Recent Advances in Alternaria Phytotoxins: A Review of Their Occurrence, Structure, Bioactivity, and Biosynthesis
Abstract
:1. Introduction
2. Host-Selective Toxins
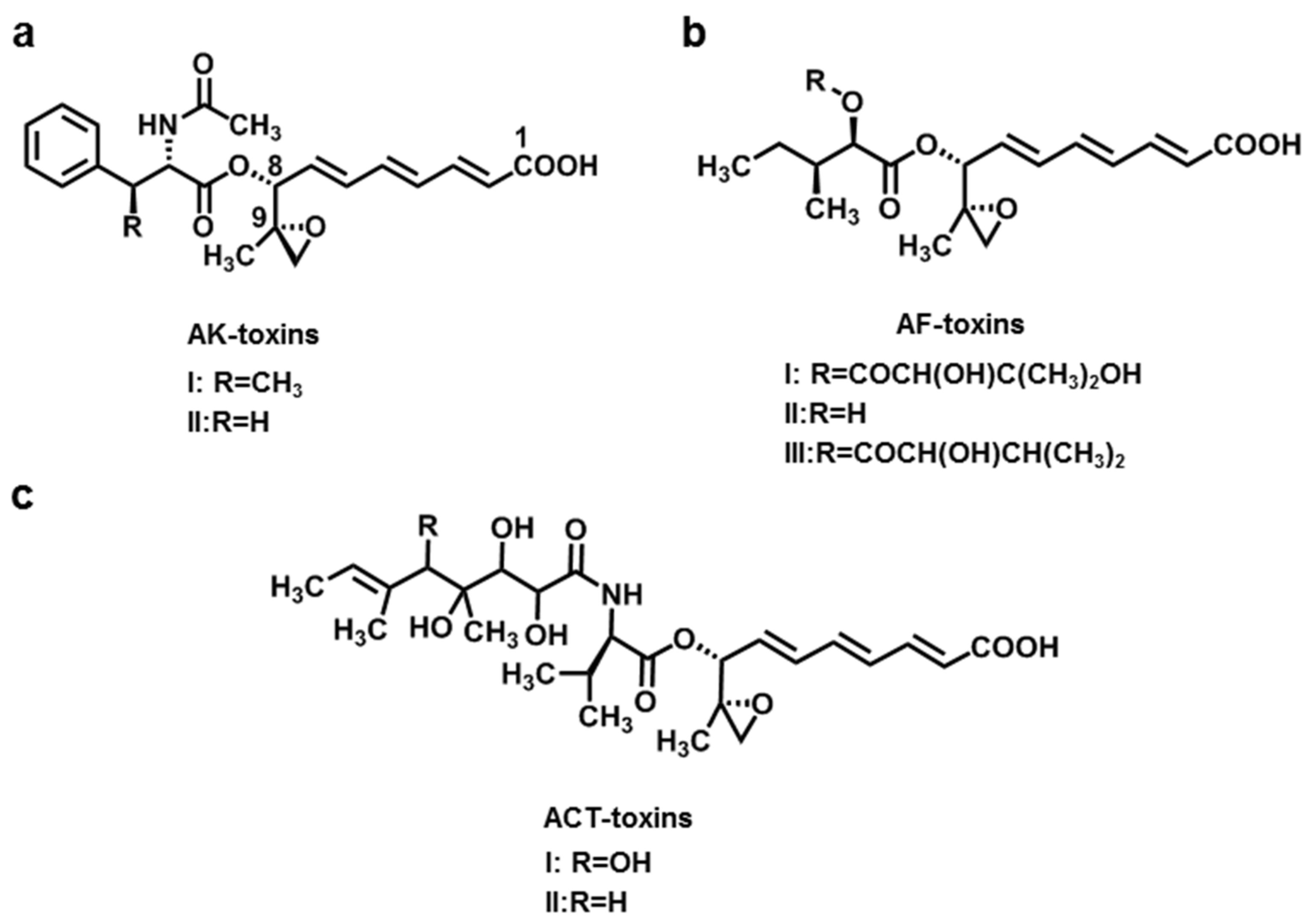
2.1. AK-Toxins, AF-Toxins, and ACT-Toxins
2.2. AAL-Toxins
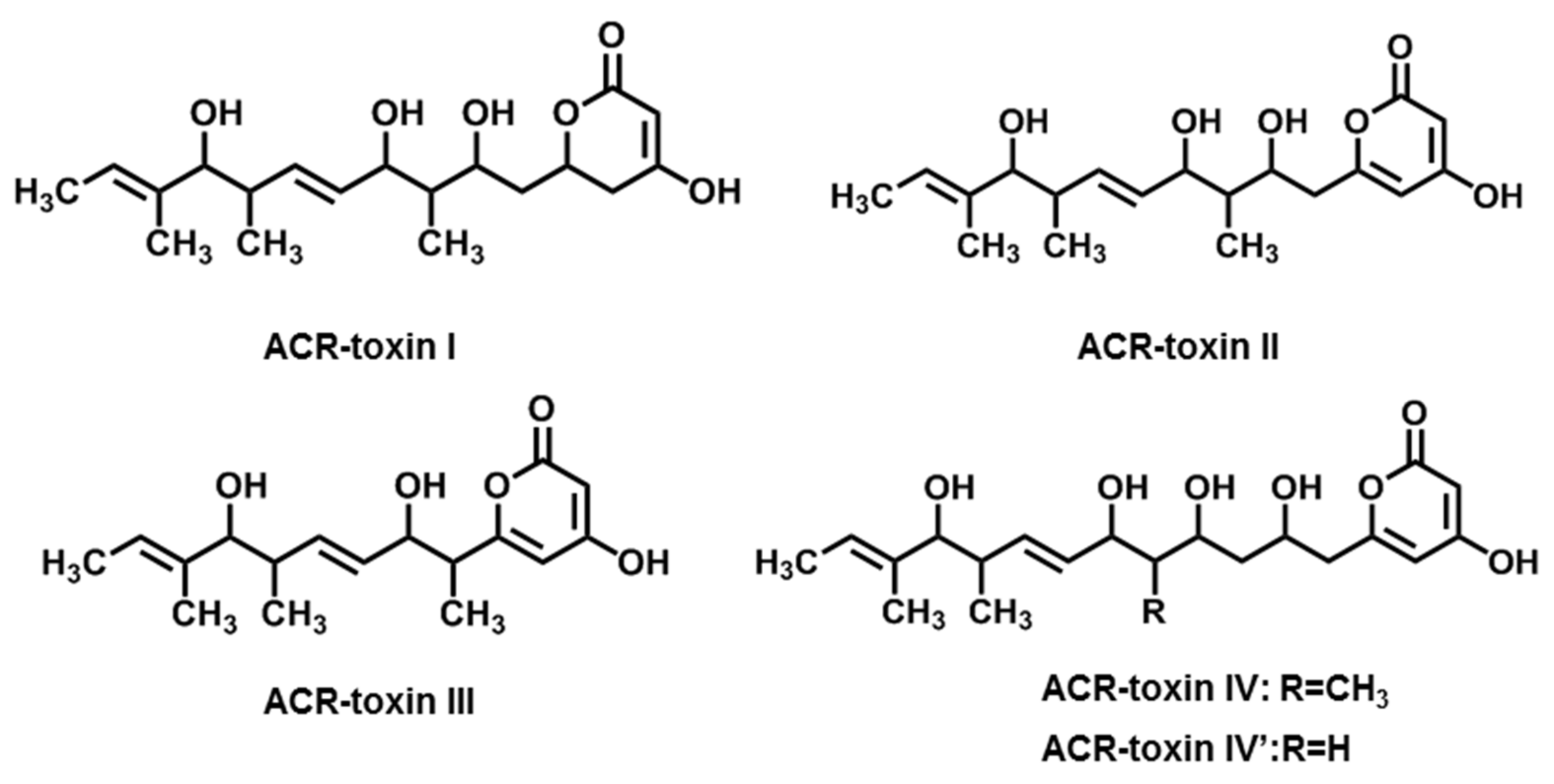
2.3. ACR-Toxins
2.4. AM-Toxins
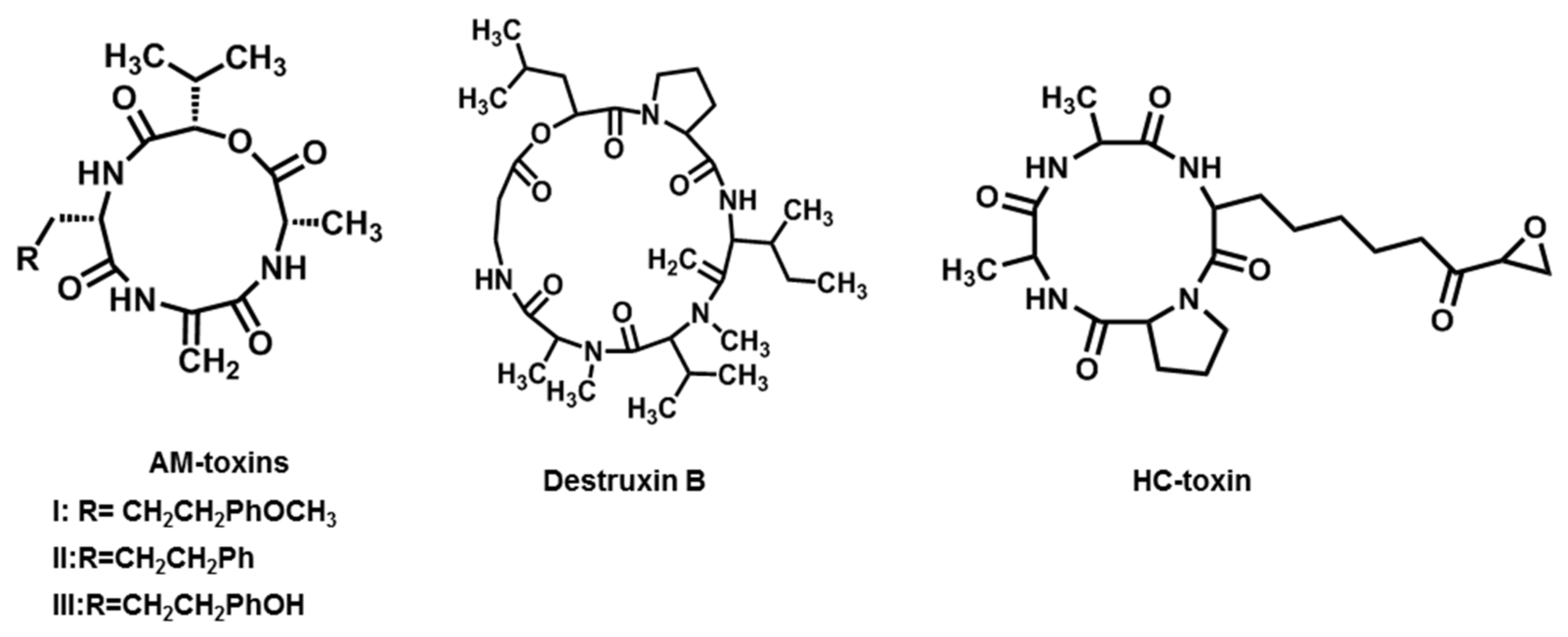
2.5. Destruxin B
2.6. HC-Toxin
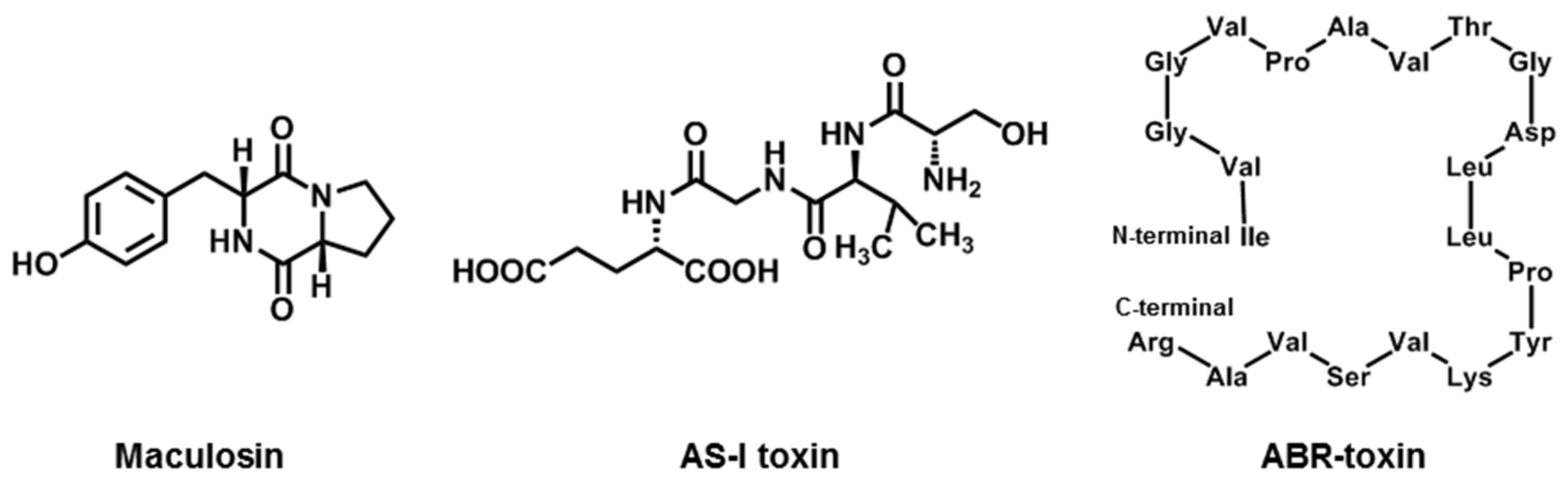
2.7. Maculosin
2.8. AS-I Toxin
2.9. ABR-Toxin
3. Non-Host-Selective Toxins
3.1. Pyranones
3.1.1. Simple Pyranones
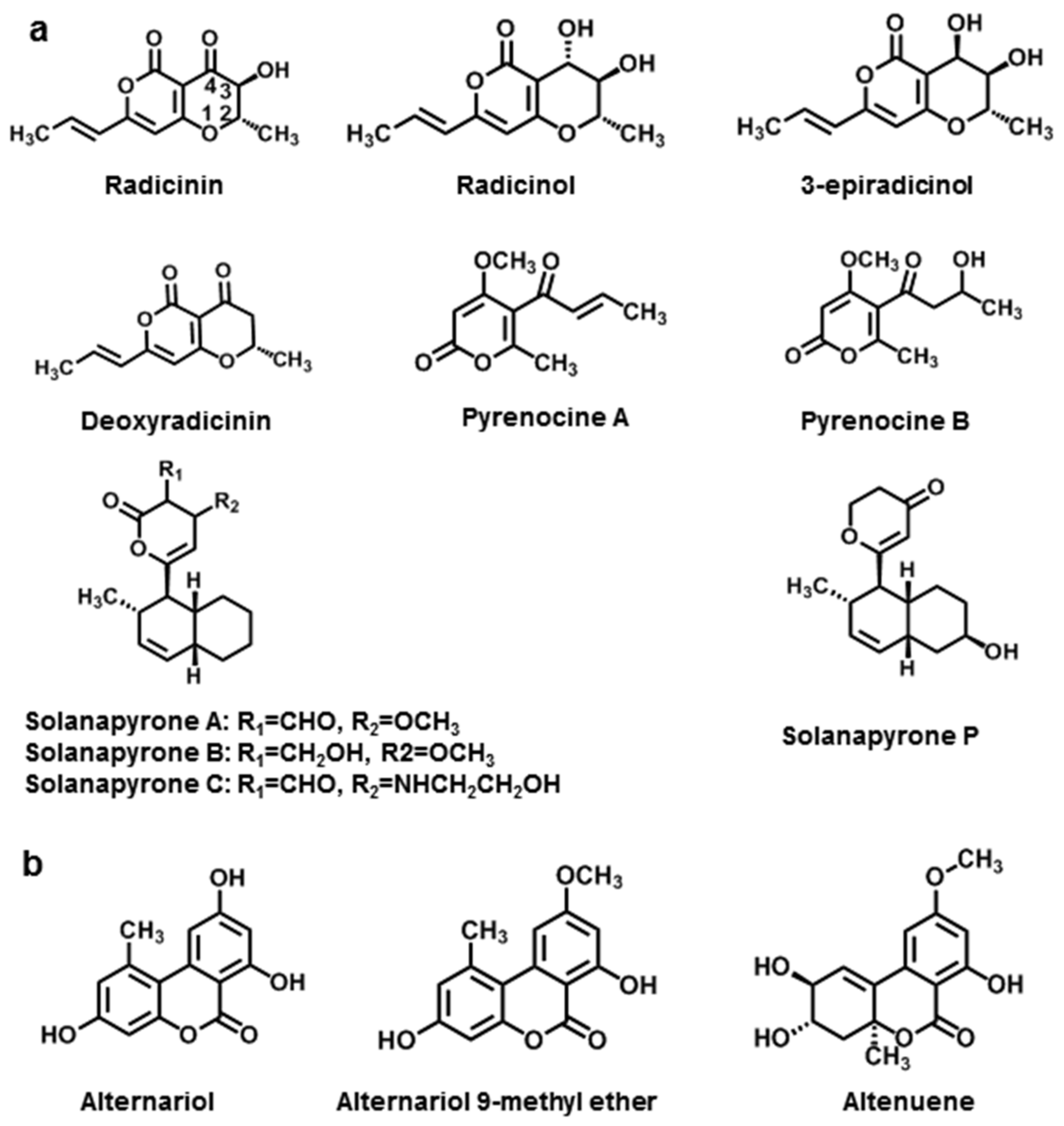
3.1.2. Dibenzopyranones
3.2. Quinones
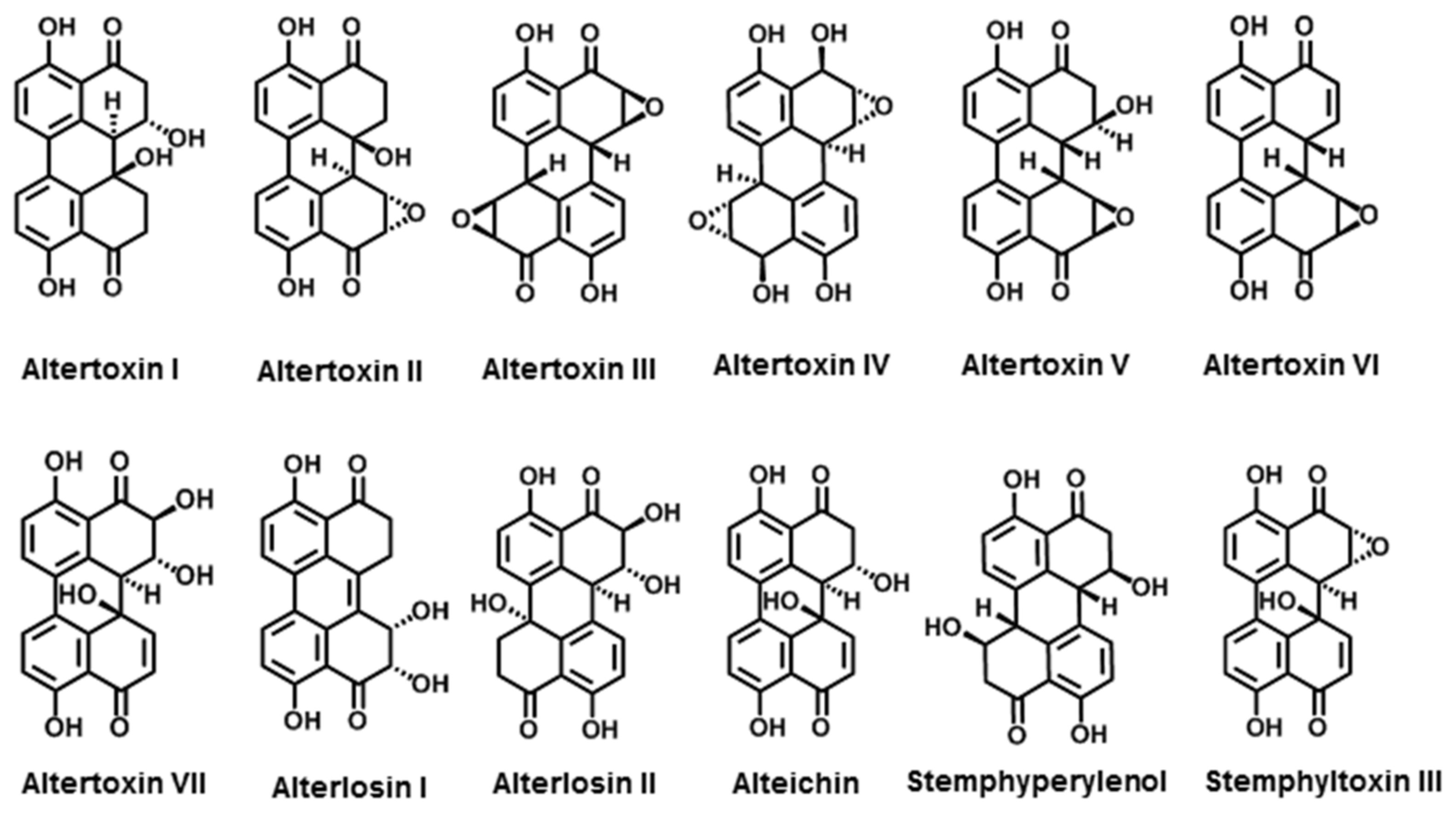
3.2.1. Perylenequinone Derivatives
3.2.2. Anthraquinone Derivatives
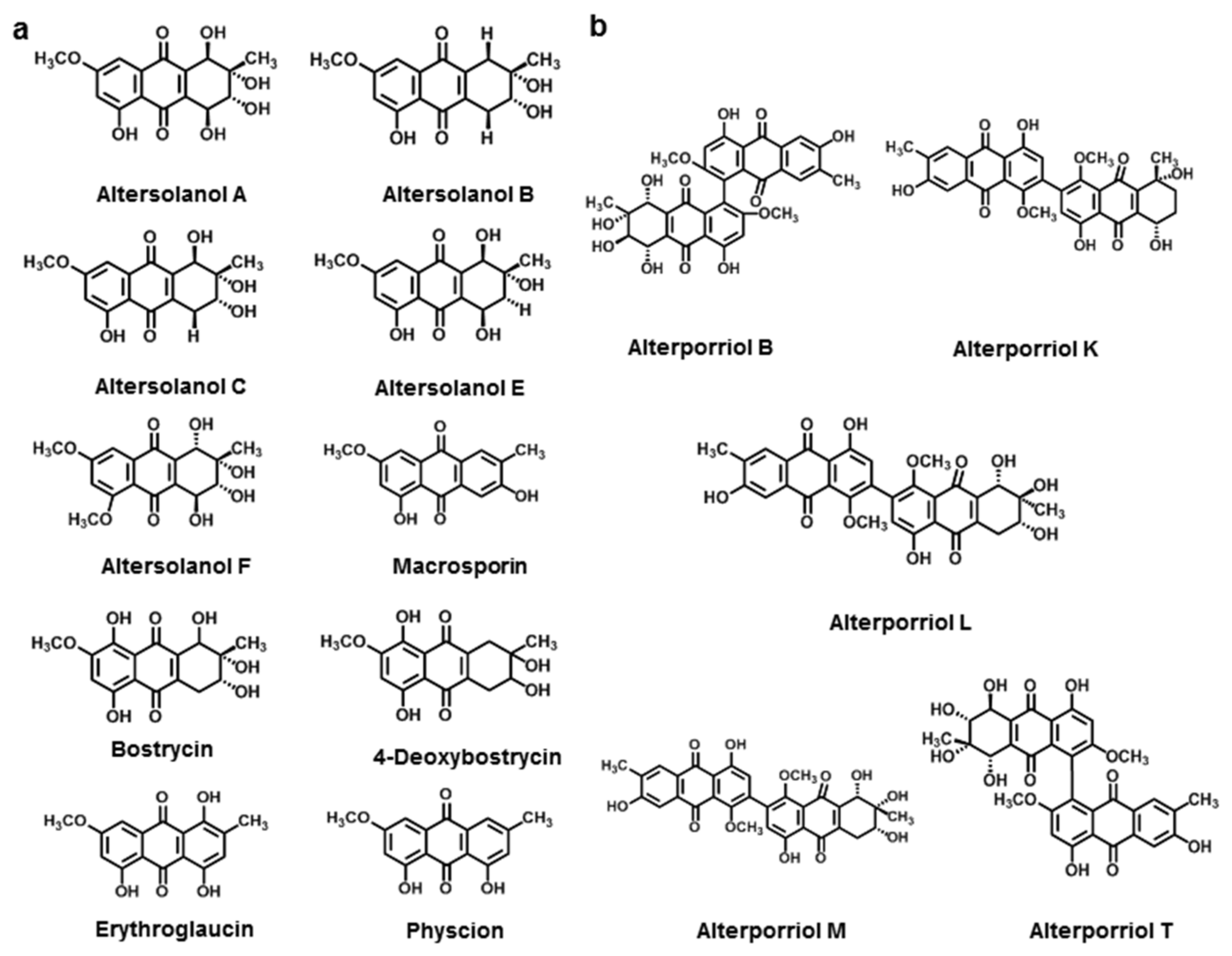
3.2.3. Bianthraquinone
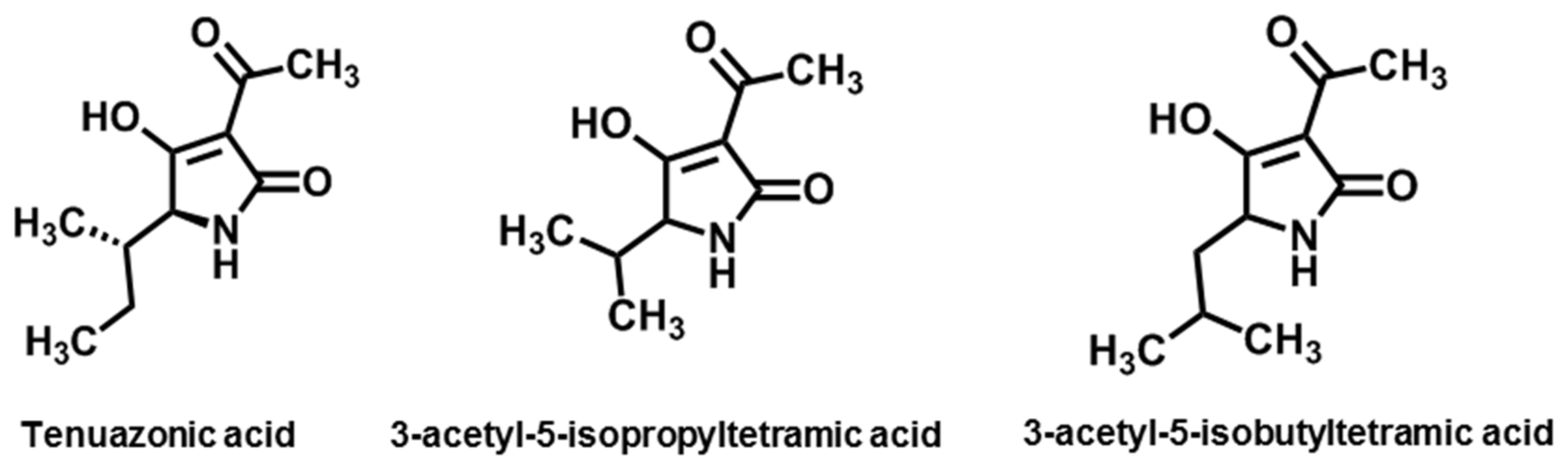
3.3. Tertramic Acids
3.4. Cyclic Peptides
3.5. Macrolides
3.6. Phenols
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomma, B.P.H.J. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.P.; Gannibal, P.B.; Peever, T.L.; Pryor, B.M. The sections of Alternaria: Formalizing species-group concepts. Mycologia 2013, 105, 530–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.B.; Patriarca, A.; Magan, N. Alternaria in food: Ecophysiology, mycotoxin production and toxicology. Mycobiology 2015, 43, 93–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woudenberg, J.H.C.; Seidl, M.F.; Groenewald, J.Z.; de Vries, M.; Stielow, J.B.; Thomma, B.P.H.J.; Crous, P.W. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Zwickel, T.; Kahlm, S.; Rychlik, M.; Muller, M. Chemotaxonomy of mycotoxigenic small-spored Alternaria fungi-do multitoxin mixtures act as an indicator for species differentiation? Front. Microbiol. 2018, 9, 1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from Alternaria fungi and their bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, S.; Kurata, M.; Harimoto, Y.; Hatta, R.; Yamamoto, M.; Akimitsu, K.; Tsuge, T. Complex regulation of secondary metabolism controlling pathogenicity in the phytopathogenic fungus Alternaria alternata. New Phytol. 2014, 202, 1297–1309. [Google Scholar] [CrossRef]
- Howlett, B.J. Secondary metabolite toxins and nutrition of plant pathogenic fungi. Curr. Opin. Plant Biol. 2006, 9, 371–375. [Google Scholar] [CrossRef]
- Tsuge, T.; Harimoto, Y.; Akimitsu, K.; Kodama, M.; Akagi, Y.; Egusa, M.; Yamamoto, M.; Otani, H. Host-selective toxins produced by the plant pathogenic fungus Alternaria alternata. FEMS Microbiol. Rev. 2013, 37, 44–66. [Google Scholar] [CrossRef]
- Nakashima, T.; Ueno, T.; Fukami, H.; Taga, T.; Masuda, H.; Osaki, K.; Otani, H.; Kohmoto, K.; Nishimura, S. Isolation and structure of AK-toxin I and II, host specific phytotoxic metabolites produced by Alternaria alternata Japanese pear pathotype. Agric. Biol. Chem. 1985, 49, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Nakatsuka, S.; Ueda, K.; Goto, T.; Yamamoto, M.; Nishimura, S.; Kohmoto, K. Structure of AF-toxin II, one of the host-specific toxins produced by Alternaria alternata strawberry pathotype. Tetrahedron Lett. 1986, 27, 2753–2756. [Google Scholar] [CrossRef]
- Mikihiro, Y.; Fumio, N.; Syoyo, N.; Keisuke, K. Studies on host-specific AF-toxins produced by Alternaria alternata strawberry pathotype causing Alternaria black spot of strawberry (3) Use of toxin for determining inheritance of disease reaction in strawberry cultivar morioka-16. Ann. Phytopath. Soc. Jpn. 1985, 51, 530–535. [Google Scholar]
- Pegg, K.G. Studies of strain of Alternaria citri pierce, the causal organism of brown spot of Emperor mandarn. Queensl. J. Agric. Anim. Sci. 1966, 23, 15–28. [Google Scholar]
- Timmer, L.W.; Solel, Z.; Orozco-Santos, M. Alternaria brown spot of mandarins. In Compendium of Citrus Diseases, 2nd ed.; Timmer, L.W., Garnsey, S.M., Graham, J.H., Eds.; Amer Phytopathological Society: Saint Paul, MI, USA, 2000; pp. 19–21. [Google Scholar]
- Akimitsu, K.; Peever, T.L.; Timmer, L.W. Molecular, ecological and evolutionary approaches to understanding Alternaria diseases of citrus. Mol. Plant Pathol. 2003, 4, 435–446. [Google Scholar] [CrossRef]
- Grogan, R.; Kimble, K.; Misaghi, I. A stem canker disease of tomato caused by Alternaria alternata f. sp. lycopersici. Phytopathology 1975, 65, 880–886. [Google Scholar] [CrossRef]
- Gilchrist, D.G. Production and nature of a host-specific toxin from Alternaria alternata f. sp. lycopersici. Phytopathology 1976, 66, 165–171. [Google Scholar] [CrossRef]
- Kohmoto, K.; Scheffer, R.; Whiteside, J. Host-Selective Toxins from Alternaria citri. Phytopathology 1979, 69, 667–671. [Google Scholar] [CrossRef] [Green Version]
- Kohmoto, K.; Akimitsu, K.; Otani, H. Correlation of resistance and susceptibility of citrus to Alternaria alternata with sensitivity to host-specific toxins. Phytopathology 1991, 81, 719–722. [Google Scholar] [CrossRef]
- Okuno, T.; Ishita, Y.; Sawai, K.; Matsumoto, T. Characterization of alternariolide, a host-specific toxin produced by Alternaria mali Roberts. Chem. Lett. 1974, 3, 635–638. [Google Scholar] [CrossRef]
- Hoffmeister, D.; Keller, N. Natural products of filamentous fungi: Enzymes, genes, and their regulation. Nat. Prod. Rep. 2007, 24, 393–416. [Google Scholar] [CrossRef]
- Tewari, J.; Bains, P. Phytotoxins produced by Alternaria brassicae and bioassay of destruxin B. In Toxins in Plant Disease Development and Evolving Biotechnology; Upadhyay, R., Ed.; Science Publishers: Enfield, NH, USA, 1997; Volume 2, pp. 21–35. [Google Scholar]
- Parada, R.Y.; Sakuno, E.; Mori, N.; Oka, K.; Egusa, M.; Kodama, M.; Otani, H. Alternaria brassicae produces a host-specific protein toxin from germinating spores on host leaves. Biochem. Cell Biol. 2008, 98, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Pringle, R.B. Amino acid composition of the host-specific toxin of Helminthosporium carbonum. Plant Physiol. 1971, 48, 756–759. [Google Scholar] [CrossRef] [Green Version]
- Kawai, M.; Rich, D.H.; Walton, J.D. The structure and conformation of HC-toxin. Biochem. Biophys. Res. Comm. 1983, 111, 398–403. [Google Scholar] [CrossRef]
- Wight, W.D.; Labuda, R.; Walton, J.D. Conservation of the genes for HC-toxin biosynthesis in Alternaria jesenskae. BMC Microbiol. 2013, 13, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.; Cranston, R. An economic evaluation of control methods for diffuse and spotted knapweed in Western Canada. Can. J. Plant Sci. 1979, 59, 375–382. [Google Scholar] [CrossRef]
- Bobylev, M.M.; Bobyleva, L.I.; Strobel, G.A. Synthesis and bioactivity of analogs of maculosin, a host-specific phytotoxin produced by Alternaria alternata on spotted knapweed (Centaurea maculosa). J. Agric. Food Chem. 1996, 44, 3960–3964. [Google Scholar] [CrossRef]
- Liakopoulou-Kyriakides, M.; Lagopodi, A.L.; Thanassoulopoulos, C.C.; Stavropoulos, G.S.; Magafa, V. Isolation and synthesis of a host-selective toxin produced by Alternaria alternate. Phytochemistry 1997, 45, 37–40. [Google Scholar] [CrossRef]
- Feng, B.; Nakastuka, S.; Goto, T.; Tsuge, T.; Nishimura, S. Biosyntheses of host-selective toxins produced by Alternaria alternata pathogens, I: (8R, 9S)-9,10-epoxy-8-hydroxy-9-methyl-deca-(2E, 4Z, 6E)-trienoic acid as a biological precursor of AK-toxins. Agric. Biol. Chem. 1990, 54, 845–848. [Google Scholar] [CrossRef]
- Gardner, J.M.; Kono, Y.; Tatum, J.H.; Suzuki, Y.; Takeuchi, S. Structure of the major component of ACRL-toxins, host-specific pathotoxic compound produced by Alternaria citri. Agric. Biol. Chem. 1985, 49, 1235–1238. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 2003, 119, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Namiki, F.; Yamamoto, M.; Nishimura, S.; Nakatsuka, S.; Goto, T.; Kohmot, K.; Otani, H. Studies on host-specific AF-toxins produced by Alternaria alternata strawberry pathotype causing Alternaria black spot of strawberry. 4. Protective effect of AF-toxin II on AF-toxin I-induced toxic action and fungal infection. Ann. Phytopath. Soc. Jpn. 1986, 52, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, N.; Yamamoto, M.; Nishimura, S.; Kohmoto, K.; Kuwada, K.; Watanabe, Y. Studies on host-specific AF-toxins produced by Alternaria alternata strawberry pathotype causing Alternaria black spot of strawberry. (1) Production of host-specific toxins and their biological activities. Ann. Phytopathol Soc. Jpn. 1984, 50, 600–609. [Google Scholar] [CrossRef] [Green Version]
- Kohmoto, K.; Itoh, Y.; Shimomura, N.; Kondoh, Y.; Otani, H.; Kodama, M.; Nishimura, S.; Nakatsuka, S. Isolation and biological activities of two host-specific toxins from the tangerine pathotype of Alternaria alternata. Phytopathology 1993, 83, 495–502. [Google Scholar] [CrossRef]
- Otani, H.; Kohmoto, K.; Nishimura, S.; Nakashima, T.; Ueno, T.; Fukami, H. Biological activities of AK-toxins I and II, host-specific toxins from Alternaria alternata Japanese pear pathotype. Ann. Phytopathol Soc. Jpn. 1985, 51, 285–293. [Google Scholar] [CrossRef]
- Park, P.; Ikeda, K. Ultrastructural analysis of responses of host and fungal cells during plant infection. J. Gen. Plant Pathol. 2008, 74, 2–14. [Google Scholar] [CrossRef]
- Meena, M.; Samal, S. Alternaria host-specific (HSTs) toxins: An overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 2019, 6, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Namiki, F.; Okamoto, H.; Katou, K.; Yamamoto, M.; Nishimura, S.; Nakatsuka, S.; Goto, T.; Kohmoto, K.; Otani, H.; Novacky, A. Studies on host-specific toxins produced by Alternaria alternata strawberry pathotype causing Alternaria black spot of strawberry (5) Effect of toxins on membrane potential of susceptible plants by means of electrophysiological analysis. Ann. Phytopathol. Soc. Jpn. 1986, 52, 610–619. [Google Scholar] [CrossRef] [Green Version]
- Otani, H.; Tomiyama, K.; Okamoto, H.; Nishimura, S.; Kohmoto, K. Effect of AK-toxin produced by Alternaria alternata Japanese pear pathotype on membrane potential of pear cells. Ann. Phytopathol. Soc. Jpn. 1989, 55, 466–468. [Google Scholar] [CrossRef] [Green Version]
- Otani, H.; Kohmoto, K.; Kodama, M.; Nishimura, S. Role of host-specific toxins in the pathogenesis of Alternaria alternata. In Molecular Strategies of Pathogens and Host Plants; Patil, S.S., Ouchi, S., Mills, D., Vance, C., Eds.; Springer: Berlin, Germany, 1991; Volume 3, pp. 139–149. [Google Scholar]
- Otani, H.; Kohmoto, K.; Kodama, M. Alternaria toxins and their effects on host plants. Can. J. Bot. 1995, 73, 453–458. [Google Scholar] [CrossRef]
- Uemura, I.; Miyagawa, H.; Ueno, T. Asymmetric total synthesis of AK-toxins. Terahedron 2002, 58, 2351–2358. [Google Scholar] [CrossRef]
- Morita, Y.; Hyon, G.; Hosogi, N.; Miyata, N.; Nakayashiki, H.; Muranaka, Y.; Inada, N.; Park, P.; Ikeda, K. Appressorium-localized NADPH oxidase B is essential for aggressiveness and pathogenicity in the host-specific, toxin-producing fungus Japanese Alternaria alternata pear pathotype. Mol. Plant Path. 2013, 4, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Chen, C.; Choo, C.; Chen, Y.; Yago, J.; Chung, K. Proper functions of peroxisomes are vital for pathogenesis of Citrus brown spot disease caused by Alternaria alternata. J. Fungi 2020, 6, 248. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, S.; Feng, B.; Goto, T.; Tsuge, T.; Nishiumra, S. Biosynthesis of host-selective toxins produced by Alternaria alternata pathogens. 2. Biosynthetic origin of (8R,9S)-9,10-epoxy-8-hydroxy-9-methyl-deca-(2E,4Z,6E)-trienoic acid, a precursor of AK-toxins produced by Alternaria alternata. Phytochemistry 1990, 29, 1529–1531. [Google Scholar] [CrossRef]
- Tanaka, A.; Shiotani, H.; Yamamoto, M.; Tsuge, T. Insertional mutagenesis and cloning of the genes required for biosynthesis of the hostspecific AK-toxin in the Japanese pear pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 1999, 12, 691–702. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, A.; Tsuge, T. Structural and functional complexity of the genomic region controlling AK-toxin biosynthesis and pathogenicity in the Japanese pear pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 2000, 13, 975–986. [Google Scholar] [CrossRef] [Green Version]
- Hatta, R.; Ito, K.; Hosaki, Y.; Tanaka, T.; Tanaka, A.; Yamamoto, M.; Akimitsu, K.; Tsuge, T. A conditionally dispensable chromosome controls host-specific pathogenicity in the fungal plant pathogen Alternaria alternata. Genetics 2002, 161, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Masunaka, A.; Tanaka, A.; Tsuge, T.; Peever, T.L.; Timmer, L.W.; Yamamoto, M.; Yamamoto, H.; Akimitsu, K. Distribution and characterization of AKT homologs in the tangerine. Phytopathology 2000, 90, 762–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masunaka, A.; Ohtani, K.; Peever, T.; Timmer, L.; Tsuge, T.; Yamamoto, M.; Yamamoto, H.; Akimitsu, K. An isolate that is pathogenic to both tangerines and rough lemon and produces two host-selective toxins, ACT- and ACR-toxins. Phytopathology 2005, 95, 241–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, Y.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.; Akimitsu, K. Functional analysis of a multicopy host-selective ACT-toxin biosynthesis gene in the tangerine pathotype of Alternaria alternata using RNA silencing. Mol. Plant-Microbe Interact. 2008, 21, 1591–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imazaki, A.; Tanaka, A.; Harimoto, Y.; Yamamoto, M.; Akimitsu, K.; Park, P.; Tsuge, T. Contribution of peroxisomes to secondary metabolism and pathogenicity in the fungal plant pathogen Alternaria alternata. Eukaryot. Cell 2010, 9, 682–694. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Ma, H.; Zheng, F.; Chen, Y.; Wang, M.; Jiao, C.; Li, H.; Gai, Y. The transcription regulator ACTR controls ACT-toxin biosynthesis and pathogenicity in the tangerine pathotype of Alternaria alternata. Microbiol. Res. 2021, 248, 126747. [Google Scholar] [CrossRef] [PubMed]
- Covert, S.F. Supernumerary chromosomes in filamentous fungi. Curr. Genet. 1998, 33, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Tanaka, T.; Hatta, R.; Yamamoto, M.; Akimitsu, K.; Tsuge, T. Dissection of the host range of the fungal plant pathogen Alternaria alternata by modification of secondary metabolism. Mol. Microbiol. 2004, 52, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Isshiki, Y.; Honda, A.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.; et al. Function of genes encoding acyl-CoA synthetase and enoyl-CoA hydratase for host-selective ACT-toxin biosynthesis in the tangerine pathotype of Alternaria alternata. Phytopathology 2009, 99, 369–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugahara, S.; Ito, Y.; Sakurai, Y.; Narikawa, T.; Sakata, Y. Varietal difference of the resistance to stem canker caused by Alternaria alternata in tomato. Res. Bull. Aichi Agric. Res. Cent. 1989, 21, 170–175. [Google Scholar]
- Bottini, A.; Gilchrist, D.; Phytotoxins, I. A 1-aminodimethylheptadecapentol from Alternaria alternata f. sp. lycopersici. Tetrahedron Lett. 1981, 22, 2719–2722. [Google Scholar] [CrossRef]
- Bottini, A.T.; Bowen, J.R.; Gilchrist, D.G. Phytotoxins. II. Characterization of a phytotoxic fraction from Alternaria alternata f. sp. lycopersici. Tetrahedron Lett. 1981, 22, 2723–2726. [Google Scholar] [CrossRef]
- Brandwagt, B.F.; Mesbah, L.A.; Takken, F.L.W.; Laurent, P.L.; Kneppers, T.J.A.; Hille, J.; Nijkamp, H.J.J. A longevity assurance gene homolog of tomato mediates resistance to Alternaria alternata f. sp. lycopersici toxins and fumonisin B1. Proc. Natl. Acad. Sci. USA 2000, 97, 4961–4966. [Google Scholar] [CrossRef] [Green Version]
- Caldas, E.D.; Jones, A.D.; Ward, B.; Winter, C.K.; Gilchrist, D.G. Structural characterization of three new AAL-toxins produced by Alternaria alternata f. sp. lycopersici. J. Agric. Food Chem. 1994, 42, 327–333. [Google Scholar] [CrossRef]
- Abbas, H.K.; Duke, S.O.; Paul, R.N.; Riley, R.T.; Tanaka, T. AAL-toxin, a potent natural herbicide which disrupts sphingolipid metabolism of plants. Pestic. Sci. 1995, 43, 181–187. [Google Scholar] [CrossRef]
- Mesbah, L.A.; Van Der Weerden, G.M.; Nijkamp, H.J.J.; Hille, J. Sensitivity among species of Solanaceae to AAL toxins produced by Alternaria alternata f.sp. lycopersici. Plant Pathol. 2000, 49, 734–741. [Google Scholar] [CrossRef] [Green Version]
- Duke, S.O.; Dayan, F.E. Modes of action of microbially-produced phytotoxins. Toxins 2011, 3, 1038–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shier, W.T.; Abbas, H.K.; Mirocha, C.J. Toxicity of the mycotoxins fumonisins B1 and B2 and Alternaria alternata f. sp. lycopersici toxin (AAL) in cultured mammalian cells. Mycopathologia 1991, 116, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616, 255–268. [Google Scholar] [CrossRef]
- Abbas, H.K.; Tanaka, T.; Shier, W.T. Biological activities of synthetic analogues of Alternaria alternata toxin (AAL-toxin) and fumonisin in plant and mammalian cell cultures. Phytochemistry 1995, 40, 1681–1689. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E. Clues to new herbicide mechanisms of action from natural sources. ACS Sym. Ser. 2013, 1141, 203–215. [Google Scholar]
- Gilchrist, D.G. Mycotoxins reveal connections between plants and animals in apoptosis and ceramide signaling. Cell Death Differ. 1997, 4, 1312–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orolaza, N.; Kawakita, K.; Doke, N. Inhibitory effect of AL-toxin produced by Alternaria alternata tomato pathotype on the biosynthesis of phosphatidylethanolamine in tomato leaves susceptible to the fungus. Ann. Phytopath. Soc. Jpn. 1992, 58, 719–725. [Google Scholar] [CrossRef]
- Shi, L.; Bielawski, J.; Mu, J.; Dong, H.; Teng, C.; Zhang, J.; Yang, X.; Tomishige, N.; Hanada, K.; Hannun, Y.A.; et al. Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis. Cell Res. 2007, 17, 1030–1040. [Google Scholar] [CrossRef]
- Shao, Z.; Zhao, Y.; Liu, L.; Chen, S.; Li, C.; Meng, F.; Liu, H.; Hu, S.; Wang, J.; Wang, Q. Overexpression of FBR41 enhances resistance to sphinganine analog mycotoxin-induced cell death and Alternaria stem canker in tomato. Plant Biotechnol. J. 2020, 18, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Abbas, H.K.; Tanaka, T.; Duke, S.O.; Porter, J.K.; Wray, E.M.; Hodges, L.; Sessions, A.E.; Wang, E.; Merrill, A.H.; Riley, A.R.T. Fumonisin-induced and AAL-toxin-induced disruption of sphingolipid metabolism with accumulation of free sphingoid gases. Plant Physiol. 1994, 106, 1085–1093. [Google Scholar] [CrossRef] [Green Version]
- Markham, J.E.; Hille, J. Host-selective toxins as agents of cell death in plant-fungus interactions. Mol. Plant Pathol. 2001, 1, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jia, C.; Liu, L.; Li, C.; Wang, Q. Involvement of jasmonates and ethylene in Alternaria alternata f. sp. lycopersici toxin-induced tomato cell death. J. Exp. Bot. 2011, 15, 5405–5418. [Google Scholar] [CrossRef] [Green Version]
- Ismaiel, A.; Papenbrock, J. Mycotoxins: Producing fungi and mechanisms of phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef] [Green Version]
- Caldas, E.D.; Sadilkova, K.; Ward, B.L.; Jones, A.D.; Winter, C.K.; Gilchrist, D.G. Biosynthetic studies of fumonisin B-1 and AAL toxins. J. Agric. Food Chem. 1998, 46, 4734–4743. [Google Scholar] [CrossRef]
- Doidge, E. A study of some Alternarias infecting citrus in South Africa. S. Afr. Dept Agric. Sci. Bull. 1929, 69, 99–112. [Google Scholar]
- Nishimura, S.; Tatano, S.; Miyamoto, Y.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Tada, Y.; Ichimura, K.; Akimitsu, K. A zinc-binding citrus protein metallothionein can act as a plant defense factor by controlling host-selective ACR-toxin production. Plant Mol. Biol. 2013, 81, 1–11. [Google Scholar] [CrossRef]
- Nishimura, S.; Kohmoto, K. Host-specific toxins and chemical structures from Alternaria species. Annu. Rev. Phytopathol. 1983, 21, 87–116. [Google Scholar] [CrossRef]
- Akimitsu, K.; Kohmoto, K.; Otani, H.; Nishimura, S. Host-specific effect of toxin from the rough lemon pathotype of Alternaria alternata on mitochondria. Plant Physiol. 1989, 89, 925–931. [Google Scholar] [CrossRef] [Green Version]
- Ohtani, K.; Yamamoto, H.; Akimitsu, K. Sensitivity to Alternaria alternata toxin in citrus because of altered mitochondrial RNA processing. Proc. Natl. Acad. Sci. USA 2002, 99, 2439–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumi, Y.; Kamei, E.; Miyamoto, Y.; Ohtani, K.; Masunaka, A.; Fukumoto, T.; Gomi, K.; Tada, Y.; Ichimura, K.; Peever, T.; et al. Role of the pathotype-specific ACRTS1 gene encoding a hydroxylase involved in the biosynthesis of host-selective ACR-toxin in the rough lemon pathotype of Alternaria alternata. Phytopathology 2012, 102, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Izumi, Y.; Ohtani, K.; Miyamoto, Y.; Masunaka, A.; Fukumoto, T.; Gomi, K.; Tada, Y.; Ichimura, K.; Peever, T.; Akimitsu, K. A polyketide synthase gene, ACRTS2, is responsible for biosynthesis of host-selective ACR-toxin in the rough lemon pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 2012, 25, 1419–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akimitsu, K.; Ohtani, K.; Shimagami, T.; Katsumoto, M.; Igarashi, C.; Tanaka, S.; Matsuoka, S.; Mochizuki, S.; Tsuge, T.; Yamamoto, M.; et al. Citrus as a molecular contact point for co-evolution of Alternaria pathogens. Physiol. Mol. Plant Pathol. 2016, 95, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Ueno, T.; Nakashima, T.; Uemota, M.; Fukami, H.; Lee, S.; Izumiya, N. Mass spectrometry of Alternaria mali toxins and related cyclodepsipeptides. Biol. Mass Spectrom. 1977, 4, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Park, P.; Nishimura, S.; Kohmoto, K.; Otani, H.; Tsujimoto, K. Two action sites of AM-toxin I produced by apple pathotype of Alternaria alternate in host cell: An ultrastructural study. Can. J. Bot. 1981, 59, 301–310. [Google Scholar] [CrossRef]
- Kohmoto, K.; Otani, H. Host recognition by toxigenic plant-pathogens. Experientia 1991, 47, 755–764. [Google Scholar] [CrossRef]
- Li, Y.; Aldwinckle, H.; Sutton, T.; Tsuge, T.; Kang, G.; Cong, P.; Cheng, G. Interactions of apple and the Alternaria alternata apple pathotype. Crit. Rev. Plant Sci. 2013, 32, 141–150. [Google Scholar] [CrossRef]
- Keller, N.; Turner, G.; Bennett, J. Fungal secondary metabolism-biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef]
- Johnson, R.D.; Johnson, L.; Itoh, Y.; Kodama, M.; Otani, H.; Kohmoto, K. Cloning and characterization of a cyclic peptide synthetase gene from Alternaria alternata apple pathotype whose product is involved in AM-toxin synthesis and pathogenicity. Mol. Plant-Microbe Interact. 2000, 13, 742–753. [Google Scholar] [CrossRef] [Green Version]
- Harimoto, Y.; Tanaka, T.; Kodama, M.; Yamamoto, M.; Otani, H.; Tsuge, T. Multiple copies of AMT2 are prerequisite for the apple pathotype of Alternaria alternata to produce enough AM-toxin for expressing pathogenicity. J. Gen. Plant Pathol. 2008, 74, 222–229. [Google Scholar] [CrossRef]
- Harimoto, Y.; Hatta, R.; Kodama, M.; Yamamoto, M.; Otani, H.; Tsuge, T. Expression profiles of genes encoded by the supernumerary chromosome controlling AM-toxin biosynthesis and pathogenicity in the apple pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 2007, 20, 1463–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bains, P.S.; Tewari, J.P. Purification, chemical characterization and host-specificity of the toxin produced by Alternaria-Brassicae. Physiol. Mol. Plant Pathol. 1987, 30, 259–271. [Google Scholar] [CrossRef]
- Buchwaldt, L.; Green, H. Phytotoxicity of destruxin B and its possible role in the pathogenesis of Alternaria brassicae. Plant Pathol. 1992, 41, 55–63. [Google Scholar] [CrossRef]
- Pedras, M.S.; Zaharia, I.; Gai, Y.; Zhou, Y.; Ward, D. In planta sequential hydroxylation and glycosylation of a fungal phytotoxin: Avoiding cell death and overcoming the fungal invader. Proc. Natl. Acad. Sci. USA 2001, 98, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Morel, E.; Païs, M.; Turpin, M.; Guyot, M. Cytotoxicity of cyclodepsipeptides on murine lymphocytes and on L 1210 leukemia cells. Biomed. Pharmacother. 1983, 37, 184–185. [Google Scholar] [PubMed]
- Sun, C.; Chen, H.; Yeh, S. Suppressive effects of metabolites from Alternaria brassicae on the hepatitis B surface antigen. Planta Med. 1994, 60, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chou, C.; Sun, C.; Yeh, S. Suppressive effects of destruxin B on hepatitis B virus surface antigen gene expression in human hepatoma cells. Antivir. Res. 1997, 34, 137–144. [Google Scholar] [CrossRef]
- Bandani, A.R.; Amiri, B.; Butt, T.M.; Gordon-Weeks, R. Effects of efrapeptin and destruxin, metabolites of entomogenous fungi, on the hydrolytic activity of a vacuolar type ATPase identified on the brush border membrane vesicles of Galleria mellonella midgut and on plant membrane bound hydrolytic enzymes. Biochim. Biophys. Acta 2001, 1510, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Tamura, S. Isolation and structure of protodestruxin from Metarrhizium anisopliae. Agric. Biol. Chem. 1972, 36, 896–898. [Google Scholar] [CrossRef]
- Jegorov, A.; Sedmera, P.; Matha, V. Biosynthesis of destruxins. Phytochemistry 1993, 33, 1403–1405. [Google Scholar] [CrossRef]
- Ullstrup, A.J. Inheritance of susceptibility to infection by Helminthosporium maydis race 1 in maize. J. Agric. Res. 1941, 63, 331–334. [Google Scholar]
- Kim, S.D.; Knoche, H.W.; Dunkle, L.D.; Mccrery, D.A.; Tomer, K.B. Structure of an amino acid analogue of the host-specific toxin from Helminthosporium carbonum. Tetrahedron Lett. 1985, 26, 969–972. [Google Scholar] [CrossRef]
- Walton, J.D. Host-selective toxins: Agents of compatibility. Plant Cell 1996, 8, 1723–1733. [Google Scholar]
- Pitkin, J.W.; Nikolskaya, A.; Ahn, J.H.; Walton, J.D. Reduced virulence caused by meiotic instability of the TOX2 chromosome of the maize pathogen Cochliobolus carbonum. Mol. Plant-Microbe Interact. 2000, 13, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Stierle, A.C.; Cardellina, J.H.; Strobel, G.A. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternata. Proc. Natl. Acad. Sci. USA 1988, 85, 8008–8013. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Strobel, G.A. Cellular protein receptors of maculosin, a host-specific phytotoxin of spotted knapweed (Centaurea maculosa L.). Biochim. Biophys. Acta-Gen. Subj. 1994, 1199, 11–19. [Google Scholar] [CrossRef]
- Lopes, S.C.D.N.; Fedorova, A.; Castanho, M.A.R.B. Cholesterol modulates maculosin’s orientation in model systems of biological membranes relevance towards putative molecular recognition. Steroids 2004, 69, 825–830. [Google Scholar] [CrossRef]
- Paudel, B.; Maharjan, R.; Rajbhandarib, P.; Aryalc, N.; Azizc, S.; Bhattaraic, K.; Barald, B.; Malla, R.; Bhattarai, H. Maculosin, a non-toxic antioxidant compound isolated from Streptomyces sp. KTM18. Pharm. Biol. 2021, 59, 933–936. [Google Scholar] [CrossRef]
- Robeson, D.J.; Gray, G.R.; Strobel, G.A. Production of the phytotoxins radicinin and radicinol by Alternaria chrysanthemi. Phytochemistry 1982, 21, 2359–2362. [Google Scholar] [CrossRef]
- Sheridan, H.; Canning, A.M. Novel radicinol derivatives from long-term cultures of Alternaria chrysanthemi. J. Nat. Prod. 1999, 62, 1568–1569. [Google Scholar] [CrossRef]
- Tal, B.; Robeson, D.J.; Burke, B.A.; Aasen, A.J. Phytotoxins from Alternara helianthi and the structures of deoxyradicinol and radianthin. Phytochemistry 1985, 24, 729–731. [Google Scholar] [CrossRef]
- Tal, B.; Robeson, D.J. The production of pyrenocines A and B by a novel Alternaria species. Z. Naturforsch. C 1986, 41, 11–12. [Google Scholar] [CrossRef] [Green Version]
- Ichihara, A.; Tazaki, H.; Sakamura, S. Solanapyrones A, B and C, phytotoxic metabolites from the fungus Alternaria solani. Tetrahedron Lett. 1983, 24, 5373–5376. [Google Scholar] [CrossRef]
- Wang, X.; Luo, X.; Xiao, J.; Zhai, M.; Yuan, Y.; Zhu, Y.; Crews, P.; Yuan, C.; Wu, Q. Pyrone derivatives from the endophytic fungus Alternaria tenuissima SP-07 of Chinese herbal medicine Salvia przewalskii. Fitoterapia 2014, 99, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Pero, R.W.; Owens, R.G.; Dale, S.W.; Harvan, D. Isolation and identification of a new toxin, altenuene, from the fungus Alternaria tenuis. Biochim. Biophys. Acta 1971, 230, 170–179. [Google Scholar] [CrossRef]
- Pero, R.W.; Posner, H.; Blois, M.; Harvan, D.; Spalding, J.W. Toxicity of metabolites produced by the “Alternaria”. Environ. Health Perspect. 1973, 4, 87–94. [Google Scholar] [CrossRef]
- Okuno, T.; Natsume, I.; Sawai, K.; Sawamura, K.; Furusaki, A.; Matsumoto, T. Structure of antifungal and phytotoxic pigments produced by Alternaria sps. Tetrahedron Lett. 1983, 24, 5653–5656. [Google Scholar] [CrossRef]
- Wu, W.; Yue, G.; Huang, Q.; Sun, L.; Zhang, W. A new compound from an endophytic fungus Alternaria tenuissima. J. Asian Nat. Prod. Res. 2014, 16, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Bashyal, B.P.; Wellensiek, B.P.; Ramakrishnan, R.; Faeth, S.H.; Ahmad, N.; Gunatilaka, A.A.L. Altertoxins with potent anti-HIV activity from Alternaria tenuissima QUE1Se, a fungal endophyte of Quercus emoryi. Bioorg. Med. Chem. 2014, 22, 6112–6116. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; Yi, T.; Ma, Q.; Xie, Q.; Zhou, L.; Chen, J.; Dai, H.; Wu, Y.; Zhao, Y. Biphenyl metabolites from the patchouli endophytic fungus Alternaria sp. PfuH1. Fitoterapia 2020, 146, 104708. [Google Scholar] [CrossRef]
- Stierle, A.C.; Cardellina, J.H.; Strobel, G.A. Phytotoxins from Alternara alternata, a pathogen of spotted knapweed. J. Nat. Prod. 1989, 52, 42–47. [Google Scholar] [CrossRef]
- Stack, M.E.; Mazzola, E.P. Stemphyltoxin III from Alternaria alternata. J. Nat. Prod. 1989, 52, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Stoessl, A. Some metabolites of Alternaria solani. Can. J. Chem. 1969, 47, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Yagi, A.; Okamura, N.; Haraguchi, H.; Abo, T.; Hashimoto, K. Antimicrobial tetrahydroanthraquinones from a strain of Alternaria solani. Phytochemistry 1993, 33, 87–91. [Google Scholar] [CrossRef]
- Charudattan, R.; Rao, K.V. Bostrycin and 4-deoxybostrycin: Two nonspecific phytotoxins produced by Alternaria eichhorniae. Appl. Environ. Microbiol. 1982, 43, 846–849. [Google Scholar] [CrossRef] [Green Version]
- Suemitsu, R.; Iwai, J.; Kawaguchi, K.; Haitani, N.; Kitagawa, N. Isolation and identification of erythroglaucin (1, 4, 5-trihydroxy-7-methoxy-2-methylanthraquinone) from the mycelium of Alternaria porri (Ellis) Ciferri. Agric. Biol. Chem. 1977, 41, 2289–2290. [Google Scholar]
- Huang, C.; Pan, J.; Chen, B.; Yu, M.; Huang, H.; Zhu, X.; Lu, Y.; She, Z.; Lin, Y. Three bianthraquinone derivatives from the mangrove endophytic fungus Alternaria sp ZJ9-6B from the South China Sea. Mar. Drugs 2011, 9, 832–843. [Google Scholar] [CrossRef]
- Chen, B.; Shen, Q.; Xun Zhu, X.; Lin, Y. The Anthraquinone derivatives from the fungus Alternaria sp. XZSBG-1 from the Saline Lake in Bange, Tibet, China. Molecules 2014, 19, 16529–16542. [Google Scholar] [CrossRef] [Green Version]
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Gatenbeck, S.; Sierankiewicz, J. Microbial production of tenuazonic acid analogues. Antimicrob. Agents Chemother. 1973, 3, 308–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, S.M.; Halloin, J.M.; Hagedorn, D.J. Production, purification, and bioassay of tentoxin. Phytopathology 1970, 60, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Liebermann, B.; Oertel, B. Bildung und isolierung des phytotoxins tentoxin aus Alternaria alternata. J. Basic Microbiol. 1983, 23, 503–511. [Google Scholar] [CrossRef]
- Kono, Y.; Gardner, J.M.; Takeuchi, S. Nonselective phytotoxins simultaneously produced with hostselective ACTG-toxins by a pathotype of Alternaria citri causing brown spot. Agric. Biol. Chem. 1986, 50, 2401–2403. [Google Scholar]
- Edwards, J.V.; Lax, A.R.; Lillehoj, E.B.; Boudreaux, G.J. Structure-activity relationships of cyclic and acyclic analogues of the phytotoxic peptide tentoxin. J. Agric. Food Chem. 1987, 35, 451–456. [Google Scholar] [CrossRef]
- Suemitsu, R.; Sano, T.; Yamamoto, M.; Arimoto, Y.; Morimatsu, F.; Nabeshima, T. Structural elucidation of alterporriol B, a novel metabolic pigment produced by Alternaria porri (Ellis) ciferri. Agric. Biol. Chem. 1984, 48, 2611–2613. [Google Scholar] [CrossRef] [Green Version]
- Duke, S.O. Tentoxin effects on variable fluorescence and P515 electrochromic absorbance changes in tentoxin-sensitive and -resistant plant. Plant Sci. 1993, 90, 119–126. [Google Scholar] [CrossRef]
- Tietjen, K.G.; Matern, U. Induction and suppression of phytoalexin biosynthesis in cultured cells of safflower, Carthamus tinctorius L.; by metabolites of Alternaria carthami Chowdhury. Arch. Bioch. Biophy. 1984, 229, 136–144. [Google Scholar] [CrossRef]
- Vurro, M.; Evidente, A.; Andolfi, A.; Zonno, M.C.; Giordano, F.; Motta, A. Brefeldin A and α, β-dehydrocurvularin, two phytotoxins from Alternaria zinniae, a biocontrol agent of Xanthium occidentale. Plant Sci. 1998, 138, 67–79. [Google Scholar] [CrossRef]
- Courtial, J.; Hamama, L.; Helesbeux, J.J.; Lecomte, M.; Renaux, Y.; Guichard, E.; Voisine, L.; Yovanopoulos, C.; Hamon, B.; Oge, L.; et al. Aldaulactone-an original phytotoxic secondary metabolite involved in the aggressiveness of Alternaria dauci on carrot. Front. Plant Sci. 2018, 9, 502. [Google Scholar] [CrossRef] [Green Version]
- Barasch, I.; Mor, H.; Netzer, D.; Kashman, Y. Production of zinniol by Alternaria dauci and its phytotoxic effect on carrot. Physiol. Plant Pathol. 1981, 19, 7–16. [Google Scholar] [CrossRef]
- Cotty, P.; Mishagi, I.; Hine, R. Production of zinniol by Alternaria tagetica and its phytotoxic effect on Tagetes erecta. Phytopathology 1983, 73, 1326–1328. [Google Scholar] [CrossRef]
- Cotty, P.; Mishagi, I. Zinniol production by Alternaria species. Phytopathology 1984, 74, 785–788. [Google Scholar] [CrossRef]
- Leyte-Lugo, M.; Richomme, P.; Poupard, P.; Pena-Rodriguez, L.M. Identification and quantification of a phytotoxic metabolite from Alternaria dauci. Molecules 2020, 25, 4003. [Google Scholar] [CrossRef]
- Gamboa-Angulo, M.M.; García-Sosa, K.; Alejos-González, F.; Escalante-Erosa, F.; Delgado-Lamas, G.; Peña-Rodríguez, L.M. Tagetolone and tagetenolone: Two phytotoxic polyketides from Alternaria tagetica. J. Agric. Food Chem. 2001, 49, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Avula, S.K.; Das, B.; Csuk, R.; Al-Rawahi, A.; Al-Harrasi, A. Recent advances in the stereoselective total synthesis of natural pyranones having long side chains. Molecules 2020, 25, 1905. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.D.; Nord, F.F. Radicinin: A new pigment from Stemphylium radicinum. Arch. Biochem. Biophys. 1953, 45, 469–470. [Google Scholar] [CrossRef]
- Sato, H.; Konoma, K.; Sakamura, S. Phytotoxins produced by onion pink root jungus, Pyrenochaeta terrestris. Agric. Biol. Chem. 1979, 43, 2409–7506. [Google Scholar]
- Masi, M.; Freda, F.; Clement, S.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Phytotoxic activity and structure-activity relationships of radicinin derivatives against the invasive weed buffelgrass (Cenchrus ciliaris). Molecules 2019, 24, 2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, H.; Ishida, T.; Otsuka, Y.; Hamasaki, T.; Ichinoe, M. Phytotoxins and related metabolites produced by Bipolaris coicis, the pathogen of Job’s tears. Phytochemistry 1997, 45, 41–45. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Vitti, C.; Girolamo, A.D.; Visconti, A.; Logrieco, A.; Fanizzi, F.P. Radicinols and radicinin phytotoxins produced by Alternaria radicina on carrots. J. Agric. Food Chem. 2004, 52, 3655–3660. [Google Scholar] [CrossRef]
- Santoro, E.; Mazzeo, G.; Marsico, G.; Masi, M.; Longhi, G.; Superchi, S.; Evidente, A.; Abbate, S. Assignment through chiroptical methods of the absolute configuration of fungal dihydropyranpyran-4-5-diones phytotoxins, potential herbicides for buffelgrass (Cenchrus ciliaris) biocontrol. Molecules 2019, 24, 3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giridharan, P.; Verekar, S.A.; Gohil, A.R.; Mishra, P.D.; Khanna, A.; Deshmukh, S.K. Antiproliferative activity of hamigerone and radicinol isolated from Bipolaris papendorfii. Biomed Res. Int. 2014, 2014, 890904. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Choi, G.J.; Kim, H.T.; Kim, H.J.; Cho, K.Y. Pathogenicity and pyrenocine production of Curvularia inaequalis isolated from zoysia grass. Plant Dis. 2000, 84, 684–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myobatake, Y.; Kamisuki, S.; Tsukuda, S.; Higashi, T.; Chinen, T.; Takemoto, K.; Hachisuka, M.; Suzuki, Y.; Takei, M.; Tsurukawa, Y.; et al. Pyrenocine A induces monopolar spindle formation and suppresses proliferation of cancer cells. Bioorg. Med. Chem. 2019, 27, 115–149. [Google Scholar] [CrossRef]
- Shishido, T.; Hachisuka, M.; Ryuzaki, K.; Miura, Y.; Tanabe, A.; Tamura, Y.; Kusayanagi, T.; Takeuchi, T.; Kamisuki, S.; Sugawara, F.; et al. EpsinR, a target for pyrenocine B, role in endogenous MHC-II-restricted antigen presentation. Eur. J. Immunol. 2014, 44, 3220–3231. [Google Scholar] [CrossRef]
- Hamid, K.; Strange, R.N. Phytotoxicity of solanapyrones A and B produced by the chickpea pathogen Ascochyta rabiei (Pass.) Labr. and the apparent metabolism of solanapyrone A by chickpea tissues. Physiol. Mol. Plant Pathol. 2000, 56, 235–244. [Google Scholar] [CrossRef]
- Mizushina, Y.; Kamisuki, S.; Kasai, N.; Shimazaki, N.; Takemura, M.; Asahara, H.; Linn, S.; Yoshida, S.; Matsukage, A.; Koiwai, O.; et al. A plant phytotoxin, solanapyrone A, is an inhibitor of DNA polymerase β and λ. J. Biol. Chem. 2002, 277, 630–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikawa, H.; Yokota, T.; Sakano, C.; Suzuki, Y.; NaYa, A.; Ichihara, A. Solanapyrones. Phytotoxins produced by Alternaria solani: Biosynthesis and isolation of minor components. Biosci. Biotenchnol. Biochem. 1998, 62, 2016–2022. [Google Scholar] [CrossRef] [Green Version]
- Kasahara, K.; Miyamoto, T.; Fujimoto, T.; Oguri, H.; Tokiwano, T.; Oikawa, H.; Tokiwano, T.; Oikawa, H.; Ebizuka, Y.; Fujii, I. Solanapyrone synthase, a possible Diels-Alderase and iterative type I polyketide synthase encoded in a biosynthetic gene cluster from Alternaria solani. ChemBioChem 2010, 11, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Park, C.M.; Park, J.J.; Akamatsu, H.O.; Peever, T.L.; Xian, M.; Gang, D.; Vandemark, G.; Chen, W. Functional analyses of the Diels-Alderase gene sol5 of Ascochyta rabiei and Alternaria solani indicate that the solanapyrone phytotoxins are not required for pathogenicity. Mol. Plant-Microbe Interact. 2015, 28, 482–496. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Dong, J.; Xie, D.; Li, Y.F.; Liu, Q. Synthesis of 2-(Trifluoromethyl)-dibenzopyranones with Rhodium(III)-catalyzed Formal anti-Michael Addition as Key Step. Adv. Synth. Catal. 2018, 360, 958–964. [Google Scholar] [CrossRef]
- Raistrick, H.; Stickings, C.E.; Thomas, R. Studies in the biochemistry of microorganisms. 90. Alternariol and alternariol monomethyl ether, metabolic products of Alternaria tenuis. Biochem. J. 1953, 55, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, P.M. Analysis of agricultural commodities and foods for Alternaria mycotoxins. J. Aoac. Int. 2001, 84, 1809–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging mycotoxins: Beyond traditionally determined food contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef] [PubMed]
- Schade, J.; King, A. Analysis of the major Alternaria toxins. J. Food Product. 1984, 47, 978–995. [Google Scholar] [CrossRef]
- Wollenhaupt, K.; Schneider, F.; Tiemann, U. Influence of alternariol (AOH) on regulator proteins of cap-dependent translation in porcine endometrial cells. Toxicol. Lett. 2008, 182, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Fehr, M.; Pahlke, G.; Fritz, J.; Christensen, M.O.; Boege, F.; Altemoller, M.; Podlech, J.; Marko, D. Alternariol acts as a topoisomerase poison, preferentially affecting the IIα isoform. Mol. Nutr. Food Res. 2009, 53, 441–451. [Google Scholar] [CrossRef]
- Solhaug, A.; Vine, L.L.; Ivanova, L.; Spilsberg, B.; Holme, J.A.; Pestka, J.; Collins, A.; Eriksen, G.S. Mechanisms involved in alternariol-induced cell cycle arrest. Mutat. Res.-Fund. Mol. Mutagen. 2012, 738, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Blanco, C.; Juan-Garcia, A.; Juan, C.; Font, G.; Ruiz, M.J. Alternariol induce toxicity via cell death and mitochondrial damage on Caco-2 cells. Food Chem. Toxicol. 2016, 88, 32–39. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Fernández-Blanco, C.; Sagratini, G.; Font, G.; Ruiz, M.J. Effects of soyasaponin I and soyasaponins-rich extract on the alternariol-induced cytotoxicity on Caco-2 cells. Food Chem. Toxicol. 2015, 77, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Bensassi, F.; Gallerne, C.; el Dein, O.S.; Hajlaoui, M.R.; Bacha, H.; Lemaire, C. Cell death induced by the Alternaria mycotoxin alternariol. Toxicol. In Vitro 2012, 26, 915–923. [Google Scholar] [CrossRef]
- Fleck, S.; Burkhardt, B.; Pfeiffer, E.; Metzler, M. Alternaria toxins: Altertoxin II is a much stronger mutagen and DNA strand breaking mycotoxin than alternariol and its methyl ether in cultured mammalian cells. Toxicol. Lett. 2012, 214, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, A.; Torgersen, M.; Holme, J.; Lagadic-Gossmann, D.; Eriksen, G. Autophagy and senescence, stress responses induced by the DNA-damaging mycotoxin alternariol. Toxicology 2014, 326, 119–129. [Google Scholar] [CrossRef]
- Solhaug, A.; Wisbech, C.; Christoffersen, T.E.; Hult, L.O.; Lea, T.; Eriksen, G.S.; Holme, J.A. The mycotoxin alternariol induces DNA damage and modify macrophage phenotype and inflammatory responses. Toxicol. Lett. 2015, 239, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Qian, Y.; Zhang, P.; Dong, W.; Qi, Y.; Guo, H. Etiological role of Alternaria alternata in human esophageal cancer. Chin Med. J. 1992, 105, 394–400. [Google Scholar]
- An, Y.; Zhao, T.; Miao, J.; Liu, G.; Zheng, Y.; Xu, Y.; Van Etten, R.L. Isolation, identification, and mutagenicity of alternariol monomethyl ether. J. Agric. Food Chem. 1989, 37, 1341–1343. [Google Scholar] [CrossRef]
- European Food Safety Authority. Panel on Contaminants in the Food Chain. Scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407–2504. [Google Scholar] [CrossRef]
- Siegel, D.; Feist, M.; Proske, M.; Koch, M.; Nehls, I. Degradation of the Alternaria mycotoxins alternariol, alternariol monomethyl ether, and altenuene upon bread baking. J. Agric. Food Chem. 2010, 58, 9622–9630. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Q.; Gao, Y.; Tang, J.; Zhang, A.; Gao, J. Secondary metabolites from the endophytic botryosphaeria dothiadea of melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J. Agric. Food Chem. 2014, 62, 3584–3590. [Google Scholar] [CrossRef]
- De Souza, G.D.; Mithofer, A.; Daolio, C.; Schneider, B.; Rodrigues-Filho, E. Identification of Alternaria alternata mycotoxins by LC-SPE-NMR and their cytotoxic effects to soybean (Glycine max) cell suspension culture. Molecules 2013, 18, 2528–2538. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Huang, L.; Liu, Y.; Toshmatov, Z.; Zhang, C.; Shao, H. Two phytotoxins isolated from the pathogenic fungus of the invasive weed Xanthium italicum. Chem. Biodivers. 2020, 7, e2000043. [Google Scholar] [CrossRef] [PubMed]
- Demuner, A.J.; Barbosa, L.C.; Miranda, A.C.M.; Geraldo, G.C.; da Silva, C.M.; Giberti, S.; Bertazzini, M.; Forlani, G. The fungal phytotoxin alternariol 9-methyl ether and some of its synthetic analogues inhibit the photosynthetic electron transport chain. J. Nat. Prod. 2013, 76, 2234–2245. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R. Studies in the biosynthesis of fungal metabolites. Biochem. J. 1961, 80, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Gatenbeck, S.; Hermodsson, S. Enzymic synthesis of the aromatic product alternariol. Acta Chem. Scand. 1965, 19, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Hiltunen, M.; Söderhäll, K. Alternariol-O-methyltransferase from Alternaria alternata: Partial purification and relation to polyketide synthesis. Exp. Mycol. 1992, 16, 44–51. [Google Scholar] [CrossRef]
- Wenderoth, M.; Garganese, F.; Schmidt-Heydt, M.; Soukup, S.T.; Ippolito, A.; Sanzani, S.M.; Fischer, R. Alternariol as virulence and colonization factor of Alternaria alternata during plant infection. Mol. Microbiol. 2019, 1, 131–146. [Google Scholar] [CrossRef]
- Kumagai, Y.; Shinkai, Y.; Miura, T.; Cho, A.K. The chemical biology of naphthoquinones and its environmental implications. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 221–247. [Google Scholar] [CrossRef]
- Hu, J.; Sarrami, F.; Li, H.; Zhang, G.; Stubbs, K.; Lacey, E.; Stewart, S.; Karton, A.; Piggott, A.; Chooi, Y. Heterologous biosynthesis of elsinochrome A sheds light on the formation of the photosensitive perylenequinone system. Chem. Sci. 2019, 10, 1457–1465. [Google Scholar] [CrossRef] [Green Version]
- Stack, M.E.; Mazzola, E.P.; Page, S.W.; Pohland, A.E.; Highet, R.J.; Tempesta, M.S.; Corley, D.G. Mutagenic perylenequinone metabolites of Alternaria alternata: Altertoxins I, II, and III. J. Nat. Prod. 1986, 49, 866–871. [Google Scholar] [CrossRef]
- Stack, M.E.; Prival, M.J. Mutagenicity of the Alternaria metabolites altertoxins-I, altertoxins-II, and altertoxins-III. Appl. Environ. Microbiol. 1986, 52, 718–722. [Google Scholar] [CrossRef] [Green Version]
- Fleck, S.C.; Sauter, F.; Pfeiffer, E.; Metzler, M.; Hartwig, A.; Köberle, B. DNA damage and repair kinetics of the Alternaria mycotoxins alternariol, altertoxin II and stemphyltoxin III in cultured cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 798–799, 27–34. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, C.; Xiao, X.; Zhang, Q.; Huang, B. New cytotoxic compounds of endophytic fungus Alternaria sp. isolated from Broussonetia papyrifera (L.) Vent. Fitoterapia 2016, 110, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Robeson, D.; Strobel, G.; Matusumoto, G.K.; Fisher, E.L.; Chen, M.; Clardy, J. Alteichin: An unusual phytotoxin from Alternaria eichorniae, a fungal pathogen of water hyacinth. Experientia 1984, 40, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Hradil, C.M.; Hallock, Y.F.; Clardy, J.; Kenfield, D.S.; Strobel, G. Phytotoxins from Alternaria cassia. Phytochemistry 1989, 28, 73–75. [Google Scholar] [CrossRef]
- Davis, V.M.; Stack, M.E. Mutagenicity of stemphylotoxin-III, A metabolite of Alternaria alternata. Appl. Environ. Microbiol. 1991, 57, 180–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, E.M.; Muller, C.E. Anthraquinones as pharmacological tools and drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef]
- Okamura, N.; Mimura, K.; Yagi, A. Altersolanol-related compounds from the culture liquid of Alternaria solani. Phytochemistry 1996, 42, 77–80. [Google Scholar] [CrossRef]
- Suemitsu, R.; Yamadaa, Y.; Sanoa, T.; Yamashitaa, K. Phytotoxic ativities of Altersolanol A, B and dactylariol, and activities of altersolanol A against Some microorganisms. Agric. Biol. Chem. 1984, 48, 2383–2384. [Google Scholar]
- Haraguchi, H.; Abo, T.; Fukuda, A.; Okamura, N.; Yagi, A. Mode of phytotoxic action of altersolanols. Phytochemistry 1996, 43, 989–992. [Google Scholar] [CrossRef]
- Evidente, A.; Rodeva, R.; Andolfi, A.; Stoyanova, Z.; Perrone, C.; Motta, A. Phytotoxic polyketides produced by Phomopsis foeniculi, a strain isolated from diseased Bulgarian fennel. Eur. J. Plant Pathol. 2011, 130, 173–182. [Google Scholar] [CrossRef]
- Mishra, P.D.; Verekar, S.A.; Deshmukh, S.K.; Joshi, K.S.; Fiebig, H.H.; Kelter, G. Altersolanol A: A selective cytotoxic anthraquinone from a Phomopsis sp. Lett. Appl. Microbiol. 2015, 60, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Trigos, A.; Mendoza, G.; Espinoza, C.; Salinas, A.; Fernandez, J.J.; Norte, M. The role of macrosporin in necrotic spots. Phytochem. Lett. 2011, 4, 122–125. [Google Scholar] [CrossRef]
- Yuan, P.; He, L.; Chen, D.; Sun, Y.; Ge, Z.; Shen, D.; Lu, Y. Proteomic characterization of Mycobacterium tuberculosis reveals potential targets of bostrycin. J. Proteom. 2020, 212, 103576. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Fang, L.; Liu, J.; Cheng, W.; Yun, M.; Yang, H. Inhibitory effects of marine fungal metabolites from the South China Sea on prostate cancer cell line DU-145. Int. J. Intern. Med. 2008, 35, 562–564. [Google Scholar]
- Chen, W.; Hou, J.; Guo, Y.; Yang, H.; Xie, C.; Lin, Y.; She, Z. Bostrycin inhibits proliferation of human lung carcinoma A549 cells via downregulation of the PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2011, 30, 17. [Google Scholar] [CrossRef] [Green Version]
- Jie, J.; Shi, L.; Yue, S.; Wang, M.; Zhang, J. Bostrycin inhibits growth of tongue squamous cell carcinoma cells by inducing mitochondrial apoptosis. Transl. Cancer Res. 2020, 9, 3926–3936. [Google Scholar] [CrossRef]
- Qin, X.; Peng, Y.; Zheng, J. In vitro and in vivo studies of the electrophilicity of physcion and its oxidative metabolites. Chem. Res. Toxicol. 2018, 31, 340–349. [Google Scholar] [CrossRef]
- Mueller, S.O.; Schmitt, M.; Dekant, W.; Stopper, H.; Schlatter, J.; Lutz, W.K. Occurrence of emodin, chrysophanol and physcion in vegetables, herbs and liquors. Genotoxicity and anti-genotoxicity of the anthraquinones and of the whole plants. Food Chem. Toxicol. 1999, 37, 481–491. [Google Scholar] [CrossRef]
- Anke, H.; Kolthoum, I.; Laatsch, H. Metabolic products of microorganisms. 192. The anthraquinones of the Aspergillus glaucus group. II. Biological activity. Arch. Microbiol. 1980, 126, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Teuscher, F.; Li, D.; Diesel, A.; Ebel, R.; Proksch, P.; Wang, B. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 2006, 69, 1622–1625. [Google Scholar] [CrossRef]
- Ohnishi, K.; Tanabe, H.; Hayashi, S.; Suemitsu, R. Biosynthesis of Alterporriol-A by Alternara porri. Biosci. Biotechnol. Biochem. 1992, 56, 42–43. [Google Scholar] [CrossRef]
- Kang, S.; Pandey, R.; Lee, C.; Sim, J.; Jeong, J.; Choi, B.; Jung, M.; Ginzburg, D.; Zhao, K.; Won, S.; et al. Genome-enabled discovery of anthraquinone biosynthesis in Senna tora. Nat. Commun. 2020, 11, 5875. [Google Scholar] [CrossRef] [PubMed]
- Royles, B.J.L. Naturally occurring tetramic acids: Structure, isolation, and synthesis. Chem. Rev. 1995, 95, 1981–2001. [Google Scholar] [CrossRef]
- Rosett, T.; Sankhala, R.H.; Stickings, C.E.; Taylor, M.E.U.; Thomas, R. Studies in the biochemistry of microorganisms. Metabolites of Alternaria tenuis auct: Culture filtrate products. Biochem. J. 1957, 67, 390–400. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Singh, K. Evaluation of prophylactic efficacy of cinnamaldehyde in murine model against Paradendryphiella arenariae mycotoxin tenuazonic acid-induced oxidative stress and organ toxicity. Sci. Rep. 2021, 11, 19420. [Google Scholar] [CrossRef]
- Stickings, C.E. Studies in the biochemistry of micro-organisms. 106. Metabolites of Alternaria tenuis auct.: The structure of tenuazonic acid. Biochem. J. 1959, 72, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, S.; Muro, H.; Nozoe, S.; Okuda, S.; Sato, Z. Isolation of 3,4-dihydro-3,4,8-trihydroxy-2(2H)-naphthalenone and tenuazonic acid from Pyricularia oryzae Cavara. Tetrahedron Lett. 1972, 1, 13–16. [Google Scholar] [CrossRef]
- Steyn, P.S.; Rabie, C.J. Characterization of magnesium and calcium tenuazonate from Phoma sorghina. Phytochemistry 1976, 15, 1977–1979. [Google Scholar] [CrossRef]
- Montemurro, N.; Visconti, A. Alternaria metabolites-chemical and biological data. In Alternaria Biology, Plant Diseases and Metabolites; Chelkowski, J., Visconti, A., Eds.; Elsevier Science: Amsterdam, The Netherlands; London, UK; New York, NY, USA; Tokyo, Japan, 1992; pp. 449–541. [Google Scholar]
- Ebbole, D. Magnaporthe as a model for understanding host-pathogen interactions. Annu. Rev. Phytopathol. 2007, 45, 437–456. [Google Scholar] [CrossRef]
- Chen, S.; Qiang, S. Recent advances in tenuazonic acid as a potential herbicide. Pestic. Biochem. Phys. 2017, 143, 252–257. [Google Scholar] [CrossRef]
- Siegel, D.; Rasenko, T.; Koch, M.; Nehls, I. Determination of the Alternaria mycotoxin tenuazonic acid in cereals by high-performance liquid chromatography-electrospray ionization ion-trap multistage mass spectrometry after derivatization with 2, 4-dinitrophenylhydrazine. J. Chromatogr. A 2009, 1216, 4582–4588. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Curtui, V.; Ackermann, Y.; Latif, H.; Usleber, E. Enzyme immunoassay for tenuazonic acid in apple and tomato products. J. Agric. Food Chem. 2011, 59, 12317–12322. [Google Scholar] [CrossRef]
- Motoyama, T. Secondary metabolites of the rice blast fungus Pyricularia oryzae: Biosynthesis and biological function. Int. J. Mol. Sci. 2020, 21, 8698. [Google Scholar] [CrossRef]
- Miller, F.; Rightel, W.; Sloan, B.; Ehrlich, J.; French, J.; Bartz, Q.; Dixon, G. Antivial activity of tenuazonic acid. Nature 1963, 2000, 1338–1339. [Google Scholar] [CrossRef] [PubMed]
- Kaczka, E.A.; Gitterman, C.O.; Dulaney, E.L.; Smith, M.C.; Hendlin, D.; Woodruff, H.; Folkers, K. Discovery of inhibitory activity of tenuazonic acid for growth of human adenocarcinoma-1. Biochem. Biophys. Res. Commun. 1964, 14, 54–57. [Google Scholar] [CrossRef]
- Gitterman, C.O. Antitumor, cytotoxic, and antibacterial activities of tenuazonic acid and congeneric tetramic acids. J. Med. Chem. 1965, 8, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cui, Z.; Gu, Y.; Liu, Y.; Wang, Q. The phytotoxicity of natural tetramic acid derivatives. Pest Manag. Sci. 2011, 67, 1059–1061. [Google Scholar] [CrossRef]
- Smith, E.R.; Fredrickson, T.N.; Hadidian, Z. Toxic effects of the sodium and the N, N′-dibenzylethylenediamine salts of tenuazonic acid. Cancer Chemother. Rep. 1968, 52, 579–585. [Google Scholar]
- Davies, N.D.; Diner, U.L.; Morgan-Jones, G. Tenuazonic acid production by Alternaria alternata and Alternaria tenuissima isolated from cotton. Appl. Environ. Microbiol. 1977, 34, 155–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigeura, H.T.; Gordon, C.N. The biological activity of tenuazonic acid. Biochemistry 1963, 2, 1132–1137. [Google Scholar] [CrossRef]
- Asam, S.; Rychlik, M. Potential health hazards due to the occurrence of the mycotoxin tenuazonic acid in infant food. Eur. Food Res. Technol. 2013, 236, 491–497. [Google Scholar] [CrossRef]
- Umetsu, M.; Chiba, S.; Ogawa, S.; Nakao, T. Immune responses in mycoplasma pneumoniae infections. Uirusu 1974, 24, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Meazza, G.; Scheffler, B.E.; Tellez, M.R.; Rimando, A.M.; Romagni, J.G.; Duke, S.O.; Nanayakkara, D.; Khan, I.A.; Abourashed, E.A.; Dayan, F.E. The inhibitory activity of natural products on plant p-hydroxyphenylpyruvate dioxygenase. Phytochemistry 2002, 59, 281–288. [Google Scholar] [CrossRef]
- Bjork, P.K.; Rasmussen, S.A.; Gjetting, S.K.; Havshoi, N.W.; Petersen, T.I.; Ipsen, J.O.; Larsen, T.O.; Fuglsang, A.T. Tenuazonic acid from Stemphylium loti inhibits the plant plasma membrane H+-ATPase by a mechanism involving the C-terminal regulatory domain. New Phytol. 2020, 226, 770–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zonno, M.; Vurro, M. Effect of fungal toxins on germination of Striga hermonthica seeds. Weed Res. 1999, 39, 15–20. [Google Scholar] [CrossRef]
- Marfori, E.C.; Kajiyama, S.I.; Fukusaki, E.I.; Kobayashi, A. Phytotoxicity of the tetramic acid metabolite trichosetin. Photochemistry 2003, 62, 715–721. [Google Scholar] [CrossRef]
- Tylkowska, K.; Grabarkiewicz-Szczesna, J.; Iwanowska, H. Production of toxins by Alternaria alternata and A. radicina and their effects on germination of carrot seeds. Seed Sci. Technol. 2003, 31, 309–316. [Google Scholar] [CrossRef]
- Zhou, B.; Qiang, S. Effect of tenuazonic acid produced by Alternaria alternata on mironucleus and karyokinesis of Vicia faba root tip cells. Chin. J. App. Environ. Biol. 2007, 13, 803–806. [Google Scholar]
- Qiang, S.; Summerell, B.A.; Li, Y. Pathogenicity of Alternaria alternata on Crofton weed (Eupatorium adenophorum). In Proceedings of the 17th Asian-Pacific Weed Science Society Conference, Bangkok, Thailand, 22–27 November 1999; pp. 556–561. [Google Scholar]
- Qiang, S.; Wan, Z.; Dong, Y.; Li, Y. Phytotoxicity of crude metabolites produced by Alternaria alternata to Crofton weed. In Sustainable Management towards the 21 Century in China, Proceedings of the 6th Weed Science Conference of China, Nanning, China, 1 March 1999; Guangxi Nationality Press Nanning: Nanning, China, 1999. [Google Scholar]
- Chen, S.; Dai, X.; Qiang, S.; Tang, Y. Effect of a nonhost-selective toxin from Alternaria alternata on chloroplast-electron transfer activity in Eupatorium adenophorum. Plant Pathol. 2005, 54, 671–677. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Wang, H.; Meng, B.; Wei, R.; Wang, L.; An, C.; Chen, S.; Yang, C.; Qiang, S. An evaluation of tenuazonic acid, a potential biobased herbicide in cotton. Pest Manag. Sci. 2019, 75, 2482–2489. [Google Scholar] [CrossRef]
- Chen, S.; Xu, X.; Dai, X.; Yang, C.; Qiang, S. Identification of tenuazonic acid as a novel type of natural photosystem II inhibitor binding in QB-site of Chlamydomonas reinhardtii. Biochim. Biophys. Acta 2007, 1767, 306–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Yin, C.; Qiang, S.; Zhou, F.; Dai, X. Chloroplastic oxidative burst induced by tenuazonic acid, a natural photosynthesis inhibitor, triggers cell necrosis in Eupatorium adenophorum Spreng. Biochim. Biophys. Acta 2010, 1797, 391–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Strasser, R.J.; Qiang, S. In vivo assessment of effect of phytotoxin tenuazonic acid on PSII reaction centers. Plant Physiol. Biochem. 2014, 84, 10–21. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, M.; Gao, L.; Yang, Q.; Kalaji, H.; Qiang, S.; Strasser, R.; Chen, S. Tenuazonic acid-triggered cell death is the essential prerequisite for Alternaria alternata (Fr.) Keissler to infect successfully host Ageratina adenophora. Cells 2021, 10, 1010. [Google Scholar] [CrossRef]
- Schobert, R.; Schlenk, A. Tetramic and tetronic acids: An update on new derivatives and biological aspects. Bioorg. Med. Chem. 2008, 16, 4203–4221. [Google Scholar] [CrossRef]
- Mo, X.; Gulder, T.A.M. Biosynthetic strategies for tetramic acid formation. Nat. Prod. Rep. 2020, 38, 1555–1566. [Google Scholar] [CrossRef]
- Collemare, J.; Billard, A.; Böhnert, H.; Lebrun, M.H. Biosynthesis of secondary metabolites in the rice blast fungus Magnaporthe grisea: The role of hybrid PKS-NRPS in pathogenicity. Mycol. Res. 2008, 112, 207–2015. [Google Scholar] [CrossRef]
- Stickings, C.; Townsend, R. Studies in the biochemistry of micro-organisms. 108. Metabolites of Alternaria tenuis Auct.: The biosynthesis of tenuazonic acid. Biochem. J. 1961, 78, 412–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, C.; Motoyama, T.; Osada, H. Biosynthesis of the mycotoxin tenuazonic acid by a fungal NRPS–PKS hybrid enzyme. Nat. Commun. 2015, 6, 8758. [Google Scholar] [CrossRef] [Green Version]
- Yun, C.; Nishimoto, K.; Motoyama, T.; Shimizu, T.; Hino, T.; Dohmae, N.; Nagano, S.; Osada, H. Unique features of the ketosynthase domain in a nonribosomal peptide synthetase–polyketide synthase hybrid enzyme, tenuazonic acid synthetase 1. J. Biol. Chem. 2020, 295, 11602–11612. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, F.; Yin, C.; Strasser, R.; Yang, C.; Qiang, S. Application of fast chlorophyll a fluorescence kinetics to probe action target of 3-acetyl-5-isopropyltetramic acid. Environ. Exp. Bot. 2011, 71, 269–279. [Google Scholar] [CrossRef]
- Chen, S.; Yin, C.; Strasser, R.; Govindjee; Yang, C.; Qiang, S. Reactive oxygen species from chloroplasts contribute to 3-acetyl-5-isopropyltetramic acid-induced leaf necrosis of Arabidopsis thaliana. Plant Physiol. Biochem. 2012, 52, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhang, Y.; Hu, L.; Ma, Y.; Gao, J. Cytotoxic metabolites produced by Alternaria No.28, an endophytic fungus isolated from Ginkgo biloba. Nat. Prod. Commun. 2009, 4, 1473–1476. [Google Scholar] [CrossRef] [Green Version]
- Lebrun, M.H.; Nicolas, L.; Boutar, M.; Gaudemer, F.; Ranomenjanahary, S.; Gaudemer, A. Relationship between the structure and the phytotoxicity of the fungal toxin tenuazonic acid. Phytochemistry 1988, 27, 77–84. [Google Scholar] [CrossRef]
- Wong, C.; Lam, H.; Song, T.; Chen, G.; Li, X. Synthesis of constrained head-to-tail cyclic tetrapeptides by an imine-induced ring-closing/contraction strategy. Angew. Chem. Int. Ed. 2013, 52, 10212–10215. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.L.; Kuyper, L.F.; Phelps, D.W.; Cordes, A.W. Structure of the cyclic tetrapeptide tentoxin. Crystal and molecular structure of the dihydro derivative. J. Chem. Soc. Chem. Commun. 1974, 184, 339–340. [Google Scholar] [CrossRef]
- Liu, Y.; Rychlik, M. Development of a stable isotope dilution LC−MS/MS method for the Alternaria toxins tentoxin, dihydrotentoxin, and isotentoxin. J. Agric. Food Chem. 2013, 61, 2970–2978. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Carrasco, Y.; Mañes, J.; Berrada, H.; Juan, C. Development and validation of a LC-ESI-MS/MS method for the determination of Alternaria toxins alternariol, alternariol methyl-ether and tentoxin in tomato and tomato-based products. Toxins 2016, 8, 328. [Google Scholar] [CrossRef] [Green Version]
- De Sa, S.V.M.; Monteiro, C.; Fernandes, J.O.; Pinto, E.; Faria, M.A.; Cunha, S.C. Emerging mycotoxins in infant and children foods: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef]
- Halloin, J.M.; Hagedorn, D.J. Effects of tentoxin on enzymic activities in cucumber and cabbage cotyledons. Mycopathologia 1975, 55, 159–162. [Google Scholar] [CrossRef]
- Schadler, D.L.; Steele, J.A.; Durbin, R.D. Some effects of tentoxin on mature and developing chloroplasts. Mycopathologia 1976, 58, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.A.; Uchytil, T.F.; Durbin, R.D.; Bhatnagar, P.; Rich, D.H. Chloroplast coupling factor 1: A species-specific receptor for tentoxin. Proc. Natl. Acad. Sci. USA 1976, 73, 2245–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groth, G. Structure of spinach chloroplast F-1-ATPase complexed with the phytopathogenic inhibitor tentoxin. Proc. Natl. Acad. Sci. USA 2002, 99, 3464–3468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santolini, J.; Haraux, F.; Sigalat, C.; Moal, G.; Andre, F. Kinetic analysis of tentoxin binding to chloroplast F-1-ATPase-A model for the overactivation process. J. Biol. Chem. 1999, 274, 849–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Williams, D.; Kandiah, E.; Fromme, P.; Chiu, P.L. Structural basis of redox modulation on chloroplast ATP synthase. Commun. Biol. 2020, 3, 842. [Google Scholar] [CrossRef] [PubMed]
- Klotz, M.G. The action of tentoxin on membrane processes in plants. Physiol. Plantarum 1988, 74, 575–582. [Google Scholar] [CrossRef]
- Ramm, K.; Ramm, M.; Liebermann, B.; Reuter, G. Studies of the biosynthesis of tentoxin by AIternaria alternata. Microbiology 1994, 140, 3257–3266. [Google Scholar] [CrossRef] [Green Version]
- Liebermann, B.; Ramm, K. N-methylation in the biosynthesis of the phytotoxin tentoxin. Phytochemistry 1991, 30, 1815–1817. [Google Scholar] [CrossRef]
- De Bruyne, L.; Van Poucke, C.; Di Mavungu, D.J.; Zainudin, N.A.I.M.; Vanhaecke, L.; De Vleesschauwer, D.; Turgeon, B.G.; De Saeger, S.; Hofte, M. Comparative chemical screening and genetic analysis reveal tentoxin as a new virulence factor in Cochliobolus miyabeanus, the causal agent of brown spot disease on rice. Mol. Plant Pathol. 2016, 17, 805–817. [Google Scholar] [CrossRef]
- Li, Y.; Han, W.; Gui, X.; Wei, T.; Tang, S.; Jin, J. Putative nonribosomal peptide synthetase and cytochrome P450 genes responsible for tentoxin biosynthesis in Alternaria alternata ZJ33. Toxins 2016, 8, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutten, T.L.M.; Knuiman, B. Brefeldin A effects on tobacco pollen tubes. Eur. J. Cell Biol. 1993, 61, 247–255. [Google Scholar]
- Driouich, A.; Jauneau, A.; Staehelin, L.A. 7-Dehydrobrefeldin A, a naturally occurring brefeldin A derivative, inhibits secretion and causes a cis-to-trans breakdown of Golgi stacks in plant cells. Plant Physiol. 1997, 113, 487–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harri, E.; LoeMer, W.; Singh, H.; Stahlin, H.; Tamm, C. Die constitution von brefeldin A. Helv. Chem. Acta 1963, 46, 1235–1243. [Google Scholar]
- Wang, J.; Huang, Y.; Fang, M.; Zhang, Y.; Zheng, Z.; Zhao, Y.; Su, W. Brefeldin A, a cytotoxin produced by Paecilomyces sp. and Aspergillus clavatus isolated from Taxus mairei and Torreya grandis. FEMS Immunol. Med. Microbiol. 2002, 34, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misumi, Y.; Miki, K.; Takatsuki, A.; Tamura, G.; Ikehara, Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J. Biol. Chem. 1986, 261, 1398–1403. [Google Scholar] [CrossRef]
- Fujiwara, T.; Oda, K.; Yokota, S.; Takatsuki, A.; Ikehara, Y. Brefeldin A causes disassembly of the golgi-complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 1986, 263, 18545–18552. [Google Scholar] [CrossRef]
- Gamboa-Angulo, M.M.; Escalante-Erosa, F.; García-Sosa, K.; Alejos-González, F.; Delgado-Lamas, G.; Peña-Podríguez, L.M. Natural zinniol derivatives from Alternaria tagetica. Isolation, synthesis, and structure-activity correlation. J. Agric. Food Chem. 2002, 50, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Berestetskii, A.O.; Yuzikhin, O.S.; Katkova, A.S.; Dobrodumov, A.V.; Sivogrivov, D.E.; Kolombet, L.V. Isolation, identification, and characteristics of the phytotoxin produced by the fungus Alternaria cirsinoxia. Appl. Biochem. Microbiol. 2010, 46, 75–79. [Google Scholar] [CrossRef]
- Thuleau, P.; Graziana, A.; Rossignol, M.; Kauss, H.; Auriol, P.; Ranjeva, R. Binding of the phytotoxin zinniol stimulates the entry of calcium into plant protoplasts. Proc. Natl. Acad. Sci. USA 1988, 85, 5932–5935. [Google Scholar] [CrossRef] [Green Version]
- Lecomte, M.; Hamama, L.; Voisine, L.; Gatto, J.; He´lesbeux, J.-J.; Séraphin, D.; Peña-Rodriguez, L.M.; Richomme, P.; Boedo, C.; Yovanopoulos, C.; et al. Partial resistance of carrot to Alternaria dauci correlates with in vitro cultured carrot cell resistance to fungal exudates. PLoS ONE 2014, 9, e101008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qui, J.A.; Castro-Concha, L.A.; García-Sosa, K.; Miranda-Ham, M.L.; Peña-Rodríguez, L.M. Is zinniol a true phytotoxin? Evaluation of its activity at the cellular level against Tagetes erecta. J. Gen. Plant Pathol. 2010, 76, 94–101. [Google Scholar] [CrossRef]
- Nukina, M.; Marumo, S. α-Acetylorcinol from Cochliobolus lunata. Agric. Biol. Chem. 1977, 41, 717. [Google Scholar] [CrossRef]
- Venkatasubbaiah, P.; Baudoin, A.B.A.M.; Chilton, W.S. Leaf spot of hemp dogbane caused by Stagonospora apocyni, and its phytotoxins. J. Phytopathol. 1992, 135, 309–316. [Google Scholar] [CrossRef]
- Peng, W.; You, F.; Li, X.L.; Jia, M.; Zheng, C.; Han, T.; Qin, L.P. A new diphenyl ether from the endophytic fungus Verticillium sp. isolated from Rehmannia glutinosa. Chin. J. Nat. Med. 2013, 11, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, A.; Boari, A.; Evidente, M.; Cimmino, A.; Vurro, M.; Ash, G.; Evidente, A. Gulypyrones A and B and phomentrioloxins B and C produced by diaporthe gulyae, a potential mycoherbicide for Saffron Thistle (Carthamus lanatus). J. Nat. Prod. 2015, 78, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Sosa, T.; Al, J.C.; Escudero, J.C. Identification and effects of interaction phytotoxic compounds from exudade of Cistus ladanifer leaves. J. Chem. Ecol. 2001, 27, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Moon, J.H.; Seong, K.Y.; Park, K.H. Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci. Biotechnol. Biochem. 1998, 62, 2273–2276. [Google Scholar] [CrossRef] [Green Version]
- Merkl, R.; Hrádková, I.; Filip, V.; Šmidrkal, J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J. Food Sci. 2010, 28, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Deba, F.; Xuan, T.D.; Yasuda, M.; Tawata, S. Herbicidal and fungicidal activities and identification of potential phytotoxins from Bidens pilosa L. var. radiata Scherff. Weed Biol. Manag. 2007, 7, 77–83. [Google Scholar] [CrossRef]
- Zuo, S.; Zhou, S.; Ye, L.; Ma, S. Synergistic and antagonistic interactions among five allelochemicals with antialgal effects on bloom-forming Microcystis aeruginosa. Ecol. Eng. 2016, 97, 486–492. [Google Scholar] [CrossRef]
- Ceylan, O.; Alic, H. Antibiofilm, antioxidant, antimutagenic activities and phenolic compounds of Allium orientale BOISS. Brazilian Arch. Biol. Technol. 2015, 58, 935–943. [Google Scholar] [CrossRef]
- Pugazhendhi, D.; Pope, G.S.; Darbre, P.D. Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J. Appl. Toxicol. 2005, 25, 301–309. [Google Scholar] [CrossRef] [PubMed]

| Toxins | Alternaria Species | Host Range | References |
|---|---|---|---|
| AK-toxins (AK-toxin I, II) | A. alternata f. sp. kikuchana (Japanese pear pathotype) | Japanese pear | [9,10,11] |
| AF-toxins (AF-toxin I, II, III) | A. alternata f. sp. Fragariae (Strawberry pathotype) | Strawberry | [12] |
| ACT-toxins (ACT-toxin I, II) | A. alternata f. sp. citri tangerine (Tangerine pathotype) | Tangerine | [13,14,15] |
| AAL-toxins (TA1, TA2, TB1, TB2, TC1, TC2, TD1, TD2, TE1, TE2) | A. alternata f. sp. lycopersici (Tomato pathotype) | Tomato | [16,17] |
| ACR-toxins (ACR-toxin I, II, III, IV, IV’) | A. alternata f. sp. citri jambhiri (Rough lemon pathotype) | Rough lemon | [18,19] |
| AM-toxins (AM-toxin I, II, III) | A. alternata f. sp. mali (Apple pathotype) | Apple | [20,21] |
| Destruxin B | A. brassicae | Brassica spp. | [22,23] |
| HC-toxin | C. carbonum and A. jesenskae | Maize | [24,25,26] |
| Maculosin | A. alternata (Spotted knapweed pathotype) | knapweed | [27,28] |
| AS-I toxin | A. alternata (Sunflower Pathotype) | Sunflower | [29] |
| ABR-toxin | A. brassicae | Brassica spp. | [23] |
| Family | Toxins | Alternaria Species | References |
|---|---|---|---|
| Pyranones | Radicinin | A. radicina | [112] |
| Radicinol | A. radicina, A. chrysanthemi | [112,113] | |
| 3-epiradicinol | A. chrysanthemi, A. longipipes | [113,114] | |
| Deoxyradicinin | A. helianthi | [114] | |
| Pyrenocine A | A. helianthi | [115] | |
| Pyrenocine B | A. helianthi | [115] | |
| Solanapyrones A | A. solani | [116] | |
| Solanapyrones B | A. solani | [116] | |
| Solanapyrones C | A. solani | [116] | |
| Solanapyrones P | A. tenuissima | [117] | |
| Alternariol | A. tenuis | [118] | |
| Alternariol 9-methyl ether | A. tenuis | [118] | |
| Altenuene | A. tenuis | [118] | |
| Quinones | Altertoxin I | A. tenuis | [119] |
| Altertoxin II | A. tenuis | [119] | |
| Altertoxin III | A. alternata | [120] | |
| Altertoxin IV | A. tenuissima | [121] | |
| Altertoxin V | A. tenuissima | [122] | |
| Altertoxin VI | A. tenuissima | [122] | |
| Altertoxin VII | Alternaria sp. PfuH1 | [123] | |
| Alterlosins I | A. alternata | [124] | |
| Alterlosins II | A. alternata | [124] | |
| Alteichin | A. eichorniae | [120] | |
| Stemphyperylenol | A. alternata | [125] | |
| Stemphyltoxin III | A. alternata | [125] | |
| Altersolanol A | A. solani | [126] | |
| Altersolanol B | A. solani | [126] | |
| Altersolanol C | A. solani | [127] | |
| Altersolanol E | A. solani | [127] | |
| Altersolanol F | A. solani | [127] | |
| Macrosporin | A. solani | [126] | |
| Bostrycin | A. eichhorniae | [128] | |
| 4-Deoxybostrycin | A. eichhorniae | [128] | |
| Physcion | A. porri | [129] | |
| Erythroglaucin | A. porri | [129] | |
| Alterporriol B | A. porri | [130] | |
| Alterporriol K | Alternaria sp. ZJ9-6B | [130] | |
| Alterporriol L | Alternaria sp. ZJ9-6B | [130] | |
| Alterporriol M | Alternaria sp. ZJ9-6B | [130] | |
| Alterporriol T | Alternaria sp. XZSBG-1 | [131] | |
| Tertramic acid | Tenuazonic acid | A. alternata, A. longipes, A. tenuissima | [132] |
| 3-acetyl-5-isopropyltetramic acid | A. tenuis | [133] | |
| 3-acetyl-5-isobutyltetramic acid | A. tenuis | [133] | |
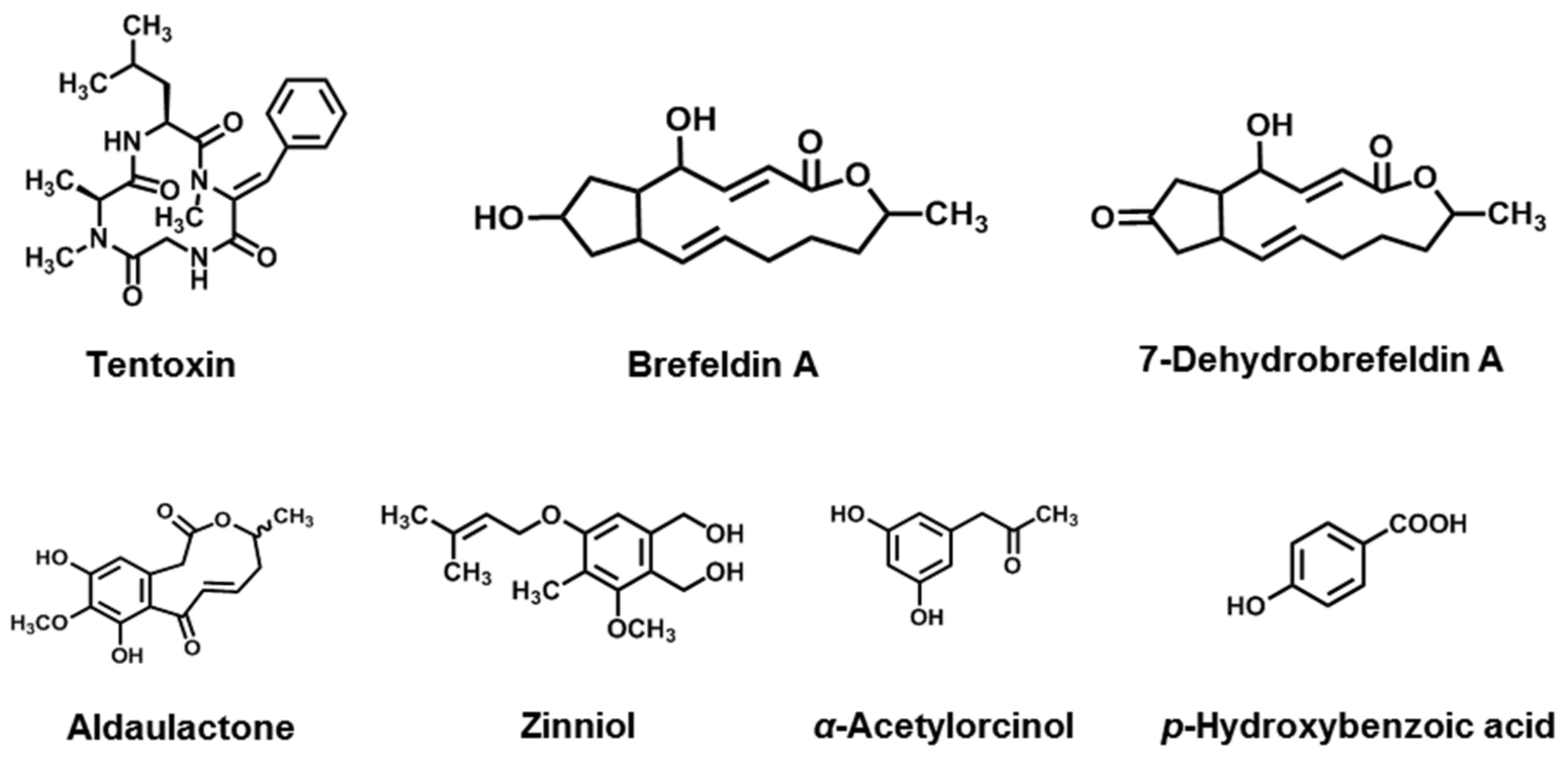
| Cyclic peptides | Tentoxin | A. alternata, A. citri, A. longipes, A. mali, A. porri, A. tenuis | [134,135,136,137,138,139] |
| Macrolides | Brefeldin A | A. carthami, A. zinnia | [140,141] |
| 7-Dehydrobrefeldin A | A. carfhami | [141] | |
| Aldaulactone | A. dauci | [142] | |
| Phenolics | Zinniol | A. zinnia, A. dauci, A. tagetica, A. solani, A. porri, A. carthami, A. macrospora, A. cichorii | [143,144,145] |
| α -Acetylorcinol | A. tenuissima, A. brassicicola, A. dauci | [146] | |
| p-Hydroxybenzoic acid | A. tagetica, A. dauci | [146,147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Guo, Y.; Luo, Z.; Gao, L.; Li, R.; Zhang, Y.; Kalaji, H.M.; Qiang, S.; Chen, S. Recent Advances in Alternaria Phytotoxins: A Review of Their Occurrence, Structure, Bioactivity, and Biosynthesis. J. Fungi 2022, 8, 168. https://doi.org/10.3390/jof8020168
Wang H, Guo Y, Luo Z, Gao L, Li R, Zhang Y, Kalaji HM, Qiang S, Chen S. Recent Advances in Alternaria Phytotoxins: A Review of Their Occurrence, Structure, Bioactivity, and Biosynthesis. Journal of Fungi. 2022; 8(2):168. https://doi.org/10.3390/jof8020168
Chicago/Turabian StyleWang, He, Yanjing Guo, Zhi Luo, Liwen Gao, Rui Li, Yaxin Zhang, Hazem M. Kalaji, Sheng Qiang, and Shiguo Chen. 2022. "Recent Advances in Alternaria Phytotoxins: A Review of Their Occurrence, Structure, Bioactivity, and Biosynthesis" Journal of Fungi 8, no. 2: 168. https://doi.org/10.3390/jof8020168
APA StyleWang, H., Guo, Y., Luo, Z., Gao, L., Li, R., Zhang, Y., Kalaji, H. M., Qiang, S., & Chen, S. (2022). Recent Advances in Alternaria Phytotoxins: A Review of Their Occurrence, Structure, Bioactivity, and Biosynthesis. Journal of Fungi, 8(2), 168. https://doi.org/10.3390/jof8020168









