks prior to experimental inoculation or co-housing Needs to be continuously pursued until the infection has been established Risk of hypersensitivity reaction or cytokine release-associated acute reactions Not exploring redundancy in the immune system or compensatory hyperactivity |
| Genetically modified animal | Selective depletion of different components of the immune response to evaluate their impact in Pneumocystis pneumonia development Recapitulating the human primary immune disorders Avoiding confounding bias seen with steroids Avoiding administration of drug to induce immunosuppression | Expensive Not exploring redundancy in the immune system or compensatory hyperactivity Restricted to specific models, especially mice Not representative of the viral induced-immunosuppression |
| Viral induced- immunosuppression | Evaluation of Pneumocystis pneumonia in a viral-induced immunosuppression context Avoiding administration of drug to induce immunosuppression Avoiding confounding bias seen with steroids | Restricted to comparisons in the context of viral induced-immunosuppression Possible only for non-human primates (ethical restrictions) |
| Strategies to implement Pneumocystis pneumonia | ||
| Passive without co-housing (only based on immunosuppression induction) | No instillation procedure to be performed No index case animals to use | Not relevant to the transmission and cycle of Pneumocystis Lack of reproducibility Inoculum not known |
| Passive by co-housing | Close to natural transmission No intervention to be performed | Need to breed pre-infected mice in the laboratory Lack of reproducibility Inoculum not known |
| Active by instillation (oropharyngeal, intranasal, transtracheal, intratracheal) | Reproducibility Control of the timing of the infection Known inoculum | Inoculated microorganisms not pure because isolated from filtered lung shreds of infected animals, possible influence on immune response (need to control) Higher inoculum than in a natural transmission Need for anesthesia and intervention by trained personnel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chesnay, A.; Paget, C.; Heuzé-Vourc’h, N.; Baranek, T.; Desoubeaux, G. Pneumocystis Pneumonia: Pitfalls and Hindrances to Establishing a Reliable Animal Model. J. Fungi 2022, 8, 129. https://doi.org/10.3390/jof8020129
Chesnay A, Paget C, Heuzé-Vourc’h N, Baranek T, Desoubeaux G. Pneumocystis Pneumonia: Pitfalls and Hindrances to Establishing a Reliable Animal Model. Journal of Fungi. 2022; 8(2):129. https://doi.org/10.3390/jof8020129
Chicago/Turabian StyleChesnay, Adélaïde, Christophe Paget, Nathalie Heuzé-Vourc’h, Thomas Baranek, and Guillaume Desoubeaux. 2022. "Pneumocystis Pneumonia: Pitfalls and Hindrances to Establishing a Reliable Animal Model" Journal of Fungi 8, no. 2: 129. https://doi.org/10.3390/jof8020129
APA StyleChesnay, A., Paget, C., Heuzé-Vourc’h, N., Baranek, T., & Desoubeaux, G. (2022). Pneumocystis Pneumonia: Pitfalls and Hindrances to Establishing a Reliable Animal Model. Journal of Fungi, 8(2), 129. https://doi.org/10.3390/jof8020129






