Deciphering the Association among Pathogenicity, Production and Polymorphisms of Capsule/Melanin in Clinical Isolates of Cryptococcus neoformans var. grubii VNI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material and Demographic and Clinical Data
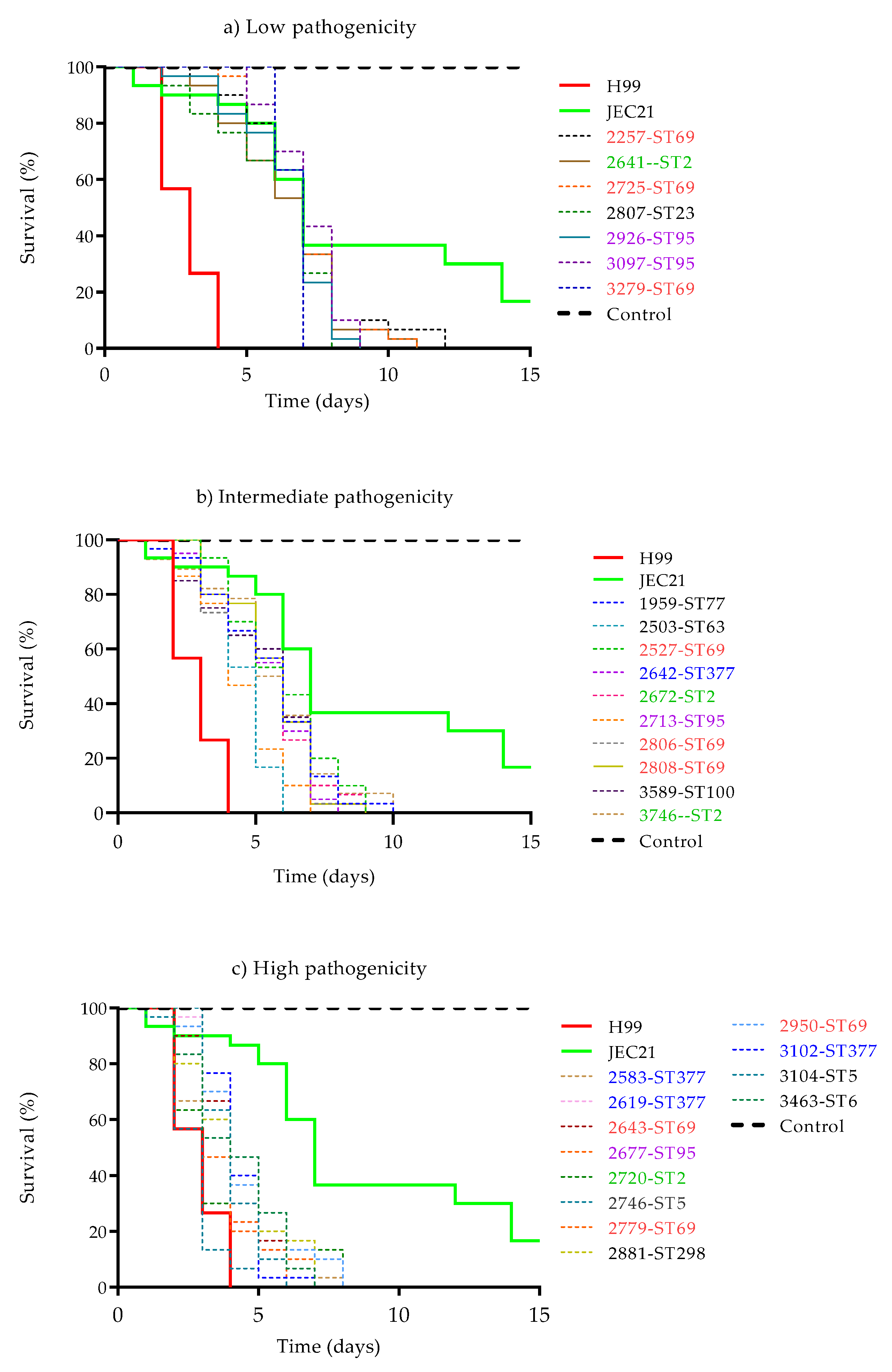
2.2. Assessment of Pathogenicity of Isolates in Galleria mellonella
2.3. Capsule Size Determination
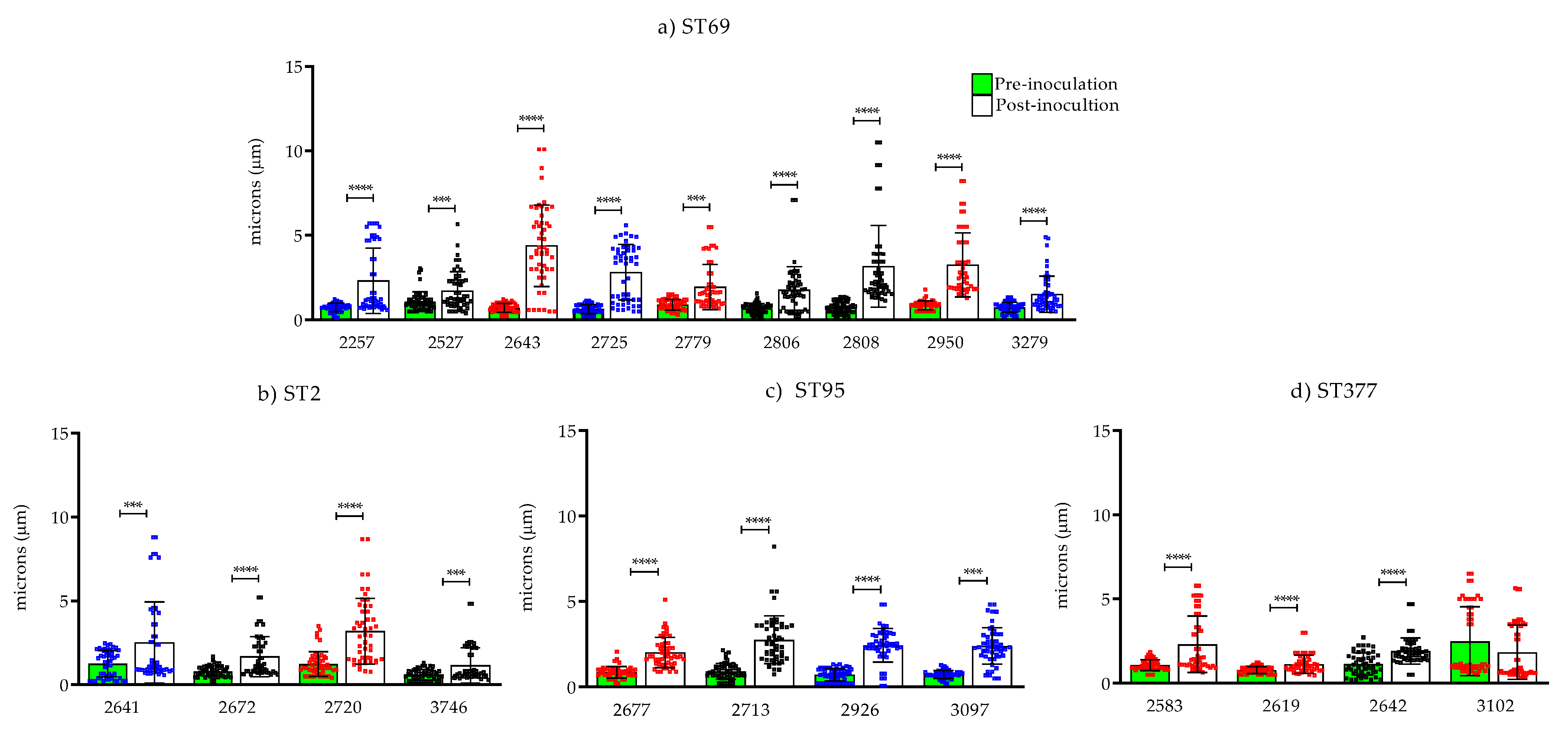
2.3.1. Effect of the Change in Capsule Size Pre- and Post-inoculation in G. mellonella
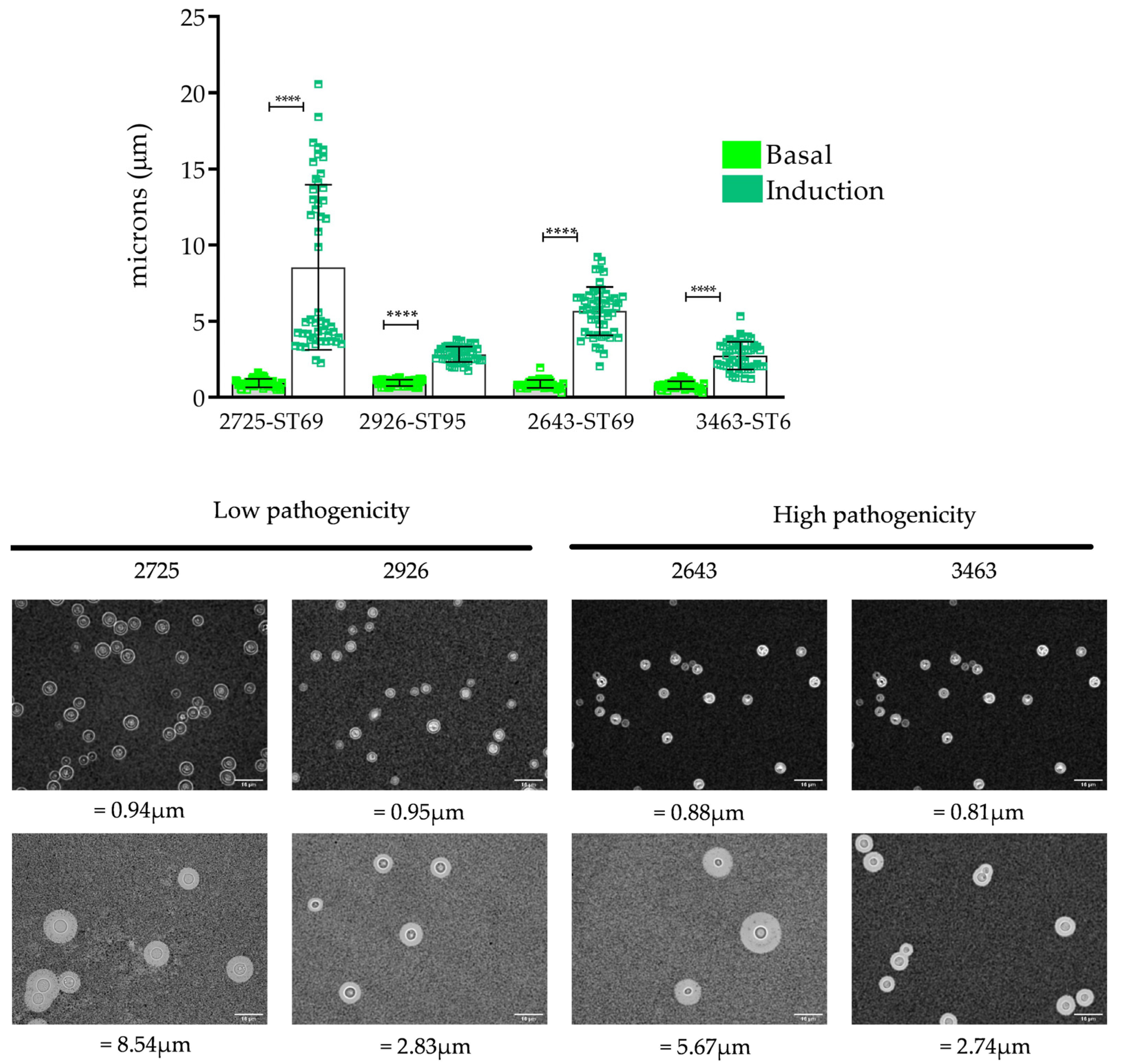
2.3.2. Capsule Induction
2.4. Melanin Production
2.5. Genome Sequencing and Data Preprocessing
2.6. Multilocus Sequence Typing
2.7. Identification and Analysis of Genetic Variants
2.8. Statistical Analysis
3. Results
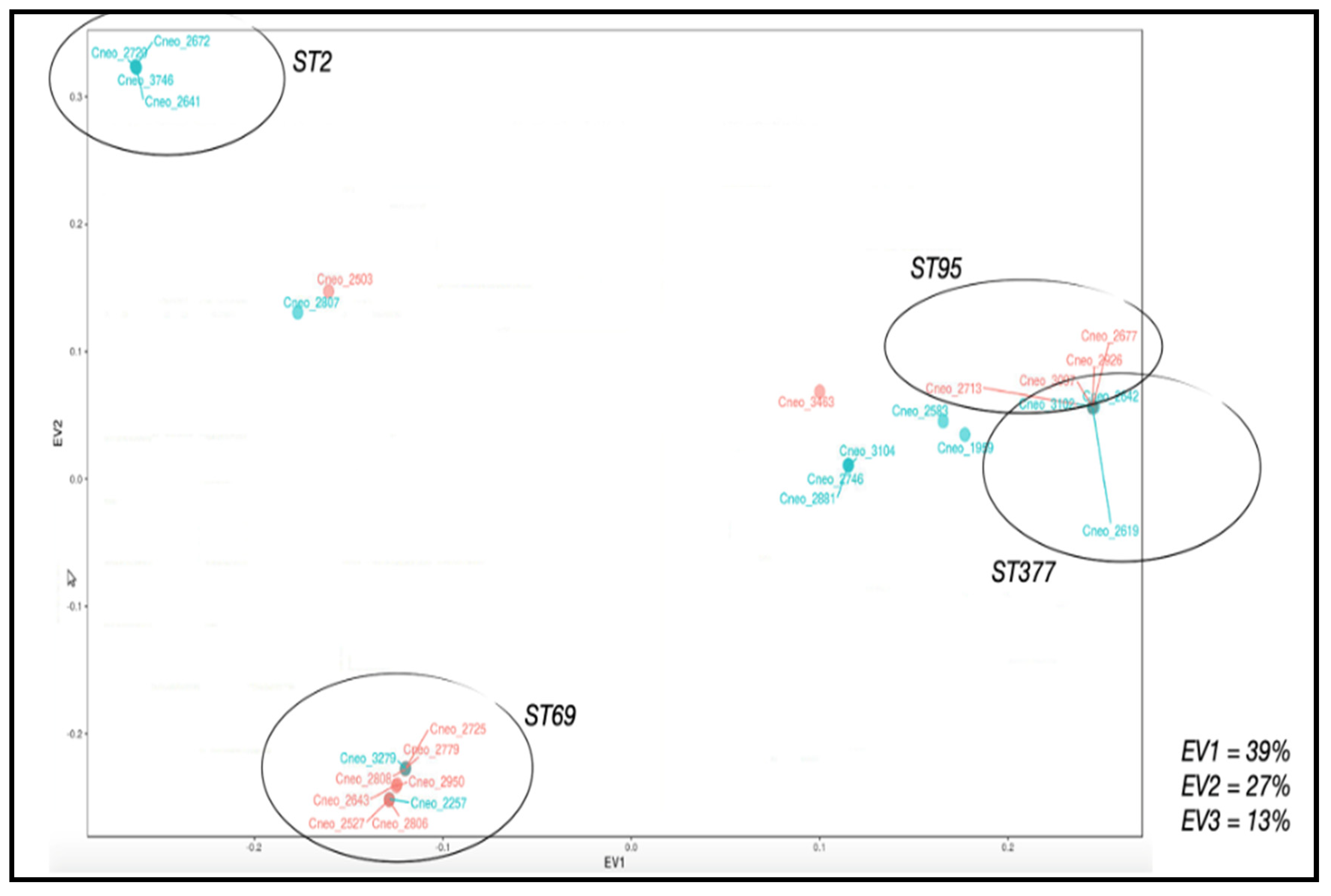
3.1. Identification of the Sequence Types of the Clinical Isolates of C. neoformans var. grubii
3.2. Assessment of Pathogenicity in G. mellonella
3.3. Determination of Capsule Size Change In Vivo Using the G. mellonella Invertebrate Model
3.4. Capsule Induction
3.5. Melanin Production
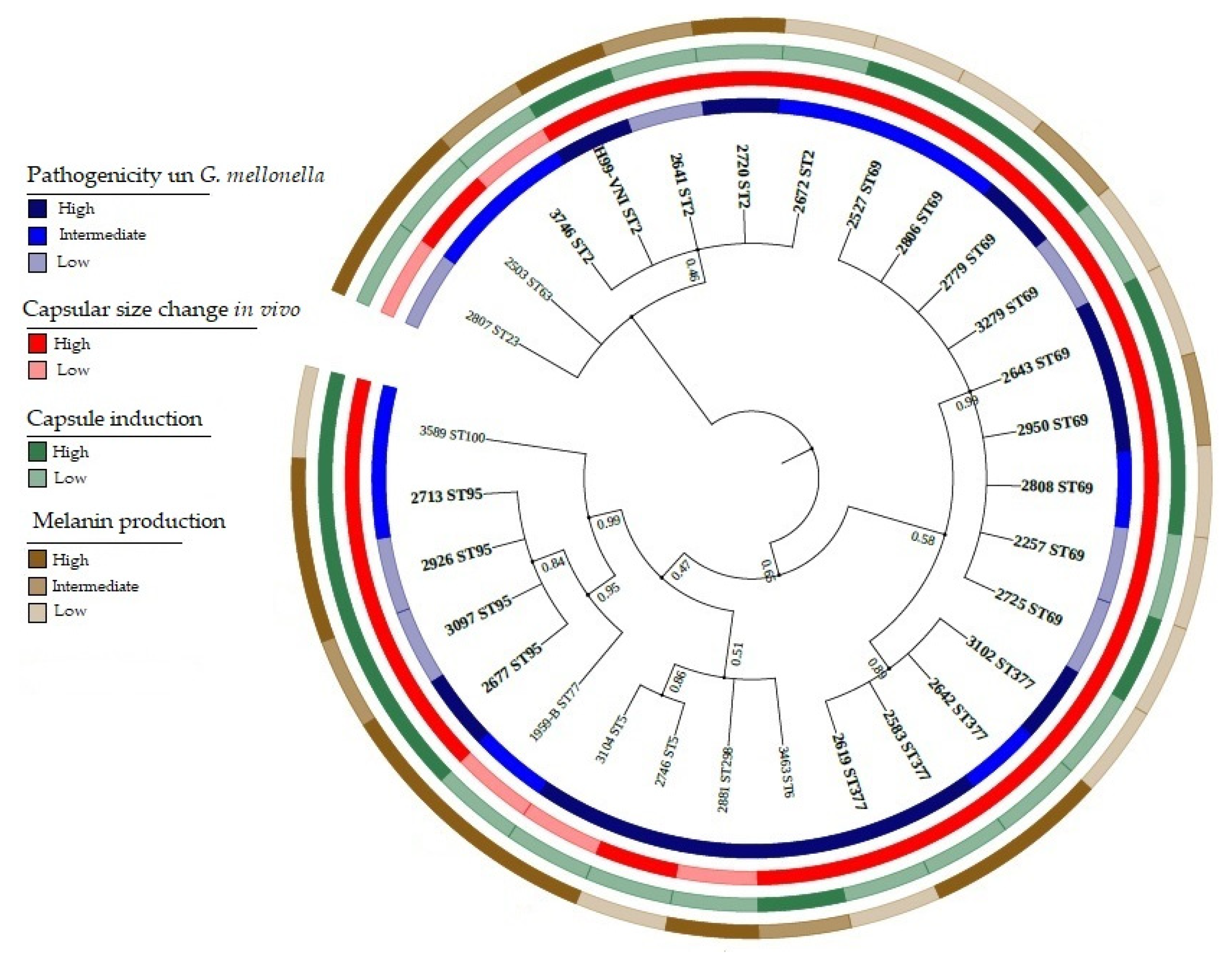
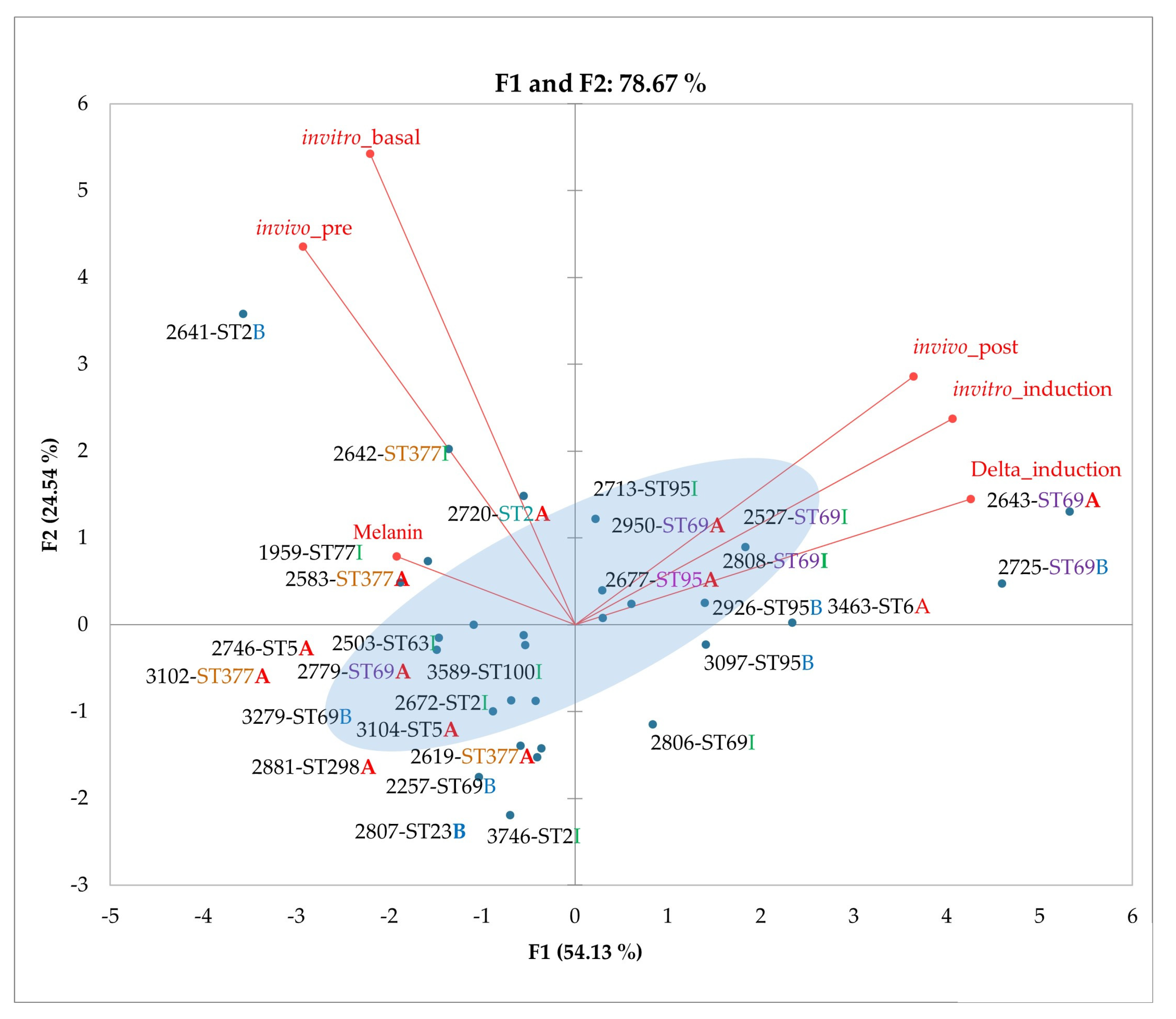
3.6. Principal Component Analysis (PCA) of Phenotypic Variables
3.7. Applications of PCA to Phenotypic and Genotypic Variable Sets
4. Discussion
4.1. Genotypic Diversity in a Group of Clinical Isolates of C. neoformans var. grubii by MLST
4.2. Pathogenicity Is Independent of Characterized Genotype in a Group of Clinical Isolates of C. neoformans var. grubii
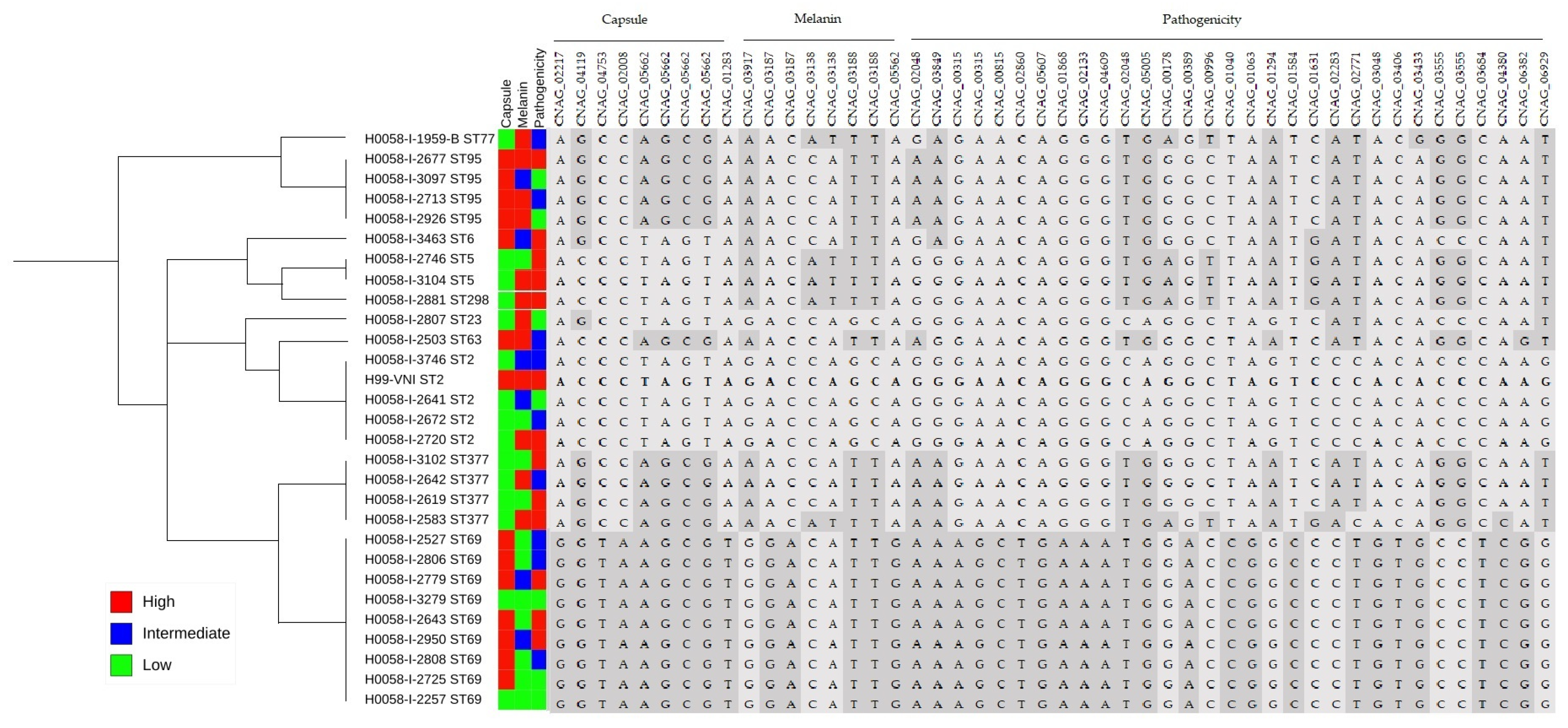
4.3. Classification of Pathogenic Isolates of C. neoformans var. grubii via the Main Virulence Factors: Capsule and Melanin
4.4. Identification of Non-Synonymous Variants Associated with Capsule and Melanin Genes That Could Influence Pathogenicity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Heitman, J. The Biology of the Cryptococcus neoformans Species Complex. Annu. Rev. Microbiol. 2006, 60, 69–105. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, O.; Martínez, I. Criptococosis. Historia natural y estado actual del tratamiento. Acta Pediatr. Mex. 2005, 26, 18–28. [Google Scholar]
- Hagen, F.; Khayhan, K.; Theelen, B.; Kolecka, A.; Polacheck, I. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 2015, 78, 16–48. [Google Scholar] [CrossRef] [Green Version]
- Barnett, J. A history of research on yeasts 14: 1 medical yeasts part 2, Cryptococcus neoformans. Yeast 2010, 27, 875–904. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.L.; Wang, Z.; Janbon, G. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb. Perspect. Med. 2014, 4, 1–27. [Google Scholar] [CrossRef]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.; Loyse, A.; Boulware, D. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Cogliati, M. Global Molecular Epidemiology of Cryptococcus neoformans and Cryptococcus gattii: An Atlas of the Molecular Types. Scientifica 2013, 2013, 675213. [Google Scholar] [CrossRef] [Green Version]
- Escandon, P.; Lizarazo, J.; Agudelo, C.; Castañeda, E. Cryptococcosis in Colombia: Compilation and Analysis of Data from Laboratory-Based Surveillance. Open Access J. Fungi 2018, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Meyer, W.; Castañeda, A.; Jackson, S.; Castañeda, E. Molecular typing of Ibero American Cryptococcus neoformans isolates. Emerg. Infect. Dis. 2003, 9, 189–195. [Google Scholar] [CrossRef]
- Viviani, M.; Cogliati, M. Molecular analysis of 311 Cryptococcus neoformans isolates from a 30-month ECMM survey of cryptococcosis in Europe. FEMS Yeast Res. 2006, 6, 614–619. [Google Scholar] [CrossRef] [Green Version]
- Perfect, J. Cryptococcus neoformans: The yeast that likes it hot. FEMS Yeast Res. 2006, 6, 463–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casadevall, A.; Perfect, J. Cryptococcus Neoformans; ASM Press: Washington, DC, USA, 1998; Volume 107, pp. 407–456. [Google Scholar]
- Idnurm, A.; Bahn, Y.S.; Nielsen, K.; Lin, X. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat. Rev. Microbiol. 2005, 3, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Janbon, G.; Ormerod, K.L.; Paulet, D.; Byrnes, E.J. Analysis of the genome and transcriptome of Cryptococcus neoformans var. grubii reveals complex RNA expression and microevolution leading to virulence attenuation. PLoS Genet. 2014, 10, e1004261. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xiao, M.; Chen, S.; Kong, F.; Dou, H.-T.; Wang, H.; Xiao, Y.-L.; Kang, M.; Sun, Z.-Y.; Hu, Z.-D.; et al. Predominance of Cryptococcus neoformans var. grubii multilocus sequence type 5 and emergence of isolates with non-wild-type minimum inhibitory concentrations to fluconazole: A multi-centre study in China. Clin. Microbiol. Infect. 2016, 22, 887.e1–887.e9. [Google Scholar] [CrossRef] [Green Version]
- Charlier, C.; Chretien, F.; Baudrimont, M.; Mordelet, E.; Lortholary, O.; Dromer, F. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood brain barrier. Am. J. Pathol. 2005, 166, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Mednick, A.J.; Nosanchuk, J.D. Melanization of Cryptococcus neoformans affects lung inflammatory responses during cryptococcal infection. Infect. Immun. 2005, 73, 2012–2019. [Google Scholar] [CrossRef] [Green Version]
- Zaragoza, O. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular surviva. Cell Microbiol. 2008, 10, 2043–2057. [Google Scholar] [CrossRef] [Green Version]
- Bose, I.; Reese, A.; Ory, J.; Janbon, G. A Yeast under Cover: The capsule of Cryptococcus neoformans. Eukaryot. Cell 2003, 2, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Casadevall, A.; Steenbergen, J.; Nosanchuk, J. Ready made virulence and “dual use” virulence factors in pathogenic environmental fungi-the Cryptococcus neoformans paradigm. Curr. Opin. Microbiol. 2003, 6, 332–337. [Google Scholar] [CrossRef]
- Huffnagle, G.; Chen, B.; Curtis, J.; Donald, M.; Trieter, R. Down regulation of the afferent phase of T cell mediated pulmonary inflammation and immunity by high melanin-producing strain of Cryptococcus neoformans. J. Immunol. 1995, 155, 3507–3516. [Google Scholar]
- McFadden, D.C.; Casadevall, A. Capsule and melanin synthesis in Cryptococcus neoformans. Med. Mycol. 2001, 39, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, W.; Aanensen, D.M.; Boekhout, T.; Cogliati, M.; Diaz, M.R.; Esposto, M.C.; Fisher, M.; Gilgado, F.; Hagen, F.; Kaocharoen, S.; et al. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med. Mycol. 2009, 47, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.C.; Kwon-Chung, K.J. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect. Immun. 1998, 66, 2230–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loftus, B.; Fung, E.; Roncaglia, P.; Rowley, D.; Amedeo, P.; Bruno, D. The Genome of the Basidiomycetous Yeast and Human Pathogen Cryptococcus neoformans. Science 2005, 25, 1321–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Meara, T.; Alspaugh, J. The Cryptococcus neoformans Capsule: A Sword and a Shield. Clin. Microbiol. Rev. 2012, 25, 387–408. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Jang, E.-H.; Lee, M.; Kim, S.-W.; Lee, Y.; Lee, K.-T.; Bahn, Y.-S. Unraveling Melanin Biosynthesis and Signaling Networks in Cryptococcus neoformans. mBio 2019, 10, e02267-19. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.; Nakayasu, E.; Oliveira, D.; Nimrichter, L.; Nosanchuk, J. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 2008, 7, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Banks, I.R.; Specht, C.A.; Donlin, M.J.; Gerik, K.J.; Levitz, S.M. A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell 2005, 4, 1902–1912. [Google Scholar] [CrossRef] [Green Version]
- Heitman, F.; Walton, F.; Idnurm, A. Novel gene functions required for melanization of the human pathogen Cryptococcus neoformans. Mol. Microbiol. 2005, 57, 1381–1396. [Google Scholar]
- Rosas, A.L.; Nosanchuk, J.; Feldmesser, M.; Cox, G.M.; McDade, H.C. Synthesis of polymerized melanin by Cryptococcus neoformans in infected rodents. Infect. Immun. 2000, 68, 2845–2853. [Google Scholar] [CrossRef] [Green Version]
- Velez, N.; Alvarado, M.; Parra-giraldo, C.M.; Sanchez-Quitian, Z.; Escandon, P.; Castañeda, E. Genotypic Diversity Is Independent of Pathogenicity in Colombian Strains of Cryptococcus neoformans and Cryptococcus gattii in Galleria mellonella. Fungi 2018, 4, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firacative, C.; Shuyao, D.; Meller, W. Galleria mellonella Model Identifies Highly Virulent Strains among All Major Molecular Types of Cryptococcus gattii. PLoS ONE 2014, 9, e1050576. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; Casadevall, A. Experimental modulation of capsule size in Cryptococcus neoformans. Biol. Proc. Online 2004, 6, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, J.; Desjardins, C.; Sykes, S.; Beale, M.; Vanhove, M. Tracing Genetic Exchange and Biogeography of Cryptococcus neoforman. Genetics 2017, 207, 327–346. [Google Scholar] [CrossRef] [Green Version]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data 2010.
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Duitama, J.; Quintero, J.C.; Cruz, D.F.; Quintero, C.; Hubmann, G.; Foulquié-Moreno, M.R.; Verstrepen, K.; Thevelein, J.; Tohme, J. An integrated framework for discovery and genotyping of genomic variants from high-throughput sequencing experiments. Nucleic Acids Res. 2014, 42, e44. [Google Scholar] [CrossRef]
- Librado, P.R.J. DnaSP v5: Un software para el análisis integral de datos de polimorfismo de ADN. Bioinformática 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Tello, D.; Gil, J.; Loaiza, C.D.; Riascos, J.J.; Cardozo, N.; Duitama, J. NGSEP3: Accurate variant calling across species and sequencing protocols. Bioinformatics 2019, 35, 4716–4723. [Google Scholar] [CrossRef] [Green Version]
- Toolkit, P. Broad Institute, GitHub Repository. 2019. Available online: http://broadinstitute.github.io/picard/ (accessed on 1 October 2021).
- Huson, D.H.; Scornavacca, C. Dendroscope 3: An interactive tool for rooted phylogenetic trees and networks. Syst. Biol. 2012, 61, 1061–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Levine, D.; Shen, J.; Gogarten, S.M.; Laurie, C.; Weir, B.S. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics 2012, 28, 3326–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samantha, D.M.; Arras, K.L.; Ormerod, P.E.; Erpf, M.I.; Espinosa, A.C.; Carpenter, R.D.; Blundell, R.D.; Stowasser, S.R.; Schulz, B.L.; Tanurdzic, M.; et al. Convergent microevolution of Cryptococcus neoformans hypervirulence in the laboratory and the clinic. Sci. Rep. 2017, 7, 17918. [Google Scholar]
- Velez, N.; Escandon, P. Multilocus sequence typing (MLST) of clinical and environmental isolates of Cryptococcus neoformans and Cryptococcus gattii in six departments of Colombia reveals high genetic diversity. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190422. [Google Scholar] [CrossRef] [PubMed]
- Beale, M.A.; Sabiiti, W.; Robertson, E.; Fuentes-Cabrejo, K.M.; O´Hanlon, S.J. Genotypic diversity is associated with clinical outcome and phenotype in cryptococcal meningitis across Southern Africa. PLoS Negl. Trop. Dis. 2015, 9, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogliati, M.; Zamfirova, R.; Tortorano, A. Molecular epidemiology of Italian clinical Cryptococcus neoformans var. grubii isolates. Med. Mycol. 2013, 51, 499–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khayhan, K.; Hagen, F.; Pan, W.; Simwami, S.; Fisher, M. Geographically Structured Populations of Cryptococcus neoformans Variety grubii in Asia Correlate with HIV Status and Show a Clonal Population Structure. PLoS ONE 2013, 8, e72222. [Google Scholar] [CrossRef]
- Simwami, S.; Khayhan, H. Low diversity Cryptococcus neoformans variety grubii multilocus sequence types from Thailand are consistent with an ancestral African origin. PLoS Pathog. 2011, 7, e10001343. [Google Scholar] [CrossRef] [Green Version]
- Dou, H.T.; Xu, Y.C.; Wang, H.Z. Molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii in China between 2007 and 2013 using multilocus sequence typing and the DiversiLab system. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 753–762. [Google Scholar] [CrossRef]
- Wiesner, D.L.; Moskalenko, O.; Corcoran, J.M.; McDonald, T.; Rolfes, M.A. Cryptococcal genotype influences immunologic response and human clinical outcome after meningitis. mBio 2012, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, D.; Santana, K.; Silva, C.; Fernandes, L.; Beserra, S. MLST reveals a clonal population structure for Cryptococcus neoformans molecular type VNI isolates from clinical sources in Amazonas, Northern-Brazil. PLoS ONE 2018, 13, e0197841. [Google Scholar]
- Sanchini, A.; Smith, I.M.; Sedlacek, L.; Schwarz, R.; Tintelnot, K. Molecular typing of clinical Cryptococcus neoformans isolates collected in Germany from 2004 to 2010. Med. Microbiol. Immunol. 2014, 203, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Litvintseva, A.; Thakur, R.; Vilgalys, R. Multilocus sequence typing reveals three genetic subpopulations of Cryptococcus neoformans var. grubii (serotype A), including a unique population in Botswana. Genetics 2006, 172, 2223–2238. [Google Scholar]
- Firacative, C.; Meyer, M.; Castañeda, E. Cryptococcus neoformans and Cryptococcus gattii Species Complexes in Latin America: A Map of Molecular Types, Genotypic Diversity, and Antifungal Susceptibility as Reported by the Latin American Cryptococcal Study Group. J. Fungi 2021, 7, 282. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, E.; Moreno, R.; Khoury, J.; Idnurm, A.; Heitman, J. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. 2005, 73, 3842–3850. [Google Scholar] [CrossRef] [Green Version]
- Brivio, M.F.; Mastore, M.; Nappi, A. A pathogenic parasite interferes with phagocytosis of insect immunocompetent cells. Dev. Comp. Immunol. 2010, 34, 991–998. [Google Scholar] [CrossRef]
- Naicker, S.D.; Magobo, R.E.; Maphanga, T.G.; Firacative, C.; van Schalkwyk, E.; Monroy-Nieto, J.; Bowers, J.; Engelthaler, D.M.; Shuping, L.; Meyer, W.; et al. Genotype, Antifungal Susceptibility, and Virulence of Clinical South African Cryptococcus neoformans Strains from National Surveillance, 2005–2009. J. Fungi 2021, 7, 338. [Google Scholar] [CrossRef]
- Helsel, M.; White, E.; Razvi, S.; Alies, B. Chemical and functional properties of metal chelators that mobilize copper to elicit fungal killing of Cryptococcus neoformans. Metallomics 2017, 1, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Nosanchuk, J.D.; Casadedevall, A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob. Agents Chemother. 2016, 50, 3519–3528. [Google Scholar] [CrossRef] [Green Version]
- Duin, D.; Casadevall, A.; Nosanchuck, J. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin. Antimicrob. Agents Chemother. 2002, 46, 3394–3400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero, R.; Robert, V.; Cardinali, G. Impact of yeast pigmentation on heat capture and latitudinal distribution. Curr. Biol. 2018, 28, 2657–2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noverr, M.C.; Williamson, P.R.; Fajardo, R.; Huffnagle, G. CNLAC1 is required for extrapulmonary dissemination of Cryptococcus neoformans but not pulmonary persistence. Infect. Immun. 2004, 72, 1693–1699. [Google Scholar] [CrossRef] [Green Version]
- Kwon-Chung, K.J.; Polacheck, I.; Popkin, T. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J. Bacteriol. 1982, 150, 1414–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas, S.D.; Bennett, J.E.; Kwon-Chung, K.J.; Perfect, J. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 1996, 184, 377–386. [Google Scholar] [CrossRef]
- Renney, H.; Oliveira, G. Faster Cryptococcus melanization increases virulence in experimental and human cryptococcosis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Sabiiti, W.; Robertson, E.; Beale, M.; Johnston, S.; Brouwer, A.E.; Loyse, A.; Jarvis, J.; Gilbert, A.S.; Fisher, M.; Harrison, T.; et al. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J. Clin. Investig. 2014, 124, 2000–2008. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.L. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [Green Version]
- Saito, H.; Tatebayashi, K. Regulation of the osmoregulatory HOG MAPK cascade in yeast. J. Biochem. 2004, 136, 267–272. [Google Scholar] [CrossRef]
- Gerstein, A.C.; Jackson, K.M.; McDonald, T.R.; Wang, Y.; Lueck, B.D.; Bohjanen, S.; Smith, K.D.; Akampurira, A.; Meya, D.B.; Xue, C.; et al. Identification of Pathogen Genomic Differences That Impact Human Immune Response and Disease during Cryptococcus neoformans Infection. mBio 2019, 10, e01440-19. [Google Scholar] [CrossRef] [Green Version]
- Olson, G.M.; Fox, D.S.; Wang, P.; Alspaugh, J.A. Role of protein O-mannosyltransferase Pmt4 in the morphogenesis and virulence of Cryptococcus neoformans. Eukaryot. Cell 2007, 6, 222–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sven, D.; Willger, J.F.; Ernst, J.; Alspaughz, A.; Lengeler, K. Characterization of the PMT Gene Family in Cryptococcus neoformans. PLoS ONE 2009, 4, e6321. [Google Scholar]
- Perfect, J.; Casadevall, A. Cryptococcosis Cryptococcosis. Infect. Dis. Clin. N. Am. 2002, 16, 837–874. [Google Scholar] [CrossRef]
- Kumar, P.; Heiss, C.; Santiago-Tirado, F.; Black, I.; Azadi, P. Pbx Proteins in Cryptococcus neoformans Cell Wall Remodeling and Capsule Assembly. Eukaryot. Cell 2014, 13, 560–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, O.; Kelly, M.; Chow, E.; Madhani, H. Parallel β-Helix Proteins Required for Accurate Capsule Polysaccharide Synthesis and Virulence in the Yeast Cryptococcus neoformans. Eukaryot. Cell 2007, 6, 630–640. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lev, S.; Saiardi, A.; Desmarini, D. Identification of a major IP5 kinase in Cryptococcus neoformans confirms that PP-IP5/IP7, not IP6, is essential for virulence. Sci. Rep. 2016, 1, 23927. [Google Scholar] [CrossRef]
- Xu, X.; Ridgway, L.; McMaster, C. Novel members of the human oxysterol-binding protein family bind phospholipids and regulate vesicle transport. J. Biol. Chem. 2001, 276, 18407–18414. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Nong, S.H.; Mansour, M.K.; Specht, C.A. Purification and characterization of a second immunoreactive mannoprotein from Cryptococcus neoformans that stimulates T-cell responses. Infect. Immun. 2002, 70, 5485–5493. [Google Scholar] [CrossRef] [Green Version]
- Kroslak, T.; Koch, T.; Kahl, E. Human phosphatidylethanolamine-binding protein facilitates heterotrimeric G protein-dependent signaling. J. Biol. Chem. 2001, 276, 39772–39778. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Steen, B.; Lian, T.; Sham, A.P.; Tam, N.; Tangen, K.L.; Kronstad, J.W. Transcriptional Regulation by Protein Kinase A in Cryptococcus neoformans. PLoS Pathog. 2007, 3, e42. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, B.; Tang, R. The Temperature-Sensitive Role of Cryptococcus neoformans ROM2 in Cell Morphogenesis. PLoS ONE 2007, 2, e368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, B.; Tegos, G.; Hamblin, M. Susceptibility of Cryptococcus neoformans to Photodynamic Inactivation Is Associated with Cell Wall Integrity. Antimicrob. Agents Chemother. 2007, 51, 2929–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claret, S.; Gatti, X.; Doignon, F.; Thoraval, D. The Rgd1p Rho GTPase-Activating Protein and the Mid2p Cell Wall Sensor Are Required at Low pH for Protein Kinase C Pathway Activation and Cell Survival in Saccharomyces cerevisiae. Eukaryot. Cell 2005, 4, 1375–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Mukaremera, L.; McDonald, T.R.; Nielsen, J.N.; Molenaar, C.J.; Akampurira, A.; Schutz, C.; Taseera, K.; Muzoora, C.; Meintjes, G.; Meya, D.B.; et al. The Mouse Inhalation Model of Cryptococcus neoformans Infection Recapitulates Strain Virulence in Humans and Shows that Closely Related Strains Can Possess Differential Virulence. Infect. Immun. 2019, 87, e00046-19. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Silva, L.E.; Ferreira-Paim, K.; Ferreira, T.B.; Vilas-Boas, A.; Mora, D.J.; Manzato, V.M.; Fonseca, F.; Buosi, K.; Andrade-Silva, J.; Prudente, B.D.S.; et al. Genotypic analysis of clinical and environmental Cryptococcus neoformans isolates from Brazil reveals the presence of VNB isolates and a correlation with biological factors. PLoS ONE 2018, 13, e0193237. [Google Scholar] [CrossRef] [Green Version]
- Day, J.N.; Qihui, S.; Thanh, L.T. Comparative genomics of Cryptococcus neoformans var. grubii associated with meningitis in HIV infected and uninfected patients in Vietnam. PLoS Negl. Trop. Dis. 2017, 11, e0005628. [Google Scholar] [CrossRef]
- Harvey, P.H.; Pagel, M.D. The Comparative Method in Evolutionary Biology; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Armitage, P.; Berry, G.; Matthews, J.N.S. Statistical Methods in Medical Research, 4th ed.; Blackwell Science: Oxford, UK, 2002. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vélez, N.; Vega-Vela, N.; Muñoz, M.; Gómez, P.; Escandón, P.; Ramírez, J.D.; Zaragoza, O.; Monteoliva Diaz, L.; Parra-Giraldo, C.-M. Deciphering the Association among Pathogenicity, Production and Polymorphisms of Capsule/Melanin in Clinical Isolates of Cryptococcus neoformans var. grubii VNI. J. Fungi 2022, 8, 245. https://doi.org/10.3390/jof8030245
Vélez N, Vega-Vela N, Muñoz M, Gómez P, Escandón P, Ramírez JD, Zaragoza O, Monteoliva Diaz L, Parra-Giraldo C-M. Deciphering the Association among Pathogenicity, Production and Polymorphisms of Capsule/Melanin in Clinical Isolates of Cryptococcus neoformans var. grubii VNI. Journal of Fungi. 2022; 8(3):245. https://doi.org/10.3390/jof8030245
Chicago/Turabian StyleVélez, Nórida, Nelson Vega-Vela, Marina Muñoz, Paola Gómez, Patricia Escandón, Juan David Ramírez, Oscar Zaragoza, Lucía Monteoliva Diaz, and Claudia-Marcela Parra-Giraldo. 2022. "Deciphering the Association among Pathogenicity, Production and Polymorphisms of Capsule/Melanin in Clinical Isolates of Cryptococcus neoformans var. grubii VNI" Journal of Fungi 8, no. 3: 245. https://doi.org/10.3390/jof8030245






