Stimulation of Hyphal Ramification and Sporulation in Funneliformis mosseae by Root Extracts Is Host Phosphorous Status-Dependent
Abstract
1. Introduction
2. Materials and Methods
2.1. AM Fungus and Plant Materials
2.2. Experiment 1: Effects of RE versus RET on F. mosseae Growth
2.2.1. RE and RET Preparation
2.2.2. Effects of RE versus RET on F. mosseae Hyphal Growth
2.3. Experiment 2: Effects of Phosphorus and Nitrogen Status on Stimulatory Effects of RET on F. mosseae Growth
2.4. Experiment 3: Effects of Host Phosphorus Status on Root Metabolomes
2.5. Statistical Analysis
3. Results
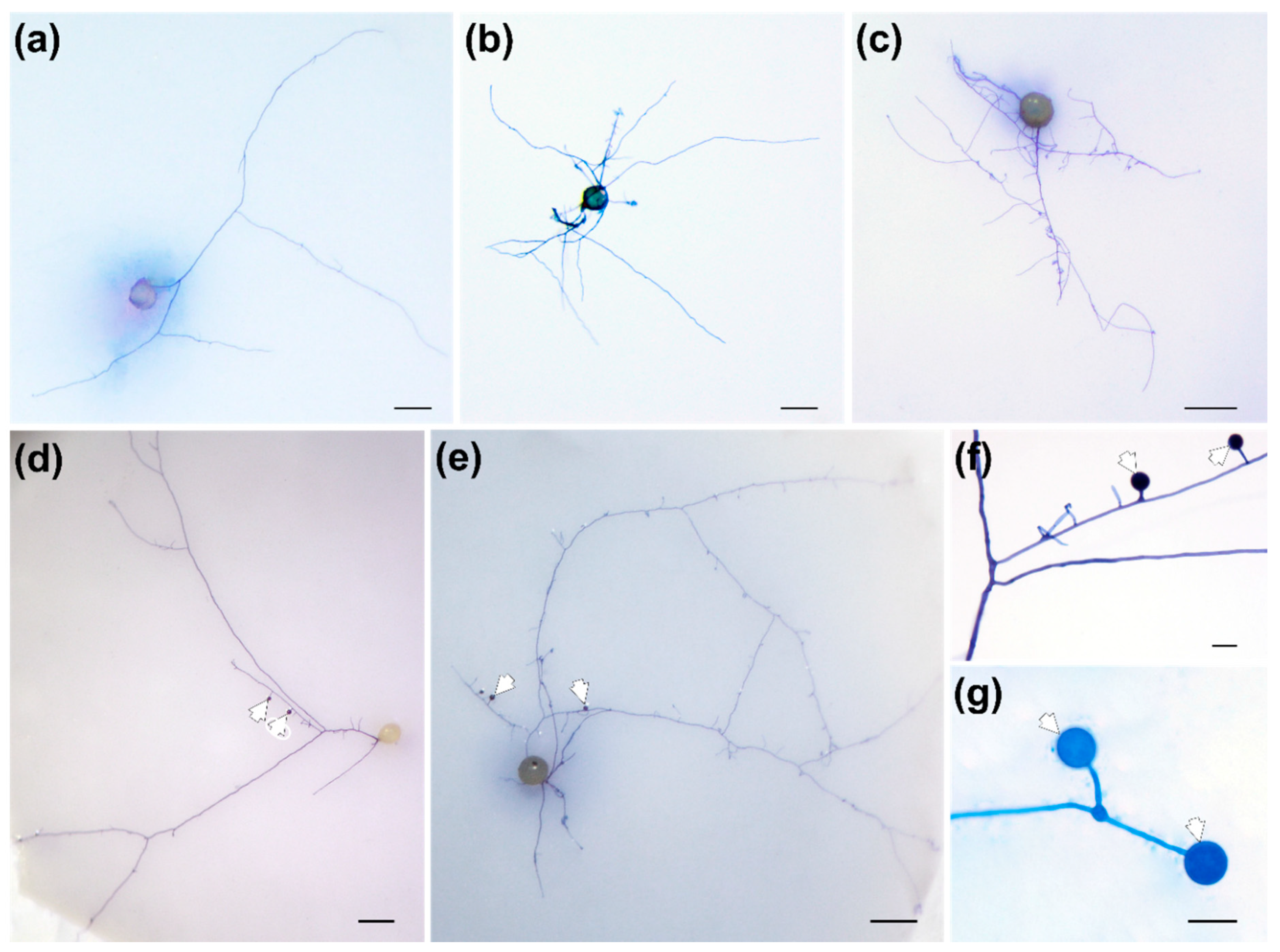
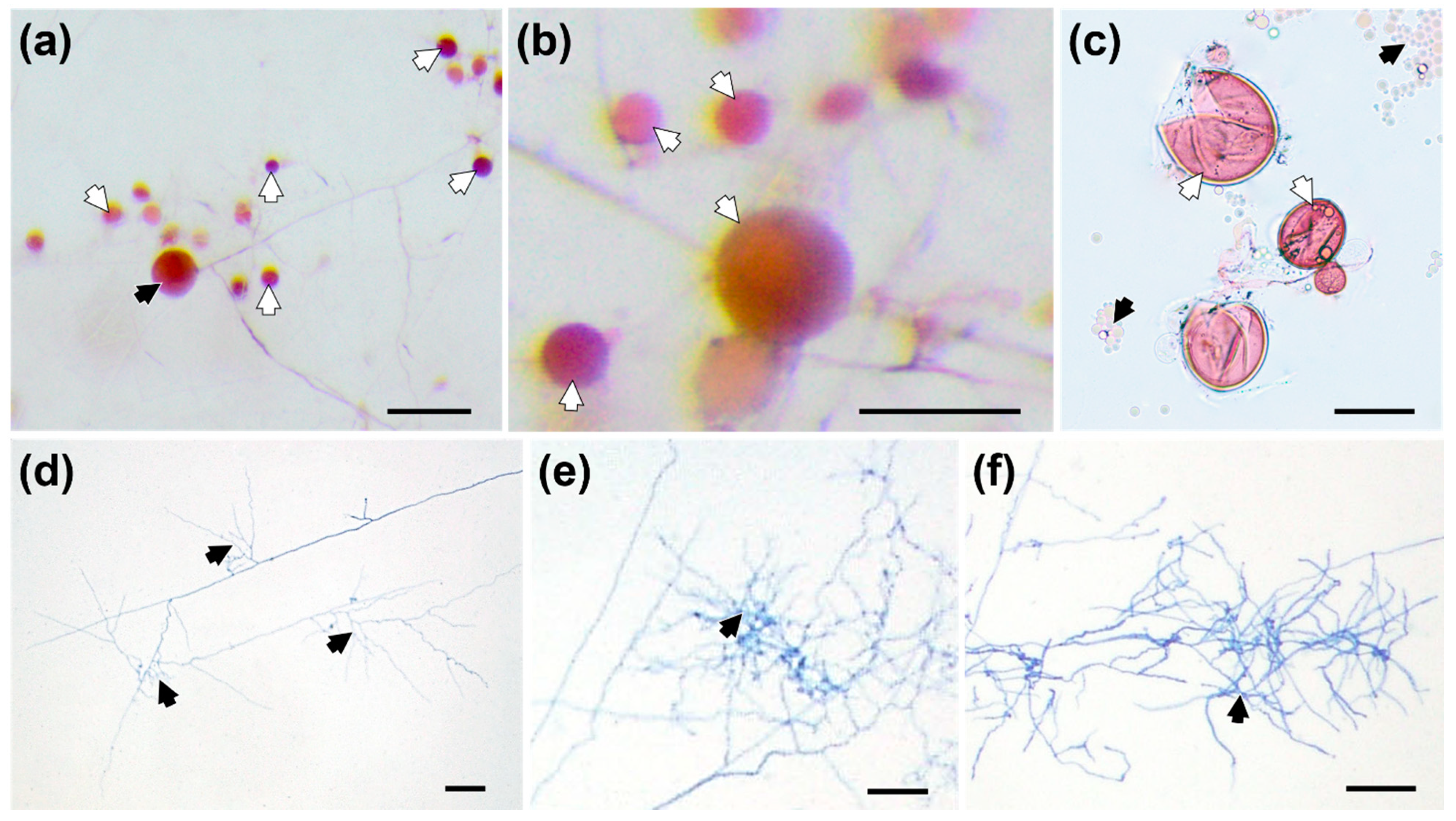
3.1. Effects of RE versus RET on F. mosseae Spore Germination and Hyphal Ramification
3.2. Effects of Phosphorus and Nitrogen Status on Stimulatory Effects of RET on F. mosseae Growth
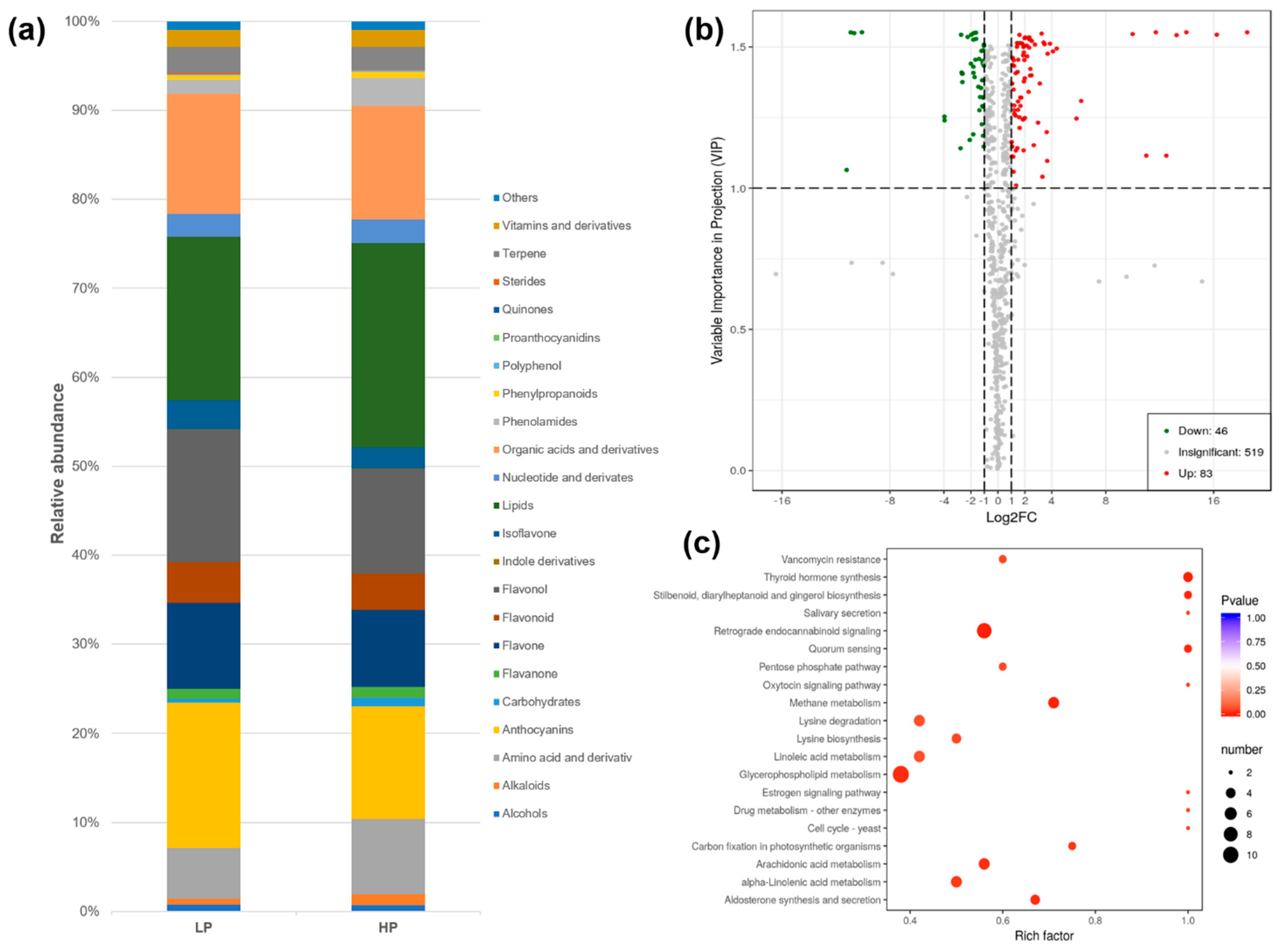
3.3. Effects of Phosphorus Status on Root Metabolomics
4. Discussion
4.1. Effects of RE versus RET on Hyphal Ramification and Differentiation of F. mosseae
4.2. Effects of Phosphorus and Nitrogen Status on Stimulation Effects of RET on F. mosseae Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, R.; Paszkowski, U. Plant carbon nourishment of arbuscular mycorrhizal fungi. Curr. Opin. Plant Biol. 2017, 39, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Bécard, G.; Douds, D.D.; Pfeffer, P.E. Extensive in vitro hyphal growth of vesicular-arbuscular mycorrhizal fungi in the presence of CO(2) and flavonols. Appl. Environ. Microb. 1992, 58, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Ghachtouli, N.E.; Paynot, M.; Martin-Tanguy, J.; Morandi, D.; Gianinazzi, S. Effect of polyamines and polyamine biosynthesis inhibitors on spore germination and hyphal growth of Glomus mosseae. Mycol. Res. 1995, 100, 597–600. [Google Scholar] [CrossRef]
- Murray, J.D.; Cousins, D.R.; Jackson, K.J.; Liu, C. Signaling at the root surface: The role of cutin monomers in mycorrhization. Mol. Plant 2013, 6, 1381–1383. [Google Scholar] [CrossRef]
- Miransari, M.; Abrishamchi, A.; Khoshbakht, K.; Niknam, V. Plant hormones as signals in arbuscular mycorrhizal symbiosis. Crit. Rev. Biotechnol. 2014, 34, 123–133. [Google Scholar] [CrossRef]
- Bücking, H.; Abubaker, J.; Govindarajulu, M.; Tala, M.; Pfeffer, P.E.; Nagahashi, G.; Lammers, P.; Shachar-Hill, Y. Root exudates stimulate the uptake and metabolism of organic carbon in germinating spores of Glomus intraradices. New Phytol. 2008, 180, 684–695. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 439, 824–827. [Google Scholar] [CrossRef]
- Kountche, B.A.; Novero, M.; Jamil, M.; Asami, T.; Bonfante, P.; Al-Babibi, S. Effect of the strigolactone analogs methyl phenlactonoates on spore germination and root colonization of arbuscular mycorrhizal fungi. Heliyon 2018, 4, e00936. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef]
- Luginbuehl, L.H.; Menard, G.N.; Kurup, S.; Van Erp, H.; Radhakrishnan, G.V.; Breakspear, A.; Oldroyd, G.E.D.; Eastmond, P.J. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 2017, 356, 1175–1178. [Google Scholar] [CrossRef]
- Rich, M.K.; Vigneron, N.; Libourel, C.; Keller, J.; Xue, L.; Hajheidari, M.; Radhakrishnan, G.V.; Ru, A.L.; Diop, S.I.; Potente, G.; et al. Lipid exchanges drove the evolution of mutualism during plant terrestrialization. Science 2021, 372, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, H.; Tsutsui, I.; Saito, K.; Kikuchi, Y.; Handa, Y.; Ezawa, T.; Hayashi, H.; Kawaguchi, M.; Akiyama, K. Stimulation of asymbiotic sporulation in arbuscular mycorrhizal fungi by fatty acids. Nat. Microbiol. 2019, 4, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Akiyama, R.; Tanaka, S.; Yano, K.; Kameoka, H.; Marui, S.; Saito, M.; Kawaguchi, M.; Akiyama, K.; Saito, K. Myristate can be used as a carbon and energy source for the asymbiotic growth of arbuscular mycorrhizal fungi. Proc. Natl. Acad. Sci. USA 2020, 117, 25779–25788. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, T.; Lewis, K.; Epstein, S.S. Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment. Science 2002, 296, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Vartoukian, S.R.; Palmer, R.M.; Wade, W.G. Strategies for culture of “unculturable” bacteria. FEMS Microbiol. Lett. 2010, 309, 1–7. [Google Scholar] [CrossRef]
- Nichols, D.; Lewis, K.; Orjala, J.; Mo, S.; Ortenberg, R.; O’Connor, P.; Zhao, C.; Vouros, P.; Kaeberlein, T.; Epstein, S.S. Short peptide induces an ‘uncultivable’ microorganism to grow in vitro. Appl. Environ. Microb. 2008, 74, 4889–4897. [Google Scholar] [CrossRef]
- Elias, K.S.; Safir, G.R. Hyphal elongation of Glomus fasciculatus in response to root exudates. Appl. Environ. Microbiol. 1987, 53, 1928–1933. [Google Scholar] [CrossRef]
- Bécard, G.; Piché, Y. Fungal growth stimulation by CO2 and root exudates in vesicular-arbuscular mycorrhizal symbiosis. Appl. Environ. Microbiol. 1989, 55, 2320–2325. [Google Scholar] [CrossRef]
- Gianinazzi-Pearson, V.; Branzanti, B.; Gianinazzi, S. In vitro enhancement of spore germination and early hyphal growth of a vesicular-arbuscular mycorrhizal fungus by host root exudates and plant flavonoids. Symbiosis 1989, 7, 243–255. [Google Scholar]
- Nair, M.; Safir, G.R.; Siqueira, J.O. Isolation and identification of vesicular-arbuscular mycorrhiza-stimulatory compounds from clover (Trifolium repens) roots. Appl. Environ. Microbiol. 1991, 57, 434–439. [Google Scholar] [CrossRef]
- Schreiner, R.P.; Koide, R.T. Stimulation of vesicular-arbuscular mycorrhizal fungi by mycotrophic and nonmycotrophic plant root systems. Appl. Environ. Microbiol. 1993, 59, 2750–2752. [Google Scholar] [CrossRef] [PubMed]
- Pinior, A.; Wyss, U.; Piché, Y.; Vierheilig, H. Plants colonized by AM fungi regulate further root colonization by AM fungi through altered root exudation. Can. J. Bot. 1999, 77, 891–897. [Google Scholar] [CrossRef]
- Gadkar, V.; David-Schwartz, R.; Nagahashi, G.; Douds, D.D., Jr.; Wininger, S.; Kapulnik, Y. Root exudate of pmi tomato mutant M161 reduces AM fungal proliferation in vitro. FEMS Microbiol. Lett. 2003, 223, 193–198. [Google Scholar] [CrossRef][Green Version]
- Paula, M.A.; Siqueira, J.O. Stimulation of hyphal growth of the VA mycorrhizal fungus Gigaspora margarita by suspension-cultured Pueraria phaseoloides cells and cell products. New Phytol. 1990, 115, 69–75. [Google Scholar] [CrossRef]
- Tsai, S.M.; Phillips, D.A. Flavonoids released naturally from alfalfa promote development of symbiotic Glomus spores in vitro. Appl. Environ. Microbiol. 1991, 57, 1485–1488. [Google Scholar] [CrossRef]
- Buee, M.; Rossignol, M.; Jauneau, A.; Ranjeva, R.; Bécard, G. The presymbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol. Plant Microbe Interact. 2000, 13, 693–698. [Google Scholar] [CrossRef]
- Nagahashi, G.; Douds, D.D., Jr. Partial separation of root exudate components and their effects upon the growth of germinated spores of AM fungi. Mycol. Res. 2000, 104, 1453–1464. [Google Scholar] [CrossRef]
- Ayling, S.M.; Smith, S.E.; Smith, F.A. Transmembrane electric potential difference of germ tubes of arbuscular mycorrhizal fungi responds to external stimuli. New Phytol. 2000, 147, 631–639. [Google Scholar] [CrossRef]
- Ramos, A.C.; Faςanha, A.R.; Feijó, J.A. Proton (H+) flux signature for the presymbiotic development of the arbuscular mycorrhizal fungi. New Phytol. 2008, 178, 177–188. [Google Scholar] [CrossRef]
- Berger, F.; Gutjahr, C. Factors affecting plant responsiveness to arbuscular mycorrhiza. Curr. Opin. Plant Biol. 2021, 59, 101994. [Google Scholar] [CrossRef]
- Kaur, S.; Suseela, V. Unraveling arbuscular mycorrhiza-induced changes in plant primary and secondary metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Breuillin, F.; Schramm, J.; Hajirezaei, M.; Ahkami, A.; Favre, P.; Druege, U.; Hause, B.; Bucher, M.; Kretzschmar, T.; Bossolini, E.; et al. Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. Plant J. 2010, 64, 1002–1017. [Google Scholar] [CrossRef] [PubMed]
- Kaeppler, S.M.; Parke, J.L.; Mueller, S.M.; Senior, L.; Stuber, C.; Tracy, W.F. Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci. 2000, 40, 358–364. [Google Scholar] [CrossRef]
- Lin, C.; Wang, Y.; Liu, M.; Li, Q.; Xiao, W.; Song, X. Effects of nitrogen deposition and phosphorus addition on arbuscular mycorrhizal fungi of Chinese fir (Cunninghamia lanceolata). Sci. Rep. 2020, 10, 12260. [Google Scholar] [CrossRef]
- Qin, J.; Wang, H.; Cao, H.; Chen, K.; Wang, X. Combined effects of phosphorus and magnesium on mycorrhizal symbiosis through altering metabolism and transport of photosynthates in soybean. Mycorrhiza 2020, 30, 285–298. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, Y.G.; Smith, F.A.; Wang, Y.; Chen, B. Arbuscular mycorrhiza enhanced arsenic resistance of both white clover (Trifolium repens Linn.) and ryegrass (Lolium perenne L.) plants in an arsenic-contaminated soil. Environ. Pollut. 2008, 155, 174–181. [Google Scholar] [CrossRef]
- Gamper, H.A.; Hartwig, U.A.; Leuchtmann, A. Mycorrhizas improve nitrogen nutrition of Trifolium repens after 8 yr of selection under elevated atmospheric CO2 partial pressure. New Phytol. 2005, 167, 531–542. [Google Scholar] [CrossRef]
- Jongen, M.; Fay, P.; Jones, M.B. Effects of elevated carbon dioxide and arbuscular mycorrhizal infection on Trifolium repens. New Phytol. 1996, 132, 413–423. [Google Scholar] [CrossRef]
- Xie, M.M.; Chen, S.M.; Zou, Y.N.; Srivastava, A.K.; Rahman, M.M.; Wu, Q.S.; Kuča, K. Effects of Rhizophagus intraradices and Rhizobium trifolii on growth and N assimilation of white clover. Plant Growth Regul. 2021, 93, 311–318. [Google Scholar] [CrossRef]
- Pawlowska, T.E.; Douds, D.D., Jr.; Charvat, I. In vitro propagation and life cycle of the arbuscular mycorrhizal fungus Glomus etunicatum. Mycol. Res. 1999, 103, 1549–1556. [Google Scholar] [CrossRef]
- Declerck, S.; Strullu, D.G.; Fortin, J.A. In Vitro Culture of Mycorrhizas; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Douds, D.D.; Schenk, N.C. Increased sporulation of vesicular-arbuscular mycorrhizal fungi by manipulation of nutrient regimes. Appl. Environ. Microbiol. 1990, 56, 314–418. [Google Scholar] [CrossRef] [PubMed]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Bécard, G.; Fortin, J.A. Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 1988, 108, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Green, N.E.; Graham, S.O.; Schenck, N.C. The influence of pH on the germination of vesicular-arbuscular mycorrhizal spores. Mycologia 1976, 68, 929–934. [Google Scholar] [CrossRef]
- Gunasekaran, P.; Sundaresan, P.; Ubalthoose Raja, N.; Lakshmanan, M. Effect of pH, temperature and nutrients on the germination of a vesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum in vitro. Proc. Indian Acad. Sci. 1987, 97, 231–234. [Google Scholar] [CrossRef]
- Sun, X.; Hu, W.; Tang, M.; Chen, H. Characterizing and handling different kinds of AM fungal spores in the rhizosphere. World J. Microbiol. Biotechnol. 2016, 32, 97. [Google Scholar] [CrossRef]
- Wang, G.M.; Stribley, D.P.; Tinker, P.B.; Walker, C. Effects of pH on arbuscular mycorrhiza I. Field observations on the long-term liming experiments at Rothamsted and Woburn. New Phytol. 1993, 124, 465–472. [Google Scholar] [CrossRef]
- Walley, F.L.; Germida, J.J. Estimating the viability of vesicular–arbuscular mycorrhizae fungal spores using tetrazolium salts as vital stains. Mycologia 1995, 87, 273–279. [Google Scholar] [CrossRef]
- Giovannetti, M.; Avio, L.; Sbrana, C.; Citernesi, A.S. Factors affecting appressorium development in the vesicular-arbuscular mycorrhizal fungus Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe. New Phytol. 1993, 123, 115–122. [Google Scholar] [CrossRef]
- Sun, X.; Tang, M. Comparison of four routinely used methods for assessing root colonization by arbuscular mycorrhizal fungi. Botany 2012, 90, 1073–1083. [Google Scholar] [CrossRef]
- Tarawaya, K.; Watanabe, S.; Yoshida, E.; Wagatsuma, T. Effect of onion (Allium cepa) root exudates on the hyphal growth of Gigaspora margarita. Mycorrhiza 1996, 6, 57–59. [Google Scholar] [CrossRef]
- Giovannetti, M.; Sbrana, C.; Citernesi, A.S.; Avio, L. Analysis of factors involved in fungal recognition responses to host-derived signals by arbuscular mycorrhizal fungi. New Phytol. 1996, 133, 65–71. [Google Scholar] [CrossRef]
- Glenn, M.G.; Chew, F.S.; Williams, P.H. Hyphal penetration of Brassica (Cruciferae) roots by a vesicular-arbuscular mycorrhizal fungus. New Phytol. 1985, 99, 463–472. [Google Scholar] [CrossRef]
- Bécard, G.; Piché, Y. Physiological factors determining vesicular-arbuscular mycorrhizal formation in host and non-host Ri T-DNA transformed roots. Can. J. Bot. 1990, 68, 1260–1264. [Google Scholar] [CrossRef]
- Giovannetti, M.; Sbrana, C.; Logi, C. Early processes involved in host recognition by arbuscular mycorrhizal fungi. New Phytol. 1994, 127, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, M.; Sbrana, C. Meeting a non-host: The behavious of AM fungi. Mycorrhiza 1998, 8, 123–130. [Google Scholar] [CrossRef]
- Ishii, T.; Shrestha, Y.H.; Kadoya, K. Effect of a sod culture system of Bahia grass (Paspalum notatum Flugge.) on vesicular arbuscular mycorrhizal formation of satsuma mandarin trees. Proc. Int. Soc. Citricul. 1996, 2, 822–824. [Google Scholar]
- Horii, S.; Matsumura, A.; Kuramoto, M.; Ishii, T. Tryptophan dimmer produced by water-stressed bahia grass is an attractant for Gigaspora margarita and Glomus caledomium. World J. Microb. Biot. 2009, 25, 1207–1215. [Google Scholar] [CrossRef]
- Cruz, A.F.; Ishii, T.; Matsumoto, I.; Kadoya, K. Seasonal changes in arbuscular mycorrhizal development and eupalitin content in Bahia grass roots grown in a satsuma mandarin orchard. J. Jpn. Soc. Hortic. Sci. 2004, 73, 529–533. [Google Scholar] [CrossRef]
- Morandi, D. Occurrence of phytoalexins and phenolic compounds in endomycorrhizal interactions, and their potential role in biological control. Plant Soil 1996, 185, 241–251. [Google Scholar] [CrossRef]
- Vierheilig, H.; Bago, B.; Albrecht, C.; Poulin, M.J.; Piche, Y. Flavonoids and arbuscular mycorrhizal fungi. In Flavonoids in the Living System; Manthey, J.A., Buslig, B.S., Eds.; Plenum Press: New York, NY, USA, 1998; pp. 9–33. [Google Scholar]
- Hildebrandt, U.; Janetta, K.; Bothe, H. Towards growth of arbuscular mycorrhizal fungi independent of a plant host. Appl. Environ. Microbiol. 2002, 68, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Scervino, J.M.; Ponce, M.A.; Monica, I.D.; Vierheilig, H.; Ocampo, J.A.; Godeas, A. Development of arbuscular mycorrhizal fungi in the presence of different patterns of Trifolium repens shoot flavonoids. J. Soil Sci. Plant Nutr. 2009, 9, 102–115. [Google Scholar] [CrossRef]
- Abdellatif, L.; Lokuruge, P.; Hamel, C. Axenic growth of the arbuscular mycorrhizal fungus Rhizophagus irregularis and growth stimulation by coculture with plant growth-promoting rhizobacteria. Mycorrhiza 2019, 29, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Blanke, V.; Renker, C.; Wagner, M.; Füllner, K.; Held, M.; Kuhn, A.J.; Buscot, F. Nitrogen supply affects arbuscular mycorrhizal colonization of Artemisia vulgaris in a phosphate-polluted field site. New Phytol. 2005, 166, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Bago, B.; Azcón-Aguilar, C.; Goulet, A.; Piché, Y. Branched absorbing structures (BAS): A feature of the extraradical mycelium of symbiotic arbuscular mycorrhizal fungi. New Phytol. 1998, 139, 375–388. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Ouziad, F.; Marner, F.J.; Bothe, H. The bacterium Paenibacillus alidus stimulates growth of the arbuscular mycorrhizal fungus Glomus intraradices up to the formation of fertile spores. FEMS Microbiol. Lett. 2006, 254, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Zhang, M.; Liang, C.; Cai, L.; Tian, J. Integration of metabolome and transcriptome analyses highlights soybean roots responding to phosphorus deficiency by modulating phosphorylated metabolite processes. Plant Physiol. Biochem. 2019, 139, 697–706. [Google Scholar] [CrossRef]
- Li, Z.; Hu, J.; Wu, Y.; Wang, J.; Song, H.; Chai, M.; Cong, L.; Miao, F.; Ma, L.; Tang, W.; et al. Integrative analysis of the metabolome and transcriptome reveal the phosphate deficiency response pathways of alfalfa. Plant Phyisol. Biochem. 2022, 170, 49–63. [Google Scholar] [CrossRef]
- Davies, F.T.; Calderón, C.M.; Huaman, Z.; Gómez, R. Influence of a flavonoid (formononetin) on mycorrhizal activity and potato crop productivity in the highlands of Peru. Sci. Hortic. 2005, 106, 318–329. [Google Scholar] [CrossRef]
- Savana da Silva, J.; Soares de Carvalho, T.; Valentim dos Santos, J.; Rose de Almeida Ribeiro, P.; Maria de Souza Moreira, F. Formononetin stimulates mycorrhizal fungi colonization on the surface of active root nodules in soybean. Symbiosis 2017, 71, 27–34. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, R.; Hu, Y.; Lin, Z. Production of isoflavone genistein in transgenic IFS tobacco roots and its role in stimulating the development of arbuscular mycorrhiza. Acta Physiol. Plant 2012, 34, 1863–1871. [Google Scholar] [CrossRef]
- Attarzadeh, M.; Balouchi, H.; Rajaie, M.; Dehnavi, M.M.; Salehi, A. Improving growth and phenolic compounds of Echinacea purpurea root by integrating biological and chemical resources of phosphorus under water deficit stress. Ind. Crops Prod. 2020, 154, 112763. [Google Scholar] [CrossRef]
- Wieser, U.; Pankow, W.; Wiemken, A. The adenylate energy charge of vesicular-arbuscular mycorrhiza of onions (Allium cepa L.). J. Plant Physiol. 1986, 124, 181–186. [Google Scholar] [CrossRef]
- Madan, R.; Pankhurst, C.; Hawke, B.; Smith, S. Use of fatty acids for identification of AM fungi and estimation of the biomass of AM spores in soil. Soil Biol. Biochem. 2002, 34, 125–128. [Google Scholar] [CrossRef]
- Stumpe, M.; Carsjens, J.G.; Stenzel, I.; Gobel, C.; Lang, I.; Pawlowski, K.; Hause, B.; Feussner, I. Lipid metabolism in arbuscular mycorrhizal roots of Medicago truncatula. Phytochemistry 2005, 66, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Halouzka, R.; Zeljković, S.C.; Klejdus, B.; Tarkowski, P. Analytical methods in strigolactone research. Plant Methods 2020, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, T.; Kohlen, W.; Sasse, J.; Borghi, L.; Schlegel, M.; Bachelier, J.B.; Reinhardt, D.; Bours, R.; Bouwmeester, H.J.; Martinoia, E. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 2012, 483, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Novero, M.; Charnikhova, T.; Ferrandino, A.; Schubert, A.; Ruyter-Spira, C.; Bonfante, P.; Lovisolo, C.; Bouwmeester, H.J.; Cardinale, F. CAROTENOID CLEAVAGE DIOXYGENASE 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus. J. Exp. Bot. 2013, 64, 1967–1981. [Google Scholar] [CrossRef]
- Dearth, S.P.; Castro, H.F.; Venice, F.; Tague, E.D.; Novero, M.; Bonfante, P.; Campagna, S.R. Metabolome changes are induced in the arbuscular mycorrhizal fungus Gigaspora margarita by germination and by its bacterial endosymbiont. Mycorrhiza 2018, 28, 421–433. [Google Scholar] [CrossRef]
- Scervino, J.M.; Gottlieb, A.; Silvani, V.A.; Pérgola, M.; Fernández, L.; Godeas, A.M. Exudates of dark septate endophyte (DSE) modulate the development of the arbuscular mycorrhizal fungus (AMF) Gigaspora rosea. Soil Biol. Biochem. 2009, 41, 1753–1756. [Google Scholar] [CrossRef]


| Treatments | Spore Germination Rate (%) | Hyphal Length (mm) | Hyphal Tips | Formation of SS | |
|---|---|---|---|---|---|
| Ratio of Germinated MS Forming SS (%) | Number of SS (per SS Forming Spore) | ||||
| Control | 48.88 ± 5.76 a | 34.20 ± 6.83 a | 23.20 ± 6.69 a | 15.00 ± 6.08 a | 1.80 ± 0.83 a |
| RE (1:5) | 66.66 ± 6.21 b | 50.80 ± 9.93 ab | 43.80 ± 7.22 bc | 20.55 ± 9.74 a | 2.00 ± 1.00 a |
| RE (1:50) | 53.88 ± 19.3 ab | 36.40 ± 5.17 a | 31.60 ± 8.68 ab | 9.44 ± 7.24 a | 1.60 ± 0.89 a |
| RET (1:5) | 70.00 ± 8.42 b | 113.80 ± 15.27 c | 56.00 ± 12.18 c | 90.00 ± 8.47 c | 5.20 ± 0.83 b |
| RET (1:50) | 55.55 ± 15.95 ab | 61.00 ± 11.85 b | 31.60 ± 11.84 ab | 44.44 ± 8.56 b | 2.40 ± 1.14 a |
| Significance (d.f. = 4) | |||||
| F | 3.362 | 48.083 | 8.890 | 82.934 | 12.222 |
| p | 0.029 * | 0.000 ** | 0.000 ** | 0.000 ** | 0.000 ** |
| Treatments | Spore Germination Rate (%) | Hyphal Length (mm) | Hyphal Tips | Formation of SS | ||
|---|---|---|---|---|---|---|
| Ratio of Germinated MS Forming SS (%) | Number of SS (per SS Forming Spore) | |||||
| LP + LN | 66.66 ± 13.02 b | 131.20 ± 37.67 b | 52.40 ± 8.56 b | 81.67 ± 14.38 b | 5.80 ± 0.84 b | |
| LP + HN | 55.55 ± 10.39 ab | 103.20 ± 29.76 ab | 53.00 ± 10.46 b | 77.78 ± 14.96 b | 5.00 ± 0.71 b | |
| HP + LN | 50.00 ± 2.77 a | 78.60 ± 15.17 a | 22.40 ± 5.94 a | 45.56 ± 5.76 a | 2.60 ± 1.14 a | |
| HP + HN | 52.77 ± 5.19 ab | 63.40 ± 16.19 a | 24.40 ± 5.81 a | 52.22 ± 5.69 a | 2.40 ± 1.14 a | |
| Significance | ||||||
| P level (d.f. = 1) | F | 6.049 | 15.260 | 68.161 | 38.323 | 44.263 |
| P | 0.026 * | 0.001 ** | 0.000 ** | 0.000 ** | 0.000 ** | |
| N level (d.f. = 1) | F | 1.111 | 3.336 | 0.134 | 0.078 | 1.316 |
| P | 0.307 ns | 0.087 ns | 0.719 ns | 0.784 ns | 0.268 ns | |
| P × N (d.f. = 1) | F | 3.086 | 0.293 | 0.039 | 1.123 | 0.474 |
| P | 0.098 ns | 0.596 ns | 0.846 ns | 0.305 ns | 0.501 ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Feng, J.; Shi, J. Stimulation of Hyphal Ramification and Sporulation in Funneliformis mosseae by Root Extracts Is Host Phosphorous Status-Dependent. J. Fungi 2022, 8, 181. https://doi.org/10.3390/jof8020181
Sun X, Feng J, Shi J. Stimulation of Hyphal Ramification and Sporulation in Funneliformis mosseae by Root Extracts Is Host Phosphorous Status-Dependent. Journal of Fungi. 2022; 8(2):181. https://doi.org/10.3390/jof8020181
Chicago/Turabian StyleSun, Xueguang, Jingwei Feng, and Jing Shi. 2022. "Stimulation of Hyphal Ramification and Sporulation in Funneliformis mosseae by Root Extracts Is Host Phosphorous Status-Dependent" Journal of Fungi 8, no. 2: 181. https://doi.org/10.3390/jof8020181
APA StyleSun, X., Feng, J., & Shi, J. (2022). Stimulation of Hyphal Ramification and Sporulation in Funneliformis mosseae by Root Extracts Is Host Phosphorous Status-Dependent. Journal of Fungi, 8(2), 181. https://doi.org/10.3390/jof8020181






