Effect of Carbon Sources on the Production of Volatile Organic Compounds by Fusarium verticillioides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Inoculum Preparation
2.2. Cultivation on Different Carbon Sources
2.3. Collection and Identification of VOCs
2.4. Statistical Analysis
3. Results
VOCs Production by F. verticillioides Strain 7600
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korpi, A.; Järnberg, J.; Pasanen, A.L. Microbial volatile organic compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef]
- Inamdar, A.A.; Morath, S.; Bennett, J.W. Fungal Volatile Organic Compounds: More than just a funky smell? Annu. Rev. Microbiol. 2020, 74, 101–116. [Google Scholar] [CrossRef]
- Strobel, G. The story of mycodiesel. Curr. Opin. Microbiol. 2014, 19, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Achimón, F.; Krapacher, C.R.; Jacquat, A.G.; Pizzolitto, R.P.; Zygadlo, J.A. Carbon sources to enhance the biosynthesis of useful secondary metabolites in Fusarium verticillioides submerged cultures. World J. Microbiol. Biotechnol. 2021, 37, 1–11. [Google Scholar] [CrossRef]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Mallette, N.; Pankratz, E.M.; Parker, A.E.; Strobel, G.A.; Busse, S.C.; Carlson, R.P.; Peyton, B.M. Evaluation of cellulose as a substrate for hydrocarbon fuel production by Ascocoryne sarcoides (NRRL 50072). J. Sustain. Bioenergy Syst. 2014, 4, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Strobel, G.A.; Knighton, B.; Kluck, K.; Ren, Y.; Livinghouse, T.; Griffin, M.; Spakowicz, D.; Sears, J. The production of myco-diesel hydrocarbons and their derivatives by the endophytic fungus Gliocladium roseum (NRRL 50072). Microbiology 2008, 154, 3319–3328. [Google Scholar] [CrossRef] [Green Version]
- Griffin, M.A.; Spakowicz, D.J.; Gianoulis, T.A.; Strobel, S.A. Volatile organic compound production by organisms in the genus Ascocoryne and a re-evaluation of myco-diesel production by NRRL 50072. Microbiology 2010, 156, 3814–3829. [Google Scholar] [CrossRef] [Green Version]
- Tomsheck, A.R.; Strobel, G.A.; Booth, E.; Geary, B.; Spakowicz, D.; Knighton, B.; Floerchinger, C.; Sears, J.; Liarzi, O.; Ezra, D. Hypoxylon sp., an endophyte of Persea indica, producing 1,8-cineole and other bioactive volatiles with fuel potential. Microb. Ecol. 2010, 60, 903–914. [Google Scholar] [CrossRef]
- Kudalkar, P.; Strobel, G.; Riyaz-Ul-Hassan, S.; Geary, B.; Sears, J. Muscodor sutura, a novel endophytic fungus with volatile antibiotic activities. Mycoscience 2012, 53, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Riyaz-Ul-Hassan, S.; Strobel, G.; Geary, B.; Sears, J. An endophytic Nodulisporium sp. from Central America producing volatile organic compounds with both biological and fuel potential. J. Microbiol. Biotechnol. 2013, 23, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Kamoun, O.; Muralitharan, G.; Belghith, H.; Gargouri, A.; Trigui-Lahiani, H. Suitable carbon sources selection and ranking for biodiesel production by oleaginous Mucor circinelloides using multi-criteria analysis approach. Fuel 2019, 257, 116117. [Google Scholar] [CrossRef]
- Raut, G.; Kamat, S.; Ravikumar, A. Trends in production and fuel properties of biodiesel from heterotrophic microbes. In Advances in Biological Science Research: A Practical Approach; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–273. [Google Scholar]
- Dörsam, S.; Fesseler, J.; Gorte, O.; Hahn, T.; Zibek, S.; Syldatk, C.; Ochsenreither, K. Sustainable carbon sources for microbial organic acid production with filamentous fungi. Biotechnol. Biofuels 2017, 10, 242. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Pan, J.J.; May, G. Endophytic Fusarium verticillioides reduces disease severity caused by Ustilago maydis on maize. FEMS Microbiol. Lett. 2009, 299, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida, M.N.; Guimarães, V.M.; Falkoski, D.L.; Visser, E.M.; Siqueira, G.A.; Milagres, A.M.F.; de Rezende, S.T. Direct ethanol production from glucose, xylose and sugarcane bagasse by the corn endophytic fungi Fusarium verticillioides and Acremonium zeae. J. Biotechnol. 2013, 168, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, O.; Ayadi, I.; Guerfali, M.; Belghith, H.; Gargouri, A.; Trigui-Lahiani, H. Fusarium verticillioides as a single-cell oil source for biodiesel production and dietary supplements. Process Saf. Environ. Prot. 2018, 118, 68–78. [Google Scholar] [CrossRef]
- Demirbas, A.; Al-Ghamdi, K. Relationships between specific gravities and higher heating values of petroleum components. Pet. Sci. Technol. 2015, 33, 732–740. [Google Scholar] [CrossRef]
- Dickschat, J.S.; Brock, N.L.; Citron, C.A.; Tudzynski, B. Biosynthesis of sesquiterpenes by the fungus Fusarium verticillioides. Chembiochem 2011, 12, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.M.; Herrfurth, C.; Irmisch, S.; Köllner, T.G.; Feussner, I.; Karlovsky, P.; Splivallo, R. Infection of corn ears by Fusarium spp. induces the emission of volatile sesquiterpenes. J. Agric. Food Chem. 2014, 62, 5226–5236. [Google Scholar] [CrossRef]
- Achimón, F.; Dambolena, J.S.; Zygadlo, J.A.; Pizzolitto, R.P. Carbon sources as factors affecting the secondary metabolism of the maize pathogen Fusarium verticillioides. LWT Food Sci. Technol. 2019, 115, 108470. [Google Scholar] [CrossRef]
- Usseglio, V.L.; Pizzolitto, R.P.; Rodriguez, C.; Zunino, M.P.; Zygadlo, J.A.; Areco, V.A.; Dambolena, J.S. Volatile organic compounds from the interaction between Fusarium verticillioides and maize kernels as a natural repellents of Sitophilus zeamais. J. Stored Prod. Res. 2017, 73, 109–114. [Google Scholar] [CrossRef]
- Achimón, F.; Brito, V.D.; Pizzolitto, R.P.; Ramirez Sanchez, A.; Gómez, E.A.; Zygadlo, J.A. Chemical composition and antifungal properties of commercial essential oils against the maize phytopathogenic fungus Fusarium verticillioides. Rev. Argent. Microbiol. 2021, 53, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Butchko, R.A.E.; Brown, D.W.; Busman, M.; Tudzynski, B.; Wiemann, P. Lae1 regulates expression of multiple secondary metabolite gene clusters in Fusarium verticillioides. Fungal Genet. Biol. 2012, 49, 602–612. [Google Scholar] [CrossRef] [Green Version]
- Jelén, H.H.; Mirocha, C.J.; Wasowicz, E.; Kaminski, E.; Mirocha, C.J.; Wa, E.; Jelen, H.H. Production of volatile sesquiterpenes by Fusarium sambucinum strains with different abilities to synthesize trichothecenes. Appl. Environ. Microbiol. 1995, 61, 3815–3820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziv, C.; Gorovits, R.; Yarden, O. Carbon source affects PKA-dependent polarity of Neurospora crassa in a CRE-1-dependent and independent manner. Fungal Genet. Biol. 2008, 45, 103–116. [Google Scholar] [CrossRef]
- Sørensen, J.L.; Giese, H. Influence of carbohydrates on secondary metabolism in Fusarium avenaceum. Toxins 2013, 5, 1655–1663. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.W.; Wu, X.; Wen, C.; Peng, B.; Peng, X.X.; Chen, X.; Li, H. Fructose promotes growth and antifungal activity of Penicillium citrinum. Protein Cell 2016, 7, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Hsu, C.S.; Mochida, I. Chemistry of Diesel Fuels; Taylor & Francis: New York, NY, USA, 2000. [Google Scholar]
- Murphy, M.J.; Taylor, J.D.; Mccormick, R.L. Compendium of Experimental Cetane Number Data; National Renewable Energy Laboratory: Golden, CO, USA, 2004; pp. 1–48. [Google Scholar]
- Guan, C.; Zhai, J.; Han, D. Cetane number prediction for hydrocarbons from molecular structural descriptors based on active subspace methodology. Fuel 2019, 249, 1–7. [Google Scholar] [CrossRef]
- Lapidus, A.L.; Smolenskii, E.A.; Bavykin, V.M.; Myshenkova, T.N.; Kondrat’Ev, L.T. Models for the calculation and prediction of the octane and cetane numbers of individual hydrocarbons. Pet. Chem. 2008, 48, 277–286. [Google Scholar] [CrossRef]
- Egolf, L.M.; Jurs, P.C. Estimation of autoignition temperatures of hydrocarbons, alcohols, and esters from molecular structure. Ind. Eng. Chem. Res. 1992, 31, 1798–1807. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.-M. The measurement of fire and explosion properties of n-pentadecane. J. Korean Soc. Saf. 2014, 29, 39–45. [Google Scholar] [CrossRef]
- Redd, M.E.; Bloxham, J.C.; Giles, N.F.; Knotts, T.A.; Wilding, W.V. A study of unexpected autoignition temperature trends for pure n-alkanes. Fuel 2021, 306, 121710. [Google Scholar] [CrossRef]
- Creton, B.; Dartiguelongue, C.; De Bruin, T.; Toulhoat, H. Prediction of the cetane number of diesel compounds using the quantitative structure property relationship. Energy and Fuels 2010, 24, 5396–5403. [Google Scholar] [CrossRef]
- Won, S.H.; Dooley, S.; Veloo, P.S.; Wang, H.; Oehlschlaeger, M.A.; Dryer, F.L.; Ju, Y. The combustion properties of 2,6,10-trimethyl dodecane and a chemical functional group analysis. Combust. Flame 2014, 161, 826–834. [Google Scholar] [CrossRef]
- Chen, C.C.; Liaw, H.J.; Kuo, Y.Y. Prediction of autoignition temperatures of organic compounds by the structural group contribution approach. J. Hazard. Mater. 2009, 162, 746–762. [Google Scholar] [CrossRef]
- Cuevas-García, R.; Téllez-Romero, J.G.; Ramírez, J.; Sarabia-Bañuelos, P.; Puente-Lee, I.; Salcedo-Luna, C.; Hernández-González, S.; Nolasco-Arizmendi, V.A. Effect of the preparation method on particle size and reaction selectivity on naphthalene hydrogenation over Ni/H-MOR catalysts. Catal. Today 2021, 360, 63–71. [Google Scholar] [CrossRef]
- Altarawneh, M.; Oluwoye, I.; Dlugogorski, B.Z. Singlet-diradical character in large PAHs triggers spontaneous-ignition of coal. Combust. Flame 2020, 212, 279–281. [Google Scholar] [CrossRef]
- Clothier, P.Q.E.; Aguda, B.D.; Moise, A.; Pritchard, H.O. How do diesel-fuel ignition improvers work? Chem. Soc. Rev. 1993, 22, 101–108. [Google Scholar] [CrossRef]
- Herbinet, O.; Husson, B.; Gall, H.L.; Battin-leclerc, F. Comparison study of the gas-phase oxidation of alkylbenzenes and alkylcyclohexanes. Chem. Eng. Sci. 2015, 131, 49–62. [Google Scholar] [CrossRef]
- Kaltschmitt, T.; Deutschmann, O. Fuel Processing for Fuel Cells, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 41. [Google Scholar]
- Demirbas, A.; Ak, N.; Aslan, A.; Sen, N. Calculation of higher heating values of hydrocarbon compounds and fatty acids. Pet. Sci. Technol. 2018, 36, 712–717. [Google Scholar] [CrossRef]
- Manjoo, R.; Deepa, S.; Yadav, A.K.; Singh, N.K. Isolation and characterization of Fusarium verticillioides NKF1 for unsaturated fatty acid production. Curr. Microbiol. 2017, 74, 1301–1305. [Google Scholar] [CrossRef]
- Park, M.O. New pathway for long-chain n-alkane synthesis via 1-alcohol in Vibrio furnissii M1. J. Bacteriol. 2005, 187, 1426–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, W.; Geng, W.; Wang, S.; Zhang, F. Biosynthesis, regulation, and engineering of microbially produced branched biofuels. Biotechnol. Biofuels 2019, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Cao, P.; Song, G.; Chen, W.; Wang, Q. Expanding the repertoire of aromatic chemicals by microbial production. J. Chem. Technol. Biotechnol. 2018, 93, 2804–2816. [Google Scholar] [CrossRef]
- Gosset, G. Production of aromatic compounds in bacteria. Curr. Opin. Biotechnol. 2009, 20, 651–658. [Google Scholar] [CrossRef]
- Noda, S.; Kondo, A. Recent advances in microbial production of aromatic chemicals and derivatives. Trends Biotechnol. 2017, 35, 785–796. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Zhang, F.; Del Cardayre, S.B.; Keasling, J.D. Microbial engineering for the production of advanced biofuels. Nature 2012, 488, 320–328. [Google Scholar] [CrossRef]
- Kang, M.K.; Nielsen, J. Biobased production of alkanes and alkenes through metabolic engineering of microorganisms. J. Ind. Microbiol. Biotechnol. 2017, 44, 613–622. [Google Scholar] [CrossRef] [Green Version]
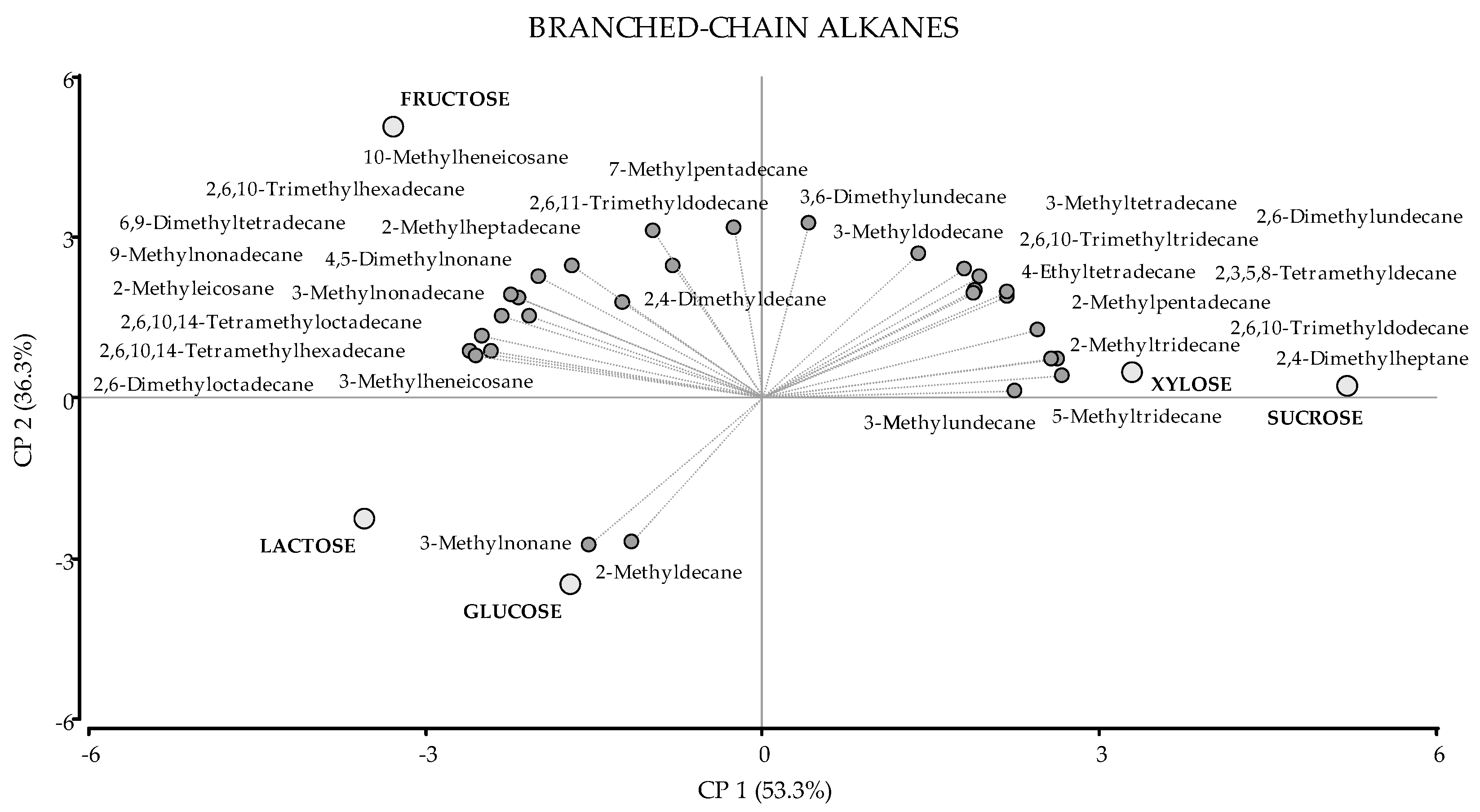
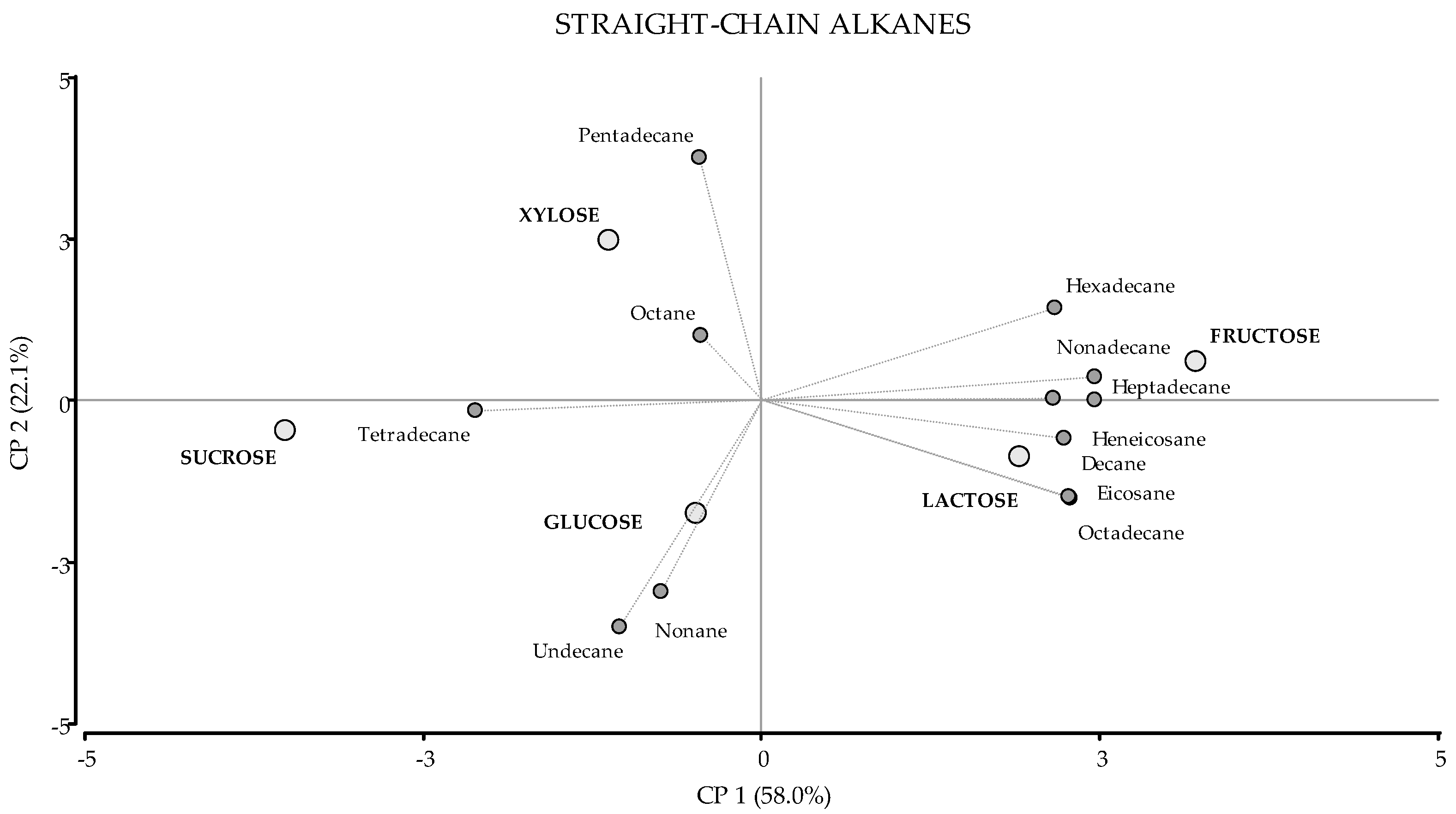


| KI (C) | KI (T) | VOC | Molecular Formula | Glucose | Fructose | Xylose | Sucrose | Lactose |
|---|---|---|---|---|---|---|---|---|
| 797 | 800 | Octane | C8H18 | 1.88 ± 0.09 | 0.94 ± 0.21 | 1.90 ± 0.21 | 0.75 ± 0.18 | 0.83 ± 0.18 |
| 899 | 900 | Nonane | C9H20 | 1.33 ± 0.07 | 0.30 ± 0.05 | 0.48 ± 0.03 | 0.65 ± 0.19 | 0.68 ± 0.09 |
| 999 | 1000 | Decane | C10H22 | 3.31 ± 0.14 | 3.88 ± 0.83 | 3.24 ± 0.49 | 3.07 ± 0.91 | 4.15 ± 0.26 |
| 1099 | 1100 | Undecane | C11H24 | 5.74 ± 0.14 | 3.26 ± 0.62 | 3.41 ± 0.45 | 4.90 ± 0.94 | 4.57 ± 0.36 |
| 1399 | 1400 | Tetradecane | C14H30 | 4.91 ± 0.34 | 4.63 ± 0.22 | 5.38 ± 0.17 | 8.00 ± 0.32 | 4.80 ± 0.52 |
| 1498 | 1500 | Pentadecane | C15H32 | 0.82 ± 0.08 | 1.41 ± 0.19 | 3.73 ± 0.06 | 1.15 ± 0.45 | 0.93 ± 0.10 |
| 1598 | 1600 | Hexadecane | C16H34 | 2.05 ± 0.05 | 2.51 ± 0.06 | 2.28 ± 0.28 | 1.38 ± 0.27 | 2.25 ± 0.13 |
| 1697 | 1700 | Heptadecane | C17H36 | 2.08 ± 0.16 | 2.85 ± 0.28 | 1.82 ± 0.14 | 1.04 ± 0.28 | 2.44 ± 0.13 |
| 1797 | 1800 | Octadecane | C18H38 | 1.09 ± 0.10 | 1.24 ± 0.08 | 0.60 ± 0.04 | 0.42 ± 0.12 | 1.16 ± 0.04 |
| 1901 | 1900 | Nonadecane | C19H40 | 0.33 ± 0.04 | 0.48 ± 0.07 | 0.33 ± 0.14 | 0.16 ± 0.06 | 0.43 ± 0.02 |
| 1998 | 2000 | Eicosane | C20H42 | 0.14 ± 0.02 | 0.21 ± 0.02 | 0.07 ± 0.01 | 0.08 ± 0.03 | 0.19 ± 0.01 |
| 2108 | 2109 | Heneicosane | C21H44 | 0.50 ± 0.11 | 1.75 ± 0.33 | 0.37 ± 0.05 | 0.44 ± 0.15 | 0.98 ± 0.15 |
| Straight-chain Alkanes | 24.18 ± 0.57 (a) | 23.46 ± 1.34 (a) | 23.61 ± 0.65 (a) | 22.04 ± 1.28 (a) | 23.41 ± 1.12 (a) | |||
| 818 | 820 | 2,4-Dimethylheptane | C9H20 | 0.52 ± 0.03 | 0.86 ± 0.11 | 0.74 ± 0.07 | 1.09 ± 0.46 | 0.21 ± 0.03 |
| 970 | 971 | 3-Methylnonane | C10H22 | 3.19 ± 0.15 | 0.10 ± 0.02 | 0.14 ± 0.01 | 0.26 ± 0.05 | 2.92 ± 0.48 |
| 1038 | 1035 | 4,5-Dimethylnonane | C11H24 | 3.30 ± 0.27 | 3.78 ± 0.30 | 2.93 ± 0.16 | 2.46 ± 0.63 | 2.44 ± 0.30 |
| 1064 | 1065 | 2-Methyldecane | C11H24 | 1.90 ± 0.14 | 0.58 ± 0.10 | 0.61 ± 0.08 | 0.69 ± 0.15 | 1.32 ± 0.05 |
| 1081 | 1085 | 2,4-Dimethyldecane | C12H26 | 1.56 ± 0.08 | 2.21 ± 0.16 | 1.54 ± 0.07 | 1.19 ± 0.21 | 1.10 ± 0.11 |
| 1170 | 1170 | 3-Methylundecane | C12H26 | 0.45 ± 0.03 | 0.40 ± 0.04 | 0.43 ± 0.02 | 0.70 ± 0.09 | 0.41 ± 0.02 |
| 1175 | 1179 | 3,6-Dimethylundecane | C13H28 | 0.07 ± 0.01 | 0.15 ± 0.02 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.07 ± 0.02 |
| 1212 | 1210 | 2,6-Dimethylundecane | C13H28 | 0.69 ± 0.05 | 1.41 ± 0.11 | 1.26 ± 0.09 | 1.66 ± 0.17 | 0.66 ± 0.14 |
| 1269 | 1271 | 3-Methyldodecane | C13H28 | 1.14 ± 0.07 | 2.43 ± 0.17 | 1.90 ± 0.12 | 2.57 ± 0.28 | 1.26 ± 0.21 |
| 1274 | 1275 | 2,6,11-Trimethyldodecane | C15H32 | 1.11 ± 0.12 | 2.21 ± 0.15 | 1.38 ± 0.10 | 1.26 ± 0.15 | 1.27 ± 0.23 |
| 1320 | 1318 | 2,3,5,8-Tetramethyldecane | C14H30 | 0.60 ± 0.04 | 1.10 ± 0.06 | 1.18 ± 0.08 | 1.52 ± 0.14 | 0.66 ± 0.10 |
| 1351 | 1348 | 5-Methyltridecane | C14H30 | 0.57 ± 0.05 | 0.63 ± 0.08 | 2.03 ± 0.12 | 3.21 ± 0.26 | 0.39 ± 0.04 |
| 1363 | 1364 | 2-Methyltridecane | C14H30 | 0.72 ± 0.08 | 1.48 ± 0.12 | 4.02 ± 0.33 | 5.98 ± 0.54 | 0.71 ± 0.10 |
| 1374 | 1368 | 2,6,10-Trimethyldodecane | C15H32 | 0.13 ± 0.01 | 0.18 ± 0.01 | 0.32 ± 0.04 | 0.45 ± 0.04 | 0.15 ± 0.02 |
| 1420 | 1419 | 2,6,10-Trimethyltridecane | C16H34 | 0.48 ± 0.06 | 1.23 ± 0.05 | 1.54 ± 0.08 | 1.71 ± 0.20 | 0.68 ± 0.03 |
| 1470 | 1470 | 3-Methyltetradecane | C15H32 | 2.18 ± 0.19 | 4.96 ± 0.53 | 6.03 ± 0.47 | 5.52 ± 1.17 | 2.14 ± 0.26 |
| 1482 | 1483 | 6,9-Dimethyltetradecane | C16H34 | 0.57 ± 0.04 | 1.36 ± 0.09 | 0.60 ± 0.06 | 0.31 ± 0.04 | 0.77 ± 0.07 |
| 1544 | 1539 | 7-Methylpentadecane | C16H34 | 1.76 ± 0.14 | 4.10 ± 0.16 | 3.25 ± 0.24 | 2.35 ± 0.55 | 2.23 ± 0.20 |
| 1550 | 1548 | 4-Ethyltetradecane | C16H34 | 0.15 ± 0.03 | 0.33 ± 0.10 | 0.66 ± 0.13 | 0.61 ± 0.21 | 0.16 ± 0.02 |
| 1562 | 1563 | 2-Methylpentadecane | C16H34 | 0.85 ± 0.02 | 1.55 ± 0.18 | 2.17 ± 0.22 | 1.64 ± 0.11 | 0.80 ± 0.04 |
| 1747 | 2,6,10-Trimethylhexadecane | C19H40 | 0.88 ± 0.05 | 1.75 ± 0.13 | 0.84 ± 0.05 | 0.54 ± 0.17 | 1.24 ± 0.07 | |
| 1762 | 1764 | 2-Methylheptadecane | C18H38 | 0.59 ± 0.04 | 1.07 ± 0.07 | 0.65 ± 0.07 | 0.41 ± 0.13 | 0.82 ± 0.04 |
| 1803 | 1810 | 2,6,10,14-Tetramethylhexadecane | C20H42 | 0.22 ± 0.01 | 0.45 ± 0.05 | 0.10 ± 0.01 | 0.14 ± 0.05 | 0.44 ± 0.04 |
| 1913 | 1910 | 2,6-Dimethyloctadecane | C20H42 | 0.78 ± 0.09 | 1.27 ± 0.16 | 0.40 ± 0.05 | 0.38 ± 0.13 | 1.14 ± 0.09 |
| 1940 | 1943 | 9-Methylnonadecane | C20H42 | 0.19 ± 0.03 | 0.49 ± 0.07 | 0.15 ± 0.01 | 0.09 ± 0.03 | 0.32 ± 0.03 |
| 1961 | 1960 | 2,6,10,14-Tetramethyloctadecane | C22H46 | 0.15 ± 0.02 | 0.31 ± 0.06 | 0.07 ± 0.01 | 0.07 ± 0.02 | 0.27 ± 0.03 |
| 1971 | 1970 | 3-Methylnonadecane | C20H42 | 0.07 ± 0.01 | 0.16 ± 0.03 | 0.04 ± 0.004 | 0.08 ± 0.03 | 0.13 ± 0.03 |
| 2040 | 2045 | 2-Methyleicosane | C21H44 | 0.10 ± 0.02 | 0.25 ± 0.06 | 0.06 ± 0.004 | 0.06 ± 0.03 | 0.20 ± 0.03 |
| 2144 | 2144 | 10-Methylheneicosane | C22H46 | 0.28 ± 0.07 | 1.01 ± 0.25 | 0.18 ± 0.02 | 0.31 ± 0.11 | 0.48 ± 0.09 |
| 2175 | 2173 | 3-Methylheneicosane | C22H46 | 0.60 ± 0.18 | 0.83 ± 0.09 | 0.23 ± 0.03 | 0.23 ± 0.04 | 0.56 ± 0.06 |
| Branched-chain Alkanes | 25.80 ± 0.67 (a) | 38.64 ± 0.62 (b) | 35.56 ± 0.63 (b) | 37.60 ± 1.67 (b) | 25.95 ± 1.84 (a) | |||
| 1238 | 1231 | Cyclohexane isothiocyanate | C7H11NS | 1.06 ± 0.09 | 1.63 ± 0.17 | 3.57 ± 0.39 | 2.32 ± 0.29 | 1.16 ± 0.21 |
| 1552 | 1550 | Decylcyclopentane | C15H30 | 0.19 ± 0.03 | 0.59 ± 0.06 | 0.27 ± 0.07 | 0.34 ± 0.06 | 0.32 ± 0.03 |
| 1660 | 1657 | Decylcyclohexane | C16H32 | 0.29 ± 0.01 | 0.29 ± 0.02 | 0.27 ± 0.04 | 0.19 ± 0.05 | 0.47 ± 0.02 |
| Cyclodecane | C10H20 | 0.39 ± 0.04 | 0.94 ± 0.05 | 0.36 ± 0.02 | 0.33 ± 0.09 | 0.65 ± 0.04 | ||
| Cyclic Alkanes | 1.93 ± 0.11 (a) | 3.45 ± 0.18 (b) | 4.47 ± 0.37 (c) | 3.18 ± 0.34 (b) | 2.60 ± 0.25 (a) | |||
| 838 | 836 | 2,4-Dimethyl-1-heptene | C9H18 | 0.79 ± 0.04 | 2.78 ± 0.14 | 1.96 ± 0.19 | 1.22 ± 0.32 | 0.51 ± 0.06 |
| 1390 | 1392 | 1-Tetradecene | C14H28 | 0.14 ± 0.01 | 0.38 ± 0.05 | 0.56 ± 0.05 | 0.80 ± 0.05 | 0.15 ± 0.02 |
| Alkenes | 0.93 ± 0.04 (a) | 3.16 ± 0.16 (c) | 2.52 ± 0.17 (b) | 2.02 ± 0.29 (b) | 0.66 ± 0.09 (a) | |||
| 857 | 855 | Ethylbenzene | C8H10 | 1.60 ± 0.12 | 0.65 ± 0.16 | 3.22 ± 0.30 | 3.77 ± 0.91 | 1.57 ± 0.24 |
| 868 | 866 | 1,3-Dimethylbenzene | C8H10 | 6.70 ± 0.57 | 1.13 ± 0.21 | 5.12 ± 0.32 | 8.13 ± 1.90 | 4.48 ± 0.60 |
| 916 | 920 | Methoxybenzene | C7H8O | 0.25 ± 0.03 | 0.20 ± 0.02 | 0.23 ± 0.03 | 0.05 ± 0.03 | 0.30 ± 0.05 |
| 953 | 953 | Propylbenzene | C9H12 | 0.76 ± 0.03 | 0.15 ± 0.02 | 0.20 ± 0.01 | 0.15 ± 0.03 | 0.53 ± 0.07 |
| 964 | 961 | 1,3,5-Trimethylbenzene | C9H12 | 6.21 ± 0.12 | 0.95 ± 0.10 | 0.95 ± 0.05 | 0.90 ± 0.20 | 5.56 ± 0.87 |
| 994 | 997 | 1,2,3-Trimethylbenzene | C9H12 | 16.31 ± 0.61 | 0.17 ± 0.03 | 0.37 ± 0.02 | 0.34 ± 0.08 | 16.50 ± 3.45 |
| 1085 | 1081 | 1-Ethyl-2,4-dimethylbenzene | C10H14 | 0.74 ± 0.04 | 0.23 ± 0.02 | 0.34 ± 0.01 | 0.30 ± 0.02 | 0.85 ± 0.02 |
| 1122 | 1117 | 1,2,4,5-Tetramethylbenzene | C10H14 | 0.85 ± 0.02 | 0.23 ± 0.03 | 1.23 ± 0.07 | 3.97 ± 0.52 | 0.73 ± 0.02 |
| 1192 | 1190 | Naphthalene | C10H8 | 0.68 ± 0.03 | 0.48 ± 0.06 | 0.95 ± 0.05 | 0.91 ± 0.14 | 0.49 ± 0.01 |
| 1233 | 1229 | Benzothiazole | C7H5NS | 0.69 ± 0.12 | 0.45 ± 0.04 | 0.56 ± 0.02 | 0.37 ± 0.04 | 0.63 ± 0.07 |
| 1250 | 1247 | 1,3-Di-tert-butylbenzene | C14H22 | 0.30 ± 0.03 | 1.79 ± 0.03 | 0.80 ± 0.05 | 0.66 ± 0.06 | 0.35 ± 0.03 |
| 1330 | 1331 | 2-Methylpropyl benzoate | C11H14O2 | 0.49 ± 0.09 | 1.05 ± 0.21 | 2.84 ± 0.56 | 1.98 ± 0.41 | 0.34 ± 0.05 |
| Benzene derivatives | 35.58 ± 0.95 (c) | 7.48 ± 0.61 (a) | 16.81 ± 0.37 (b) | 21.53 ± 3.12 (b) | 32.33 ± 3.76 (c) | |||
| 1878 | 1880 | 1-Hexadecanol | C16H34O | 1.00 ± 0.08 | 1.58 ± 0.15 | 0.64 ± 0.15 | 0.67 ± 0.33 | 1.47 ± 0.04 |
| 2072 | 2070 | 1-Octadecanol | C18H38O | 0.19 ± 0.02 | 0.79 ± 0.13 | 0.23 ± 0.06 | 0.49 ± 0.25 | 0.74 ± 0.03 |
| Straight-chain Alcohols | 1.19 ± 0.10 (a) | 2.37 ± 0.20 (b) | 0.87 ± 0.20 (a) | 1.16 ± 0.57 (a) | 2.21 ± 0.05 (b) | |||
| 1027 | 1020 | 2-Ethyl-1-hexanol | C8H18O | 1.79 ± 0.20 | 2.19 ± 0.18 | 4.08 ± 0.09 | 2.18 ± 0.34 | 2.08 ± 0.08 |
| 1227 | 1229 | 4,8-Dimethyl-1-nonanol | C11H24O | 0.03 ± 0.002 | 0.03 ± 0.004 | 0.09 ± 0.01 | 0.24 ± 0.03 | 0.03 ± 0.01 |
| 1283 | 1277 | 2-Butyl-1-octanol | C12H26O | 0.48 ± 0.03 | 1.06 ± 0.07 | 0.97 ± 0.05 | 0.90 ± 0.07 | 0.49 ± 0.07 |
| 1300 | 1293 | 2-Methyl-1-decanol | C11H24O | 0.66 ± 0.04 | 2.81 ± 0.18 | 1.95 ± 0.08 | 1.65 ± 0.07 | 0.98 ± 0.09 |
| 1316 | 1320 | 5,9-Dimethyl-1-decanol | C12H26O | 0.51 ± 0.12 | 1.82 ± 0.12 | 1.51 ± 0.06 | 1.40 ± 0.06 | 0.60 ± 0.06 |
| 1487 | 1492 | 11-Methyldodecanol | C13H28O | 1.51 ± 0.12 | 3.19 ± 0.19 | 0.91 ± 0.12 | 0.98 ± 0.14 | 2.02 ± 0.17 |
| 1515 | 1510 | 2-Hexyl-1-decanol | C16H34O | 0.25 ± 0.01 | 0.69 ± 0.04 | 0.73 ± 0.07 | 0.42 ± 0.08 | 0.46 ± 0.03 |
| 1525 | 1520 | 2-Octyl-1-decanol | C18H38O | 0.50 ± 0.05 | 1.18 ± 0.09 | 1.45 ± 0.16 | 1.05 ± 0.25 | 0.55 ± 0.07 |
| 1995 | 1989 | 2-Hexyl-1-dodecanol | C18H38O | 0.75 ± 0.14 | 1.01 ± 0.15 | 0.44 ± 0.06 | 0.22 ± 0.03 | 0.85 ± 0.10 |
| 2054 | 2060 | 9-Octadecen-1-ol | C18H36O | 0.16 ± 0.03 | 2.21 ± 0.21 | 0.74 ± 0.24 | 1.23 ± 0.62 | 0.22 ± 0.03 |
| 2188 | 2188 | 2-Octyl-1-dodecanol | C20H42O | 0.18 ± 0.04 | 0.52 ± 0.05 | 0.22 ± 0.03 | 0.17 ± 0.06 | 0.26 ± 0.04 |
| Branched-chain Alcohols | 6.82 ± 0.25 (a) | 16.71 ± 0.39 (e) | 13.09 ± 0.20 (d) | 10.44 ± 0.89 (c) | 8.54 ± 0.59 (b) | |||
| 1473 | 1475 | Decanoic acid, 2-propenyl ester | C13H24O2 | 0.51 ± 0.03 | 1.39 ± 0.03 | 1.09 ± 0.07 | 0.89 ± 0.15 | 0.68 ± 0.03 |
| 1887 | 1886 | Pentadecanoic acid, 14-methyl-, methyl ester | C17H34O2 | 0.17 ± 0.02 | 0.35 ± 0.04 | 0.12 ± 0.02 | 0.09 ± 0.03 | 0.25 ± 0.01 |
| 1918 | 1914 | Pentadecanoic acid, isopropyl ester | C18H36O2 | 0.26 ± 0.03 | 0.88 ± 0.03 | 0.15 ± 0.02 | 0.13 ± 0.04 | 0.34 ± 0.03 |
| - | 2382 | Hexanedioic acid, bis (2-ethylhexyl) ester | C22H42O4 | 0.45 ± 0.06 | 0.58 ± 0.13 | 0.18 ± 0.04 | 0.12 ± 0.03 | 0.48 ± 0.17 |
| 1605 | 1606 | Dodecanoic acid, methyl ester | C13H26O2 | 2.10 ± 0.14 | 1.32 ± 0.10 | 1.39 ± 0.24 | 0.77 ± 0.25 | 2.55 ± 0.15 |
| Esters | 3.49 ± 0.20 (b) | 4.52 ± 0.21 (c) | 2.93 ± 0.20 (b) | 2.00 ± 0.47 (a) | 4.30 ± 0.17 (c) | |||
| 982 | 983 | 3-Octanone | C8H16O | - | 0.08 ± 0.004 | 0.08 ± 0.01 | - | - |
| 1204 | 1204 | Decanal | C10H20O | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.06 ± 0.003 | 0.05 ± 0.01 |
| 1815 | 1816 | Hexadecanal | C16H32O | 0.13 ± 0.04 | 0.12 ± 0.01 | 0.09 ± 0.01 | 0.04 ± 0.01 | 0.08 ± 0.01 |
| Ketones and Aldehydes | 0.21 ± 0.04 (b) | 0.27 ± 0.01 (b) | 0.25 ± 0.02 (b) | 0.10 ± 0.01 (a) | 0.13 ± 0.01 (a) | |||
| VOC | CN * | AIT (°C) * | BP (°C) * | Reference |
|---|---|---|---|---|
| Octane | 64.0 | 220 | 126 | [30,33,34] |
| Nonane | 72.0 | 206 | 151 | [30,33,34] |
| Decane | 77.0 | 208 | 174 | [30,33,34] |
| Undecane | 79.0 | 198 | 196 | [30,33,34] |
| Dodecane | 80.0 | 204 | 216 | [30,33,34] |
| Tetradecane | 96.0 | 202 | 253 | [30,33,34] |
| Pentadecane | 98.0 | 195 | 267 | [30,34,35] |
| Hexadecane | 100.0 | 202 | 287 | [30,33,34] |
| Heptadecane | 105.0 | 203 | 302 | [30,34,36] |
| Octadecane | 110.0 | 235 | 317 | [30,33,34] |
| Nonadecane | 110.0 | 230 | 330 | [30,33,34] |
| Eicosane | 110.0 | 232 | 343 | [30,34,36] |
| Heneicosane | N/A | 220 | 356 | [34,36] |
| 2-Methylpentane | 34.5 | 303 | 60 | [31,33,34] |
| 2,3-Dimethylpentane | 21.9 | 337 | 90 | [31,33,34] |
| 2,2,4-Trimethylpentane | 17.8 | 418 | 99 | [31,33,34] |
| 3-Ethyldecane | 48.0 | N/A | 209 | [31,34] |
| 4-Propyldecane | 39.0 | N/A | 236 | [31,34] |
| 2-Methylheptane | 52.6 | N/A | 116 | [31,34] |
| 2,4-Dimethylheptane | 31.0 | N/A | 134 | [32,34] |
| 3-Methylnonane | 56.0 | N/A | 168 | [34,37] |
| 3-Methylundecane | 69.0 | N/A | 210 | [34,37] |
| 2,6-Dimethylundecane | 50.0 | N/A | 218 | [34,37] |
| 2,6,10-Trimethyldodecane | 59.1 | N/A | 248 | [34,38] |
| 4-Ethyltetradecane | 73.0 | N/A | 282 | [34,37] |
| 2-methylpentadecane | 100.0 | N/A | 282 | [34,37] |
| 2-Methylheptadecane | 101.0 | N/A | 311 | [34,37] |
| 3-Methylheneicosane | N/A | N/A | 376 | [34] |
| 1-Tetradecene | 83 | 235 | 247 | [29,33,34] |
| Ethylbenzene | 6.3 | 432 | 136 | [31,33,34] |
| 1,3-Dimethylbenzene | 7.0 | 527 | 139 | [31,34,39] |
| Propylbenzene | 16.0 | 456 | 159 | [31,33,34] |
| Naphthalene | 1.0 | 526 | 218 | [34,40,41] |
| 1,2,3-Trimethylbenzene | 10.1 | 470 | 176 | [31,33,34] |
| 1,2,4-Trimethylbenzene | 8.9 | 515 | 169 | [31,33,34] |
| 1,2,4,5-Tetramethylbenzene | 1.0 | N/A | 194 | [31,34] |
| Cyclohexane | 20.0 | 260 | 80.7 | [31,33,34] |
| Butylcyclohexane | 47.8 | 246 | 181 | [31,33,34] |
| 1-Hexadecanol | 68.0 | N/A | 249 | [30,34] |
| 1-Octadecanol | 81.0 | 177 | 210 | [30,34] |
| 2-Ethyl-1-hexanol | 23.4 | 288 | 184 | [30,33,34] |
| 3-Octanona | 35.2 | N/A | 167 | [30,34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achimón, F.; Brito, V.D.; Pizzolitto, R.P.; Zygadlo, J.A. Effect of Carbon Sources on the Production of Volatile Organic Compounds by Fusarium verticillioides. J. Fungi 2022, 8, 158. https://doi.org/10.3390/jof8020158
Achimón F, Brito VD, Pizzolitto RP, Zygadlo JA. Effect of Carbon Sources on the Production of Volatile Organic Compounds by Fusarium verticillioides. Journal of Fungi. 2022; 8(2):158. https://doi.org/10.3390/jof8020158
Chicago/Turabian StyleAchimón, Fernanda, Vanessa D. Brito, Romina P. Pizzolitto, and Julio A. Zygadlo. 2022. "Effect of Carbon Sources on the Production of Volatile Organic Compounds by Fusarium verticillioides" Journal of Fungi 8, no. 2: 158. https://doi.org/10.3390/jof8020158







