Incidence of Candidemia Is Higher in COVID-19 versus Non-COVID-19 Patients, but Not Driven by Intrahospital Transmission
Abstract
:1. Introduction
2. Materials and Methods
2.1. Definitions
2.2. Clinical Data
2.3. Microbiological Procedures
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
3.1. Incidence of Candidemia in Patients with and without COVID-19
3.2. Comparison between Candidemia Patients with and without COVID-19
3.3. Comparison between COVID-19 and Non-COVID-19 Patients with Candidemia in the Intensive Care Unit
3.4. Serological (1, 3)-β-D-Glucan Results
3.5. Involved Species and Antifungal Susceptibilities
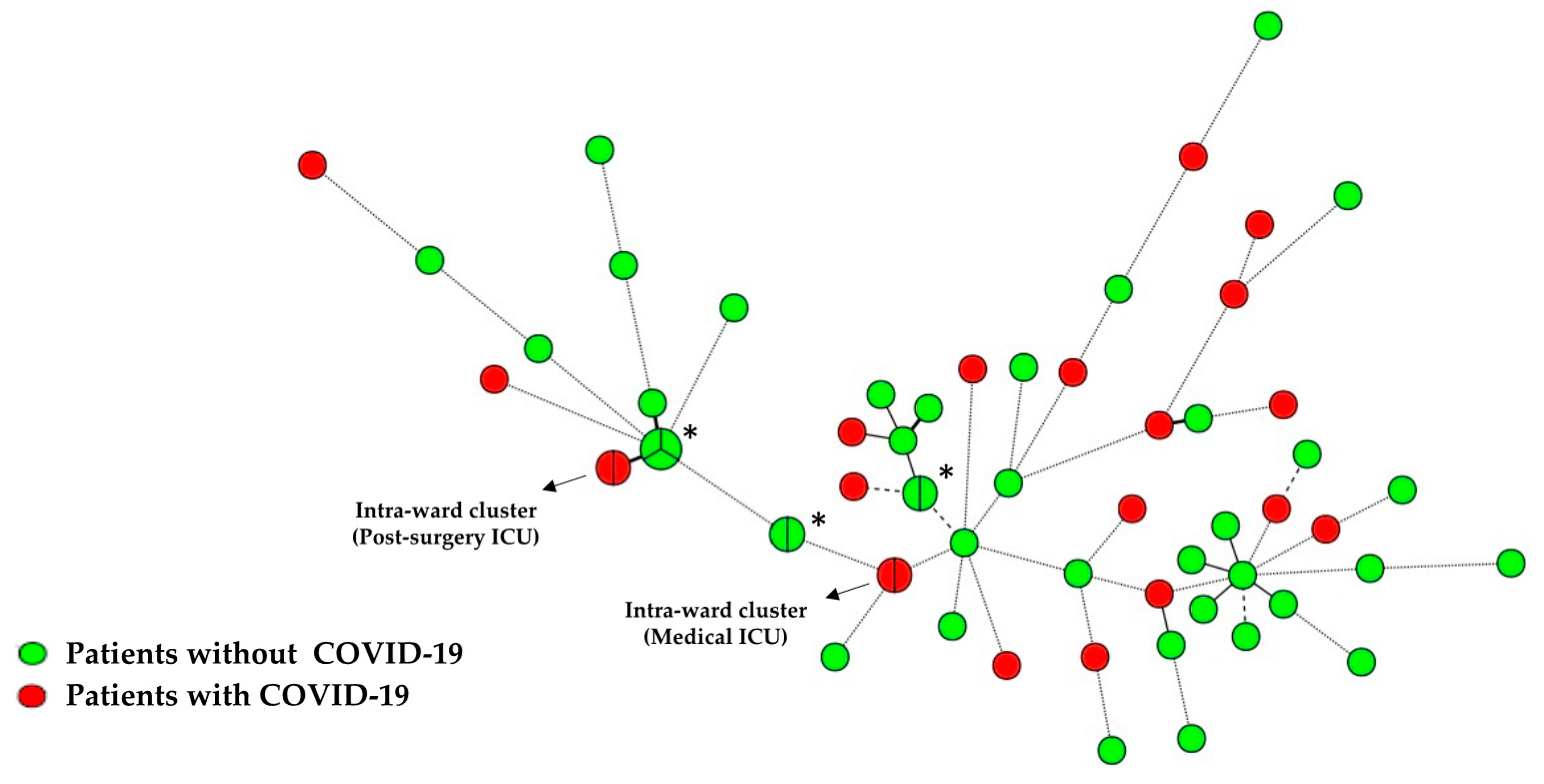
3.6. Candida Isolates Genotyping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, G.; Liang, G.; Liu, W. Fungal Co-infections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia 2020, 185, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Nori, P.; Cowman, K.; Chen, V.; Bartash, R.; Szymczak, W.; Madaline, T.; Punjabi Katiyar, C.; Jain, R.; Aldrich, M.; Weston, G.; et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect. Control Hosp. Epidemiol. 2021, 42, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-Garcia, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Kollef, M.H.; Timsit, J.F. Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med. 2020, 46, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Gangneux, J.P.; Bougnoux, M.E.; Dannaoui, E.; Cornet, M.; Zahar, J.R. Invasive fungal diseases during COVID-19: We should be prepared. J. Mycol. Med. 2020, 30, 100971. [Google Scholar] [CrossRef]
- Salmanton-Garcia, J.; Sprute, R.; Stemler, J.; Bartoletti, M.; Dupont, D.; Valerio, M.; Garcia-Vidal, C.; Falces-Romero, I.; Machado, M.; de la Villa, S.; et al. COVID-19-Associated Pulmonary Aspergillosis, March–August 2020. Emerg. Infect. Dis. 2021, 27, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Florl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Machado, M.; Valerio, M.; Alvarez-Uria, A.; Olmedo, M.; Veintimilla, C.; Padilla, B.; De la Villa, S.; Guinea, J.; Escribano, P.; Ruiz-Serrano, M.J.; et al. Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity. Mycoses 2021, 64, 132–143. [Google Scholar] [CrossRef]
- Alataby, H.; Atemnkeng, F.; Bains, S.S.; Kenne, F.M.; Diaz, K.; Nfonoyim, J. A COVID-19 Case Complicated by Candida dubliniensis and Klebsiella pneumoniae-Carbapenem-Resistant Enterobacteriaceae. J. Med. Cases 2020, 11, 403–406. [Google Scholar] [CrossRef]
- Gorkem, A.; Sav, H.; Kaan, O.; Eren, E. Coronavirus disease and candidemia infection: A case report. J. Mycol. Med. 2021, 31, 101155. [Google Scholar] [CrossRef] [PubMed]
- Awada, B.; Alam, W.; Chalfoun, M.; Araj, G.; Bizri, A.R. COVID-19 and Candida duobushaemulonii superinfection: A case report. J. Mycol. Med. 2021, 31, 101168. [Google Scholar] [CrossRef]
- Martins, A.C.; Psaltikidis, E.M.; de Lima, T.C.; Fagnani, R.; Schreiber, A.Z.; Conterno, L.O.; Kamei, K.; Watanabe, A.; Trabasso, P.; Resende, M.R.; et al. COVID-19 and invasive fungal coinfections: A case series at a Brazilian referral hospital. J. Mycol. Med. 2021, 31, 101175. [Google Scholar] [CrossRef]
- Nucci, M.; Barreiros, G.; Guimaraes, L.F.; Deriquehem, V.A.S.; Castineiras, A.C.; Nouer, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021, 64, 152–156. [Google Scholar] [CrossRef]
- Bishburg, E.; Okoh, A.; Nagarakanti, S.R.; Lindner, M.; Migliore, C.; Patel, P. Fungemia in COVID-19 ICU patients, a single medical center experience. J. Med. Virol. 2021, 93, 2810–2814. [Google Scholar] [CrossRef]
- Arastehfar, A.; Shaban, T.; Zarrinfar, H.; Roudbary, M.; Ghazanfari, M.; Hedayati, M.T.; Sedaghat, A.; Ilkit, M.; Najafzadeh, M.J.; Perlin, D.S. Candidemia among Iranian Patients with Severe COVID-19 Admitted to ICUs. J. Fungi 2021, 7, 280. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-Resistant Candida auris Infections in Critically Ill Coronavirus Disease Patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694–2696. [Google Scholar] [CrossRef] [PubMed]
- Seagle, E.E.; Jackson, B.R.; Lockhart, S.R.; Georgacopoulos, O.; Nunnally, N.S.; Roland, J.; Barter, D.M.; Johnston, H.L.; Czaja, C.A.; Kayalioglu, H.; et al. The landscape of candidemia during the COVID-19 pandemic. Clin. Infect. Dis. 2021, 74, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Macauley, P.; Epelbaum, O. Epidemiology and Mycology of Candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: Overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses 2021, 64, 634–640. [Google Scholar] [CrossRef]
- Kayaaslan, B.; Eser, F.; Kaya Kalem, A.; Bilgic, Z.; Asilturk, D.; Hasanoglu, I.; Ayhan, M.; Tezer Tekce, Y.; Erdem, D.; Turan, S.; et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses 2021, 64, 1083–1091. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Germinario, B.N.; Ferrante, M.; Frangi, C.; Li Voti, R.; Muccini, C.; Ripa, M.; Group, C.O.-B.S. Candidemia in COVID-19 patients: Incidence and characteristics in a prospective cohort compared to historical non-COVID-19 controls. Clin. Infect. Dis. 2020, 30, ciaa1594. [Google Scholar] [CrossRef]
- Riche, C.V.W.; Cassol, R.; Pasqualotto, A.C. Is the Frequency of Candidemia Increasing in COVID-19 Patients Receiving Corticosteroids? J. Fungi 2020, 6, 286. [Google Scholar] [CrossRef]
- Guisado-Gil, A.B.; Infante-Dominguez, C.; Penalva, G.; Praena, J.; Roca, C.; Navarro-Amuedo, M.D.; Aguilar-Guisado, M.; Espinosa-Aguilera, N.; Poyato-Borrego, M.; Romero-Rodriguez, N.; et al. Impact of the COVID-19 Pandemic on Antimicrobial Consumption and Hospital-Acquired Candidemia and Multidrug-Resistant Bloodstream Infections. Antibiotics 2020, 9, 816. [Google Scholar] [CrossRef]
- Agnelli, C.; Valerio, M.; Bouza, E.; Vena, A.; Guinea, J.; Del Carmen Martinez-Jimenez, M.; Marcos-Zambrano, L.J.; Escribano, P.; Munoz, P.; Group, C.S. Persistent Candidemia in adults: Underlying causes and clinical significance in the antifungal stewardship era. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 607–614. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Friberg, N.; Mares, M.; Kahlmeter, G.; Meletiadis, J.; Guinea, J.; on behalf of the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). How to interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin. Microbiol. Infect. 2020, 26, 1464–1472. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Gomez, A.; Sanchez-Carrillo, C.; Bouza, E.; Munoz, P.; Escribano, P.; Guinea, J. Isavuconazole is highly active in vitro against Candida species isolates but shows trailing effect. Clin. Microbiol. Infect. 2020, 26, 1589–1592. [Google Scholar] [CrossRef]
- Mesquida, A.; Diaz-Garcia, J.; Sanchez-Carrillo, C.; Munoz, P.; Escribano, P.; Guinea, J. In vitro activity of ibrexafungerp against Candida species isolated from blood cultures. Determination of wild type populations using the EUCAST method. Clin. Microbiol. Infect. 2022, 28, 140.E1–140.E4. [Google Scholar] [CrossRef]
- Guinea, J.; Arendrup, M.C.; Canton, R.; Canton, E.; Garcia-Rodriguez, J.; Gomez, A.; de la Pedrosa, E.G.G.; Hare, R.K.; Orden, B.; Sanguinetti, M.; et al. Genotyping Reveals High Clonal Diversity and Widespread Genotypes of Candida Causing Candidemia at Distant Geographical Areas. Front. Cell. Infect. Microbiol. 2020, 10, 166. [Google Scholar] [CrossRef]
- De Carolis, E.; Marchionni, F.; Torelli, R.; Angela, M.G.; Pagano, L.; Murri, R.; De Pascale, G.; De Angelis, G.; Sanguinetti, M.; Posteraro, B. Comparative performance evaluation of Wako beta-glucan test and Fungitell assay for the diagnosis of invasive fungal diseases. PLoS ONE 2020, 15, e0236095. [Google Scholar] [CrossRef]
- Kordalewska, M.; Guerrero, K.D.; Garcia-Rubio, R.; Jimenez-Ortigosa, C.; Mediavilla, J.R.; Cunningham, M.H.; Hollis, F.; Hong, T.; Chow, K.F.; Kreiswirth, B.N.; et al. Antifungal Drug Susceptibility and Genetic Characterization of Fungi Recovered from COVID-19 Patients. J. Fungi 2021, 7, 552. [Google Scholar] [CrossRef]
- Rajni, E.; Singh, A.; Tarai, B.; Jain, K.; Shankar, R.; Pawar, K.; Mamoria, V.; Chowdhary, A. A High Frequency of Candida auris Blood Stream Infections in Coronavirus Disease 2019 Patients Admitted to Intensive Care Units, Northwestern India: A Case Control Study. Open Forum Infect. Dis. 2021, 8, ofab452. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; de la Villa, S.; Garcia-Ramos, S.; Padilla, B.; Garcia-Olivares, P.; Pinero, P.; Garrido, A.; Hortal, J.; Munoz, P.; Caamano, E.; et al. COVID-19 associated infections in the ICU setting: A retrospective analysis in a tertiary-care hospital. Enferm. Infecc. Microbiol. Clin. 2021. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; De Maria, A.; Dentone, C.; Di Biagio, A.; et al. Bloodstream infections in critically ill patients with COVID-19. Eur. J. Clin. Investig. 2020, 50, e13319. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef]
- Antinori, S.; Bonazzetti, C.; Gubertini, G.; Capetti, A.; Pagani, C.; Morena, V.; Rimoldi, S.; Galimberti, L.; Sarzi-Puttini, P.; Ridolfo, A.L. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: An increased risk for candidemia? Autoimmun. Rev. 2020, 19, 102564. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, S.; Papachatzakis, I.; Gavrielatou, E.; Ntaidou, T.; Ischaki, E.; Malachias, S.; Vrettou, C.; Nichlos, C.; Kanavou, A.; Zervakis, D.; et al. ICU-acquired bloodstream infections in critically ill patients with COVID-19. J. Hosp. Infect. 2021, 107, 95–97. [Google Scholar] [CrossRef]
- Pittiruti, M.; Pinelli, F.; COVID, G.A.W.G.f.V.A.i. Recommendations for the use of vascular access in the COVID-19 patients: An Italian perspective. Crit. Care 2020, 24, 269. [Google Scholar] [CrossRef]
- Perez-Granda, M.J.; Carrillo, C.S.; Rabadan, P.M.; Valerio, M.; Olmedo, M.; Munoz, P.; Bouza, E. Increase in the frequency of catheter-related bloodstream infections during the COVID-19 pandemic: A plea for control. J. Hosp. Infect. 2022, 119, 149–154. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin. Infect. Dis. 2020, 73, e1634–e1644. [Google Scholar] [CrossRef]
- Diaz-Garcia, J.; Mesquida, A.; Sanchez-Carrillo, C.; Reigadas, E.; Munoz, P.; Escribano, P.; Guinea, J. Monitoring the Epidemiology and Antifungal Resistance of Yeasts Causing Fungemia in a Tertiary Care Hospital in Madrid, Spain: Any Relevant Changes in the Last 13 Years? Antimicrob. Agents Chemother. 2021, 65, e01827-20. [Google Scholar] [CrossRef]
- Diaz-Garcia, J.; Mesquida, A.; Gomez, A.; Machado, M.; Martin-Rabadan, P.; Alcala, L.; Sanchez-Carrillo, C.; Reigadas, E.; Vicente, T.; Munoz, P.; et al. Antifungal susceptibility testing identifies the abdominal cavity as a source of Candida glabrata resistant isolates. Antimicrob. Agents Chemother. 2021, 65, e01249-21. [Google Scholar] [CrossRef]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef] [Green Version]
- Jallow, S.; Govender, N.P. Ibrexafungerp: A First-in-Class Oral Triterpenoid Glucan Synthase Inhibitor. J. Fungi 2021, 7, 163. [Google Scholar] [CrossRef]
- Arastehfar, A.; Daneshnia, F.; Hilmioglu-Polat, S.; Fang, W.; Yasar, M.; Polat, F.; Metin, D.Y.; Rigole, P.; Coenye, T.; Ilkit, M.; et al. First Report of Candidemia Clonal Outbreak Caused by Emerging Fluconazole-Resistant Candida parapsilosis Isolates Harboring Y132F and/or Y132F + K143R in Turkey. Antimicrob. Agents Chemother. 2020, 64, e01001-20. [Google Scholar] [CrossRef]
- Escribano, P.; Sanchez-Carrillo, C.; Munoz, P.; Bouza, E.; Guinea, J. Reduction in Percentage of Clusters of Candida albicans and Candida parapsilosis Causing Candidemia in a General Hospital in Madrid, Spain. J. Clin. Microbiol. 2018, 56, e00574-18. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, R.; Rappold, E.; Bogdan, C.; Held, J. Comparative Analysis of the Wako beta-Glucan Test and the Fungitell Assay for Diagnosis of Candidemia and Pneumocystis jirovecii Pneumonia. J. Clin. Microbiol. 2018, 56, e00464-18. [Google Scholar] [CrossRef] [Green Version]
- Agnelli, C.; Bouza, E.; Del Carmen Martinez-Jimenez, M.; Navarro, R.; Valerio, M.; Machado, M.; Guinea, J.; Sanchez-Carrillo, C.; Alonso, R.; Munoz, P. Clinical Relevance and Prognostic Value of Persistently Negative (1,3)-beta-D-Glucan in Adults with Candidemia: A 5-year Experience in a Tertiary Hospital. Clin. Infect. Dis. 2020, 70, 1925–1932. [Google Scholar] [CrossRef]
- Koehler, P.; Stecher, M.; Cornely, O.A.; Koehler, D.; Vehreschild, M.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J.J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef]
| Variables Studied | COVID-19 Patients n = 32 (31.1%) | Non-COVID-19 Patients n = 71 (68.9%) | p |
|---|---|---|---|
| Incidence per 1000 admissions (95% CI) | 4.73 (3.24–6.67) | 0.85 (0.67–1.08) | <0.01 |
| Incidence density per 10,000 days of hospital stay | 3.22 (2.20–4.50) | 1.14 (0.90–1.40) | <0.01 |
| (95% CI) | |||
| Age—median (IQR) | 65.5 (58.0–73.8) | 65.0 (56.0–74.0) | 0.9 |
| Gender (male %) | 23 (71.9) | 44 (62.0) | 0.33 |
| Comorbidity | |||
| Cardiovascular | 15 (46.9) | 39 (54.9) | 0.45 |
| Solid tumor | 8 (25.0) | 30 (42.3) | 0.09 |
| Neurologic disease | 7 (21.9) | 24 (33.8) | 0.22 |
| Diabetes mellitus | 7 (21.9) | 28 (39.4) | 0.08 |
| Gastrointestinal disease | 6 (18.8) | 31 (43.7) | 0.01 |
| Hemodialysis | 6 (18.8) | 14 (19.7) | 1 |
| Chronic kidney disease | 5 (15.6) | 21 (29.6) | 0.13 |
| Pulmonary disease | 4 (12.5) | 19 (26.8) | 0.13 |
| Liver disease | 2 (6.2) | 20 (28.2) | 0.01 |
| SOT recipients | 2 (6.2) | 9 (12.7) | 0.49 |
| Hematological malignancy | 1 (3.1) | 7 (9.9) | 0.24 |
| HIV | 1 (3.1) | 4 (5.6) | 1 |
| Hospital setting at candidemia diagnosis | |||
| ICU | 23 (71.9) | 23 (32.4) | <0.01 |
| Medical ward | 5 (15.6) | 34 (47.9) | <0.01 |
| Surgical ward | 4 (12.5) | 14 (19.7) | 0.57 |
| Risk factors for candidemia | |||
| Total parenteral nutrition | 32 (100) | 46 (64.8) | <0.01 |
| Broad-spectrum antibiotics | 31 (96.9) | 66 (93.0) | 0.43 |
| Central venous catheter | 30 (93.8) | 50 (70.4) | <0.01 |
| Corticosteroid therapy | 27 (84.4) | 29 (40.8) | <0.01 |
| Previous ICU admission | 25 (78.1) | 17 (42.5) | <0.01 |
| Previous colonization (six months) | 22 (68.8) | 35 (49.3) | 0.06 |
| Abdominal surgery | 3 (9.4) | 25 (35.2) | <0.01 |
| Previous or concomitant infections | |||
| Low respiratory tract infections (other than COVID-19) | 11 (34.4) | 8 (11.3) | <0.01 |
| Bloodstream infection | 10 (31.2) | 14 (19.7) | 0.20 |
| CMV reactivation | 7 (21.9) | 8 (11.3) | 0.22 |
| Urinary tract infections | 5 (15.6) | 11 (15.5) | 0.98 |
| Other infections | 5 (15.6) | 11 (15.5) | 0.98 |
| Catheter-related candidemia | 26 (81.2) | 43 (60.6) | 0.03 |
| Persistent candidemia | 5 (15.6) | 8 (11.3) | 0.54 |
| Days with CVC previous candidemia, median (IQR) | 18.0 (12.0–26.3) | 16.5 (12.0–42.0) | 0.54 |
| First antifungal therapy | |||
| Echinocandins | 26 (81.2) | 34 (47.9) | <0.01 |
| Fluconazole | 4 (12.5) | 29 (40.8) | <0.01 |
| Complications | |||
| Septic shock | 14 (43.8) | 15 (21.1) | 0.02 |
| Thrombophlebitis | 3 (9.4) | 7 (9.9) | 1 |
| Ocular impairment | 3 (9.4) | 6 (8.5) | 1 |
| Outcome | |||
| Overall mortality | 20 (62.5) | 33 (46.5) | 0.13 |
| Seven-day mortality | 9 (28.1) | 16 (22.5) | 0.54 |
| 30-day mortality | 19 (59.4) | 29 (40.8) | 0.08 |
| Days from diagnosis of candidemia until death, median (IQR) | 8 (4–23) | 9.5 (4.0–20.0) | 0.89 |
| Hospital stay, median number of days (IQR) | 50 (34.2–85) | 40 (19–59) | 0.02 |
| Candida species * | |||
| Candida albicans | 22 (68.8) | 40 (56.3) | 0.23 |
| Candida tropicalis | 4 (12.5) | 6 (8.5) | 0.49 |
| Candida glabrata | 3 (9.4) | 9 (12.7) | 0.75 |
| Candida parapsilosis | 2 (6.2) | 14 (19.7) | 0.14 |
| Candida kefyr | 1 (3.1) | 0 | - |
| Candida krusei | 0 | 5 (7.0) | - |
| Variables Studied | COVID-19 Patients n = 23 | Non-COVID-19 Patients n = 23 | p |
|---|---|---|---|
| Incidence per 1000 admissions (95% CI) | 59.1 (37.4–88.7) | 3.5 (2.2–5.2) | <0.01 |
| Age—median (IQR) | 65 (57.7–74.2) | 63 (54.5–70.0) | 0.49 |
| Gender (male %) | 20 (87.0) | 18 (78.3) | 0.7 |
| Comorbidity | |||
| Cardiovascular | 11 (47.8) | 14 (60.9) | 0.37 |
| Solid tumor | 6 (26.1) | 5 (21.7) | 0.73 |
| Hemodialysis | 6 (26.1) | 13 (56.5) | 0.04 |
| Chronic kidney disease | 4 (17.4) | 10 (43.5) | 0.06 |
| Gastrointestinal disease | 3 (13.0) | 10 (43.5) | 0.02 |
| Diabetes mellitus | 2 (8.7) | 8 (34.8) | 0.03 |
| Liver disease | 2 (8.7) | 6 (26.1) | 0.24 |
| Neurologic disease | 2 (8.7) | 9 (39.1) | 0.02 |
| Pulmonary disease | 2 (8.7) | 5 (21.7) | 0.41 |
| SOT recipients | 2 (8.7) | 3 (13.0) | 1 |
| Hematological malignancy | 1 (4.3) | 4 (17.4) | 0.35 |
| HIV | 1 (4.3) | 1 (4.3) | 1 |
| Risk factors for candidemia | |||
| Total parenteral nutrition | 23 (100) | 22 (95.7) | 1 |
| Central venous catheter | 22 (95.7) | 23 (100) | 1 |
| Corticosteroid therapy | 22 (95.7) | 17 (73.9) | 0.09 |
| Broad-spectrum antibiotics | 22 (95.7) | 23 (100) | 1 |
| Previous colonization (six months) | 15 (65.2) | 20 (87.0) | 0.08 |
| Abdominal surgery | 2 (8.7) | 5 (21.7) | 0.41 |
| Catheter-related candidemia | 18 (78.3) | 15 (65.2) | 0.33 |
| Persistent candidemia | 4 (17.4) | 4 (17.4) | 1 |
| Previous or concomitant infections | |||
| Bloodstream infection | 8 (34.8) | 3 (13.0) | 0.08 |
| Ventilator-associated pneumonia | 8 (34.8) | 4 (17.4) | 0.18 |
| CMV reactivation | 7 (30.4) | 5 (21.7) | 0.5 |
| Days with CVC previous candidemia—median (IQR) | 14 (11.2–19.5) | 15.5 (11.2–22.0) | 0.62 |
| Days from ICU admission until candidemia episode, median (IQR) | 19 (13.7–23.0) | 16.5 (11.2–29.5) | 0.99 |
| Complications | |||
| Septic shock | 14 (60.9) | 11 (47.8) | 0.37 |
| Ocular impairment | 3 (13.0) | 3 (13.0) | 1 |
| Thrombophlebitis | 2 (8.7) | 2 (8.7) | 1 |
| Outcome | |||
| Overall mortality | 17 (73.9) | 14 (60.9) | 0.34 |
| Seven-day mortality | 6 (26.1) | 8 (34.8) | 0.52 |
| 30-day mortality | 16 (69.6) | 11 (47.8) | 0.13 |
| Days from diagnosis of candidemia until death, median (IQR) | 14 (4.5–24.5) | 6.5 (3.0–39.7) | 0.79 |
| Candida species | |||
| Candida albicans | 17 (73.9) | 12 (52.2) | 0.13 |
| Candida non-albicans | 6 (26.1) | 11 (47.8) | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, M.; Estévez, A.; Sánchez-Carrillo, C.; Guinea, J.; Escribano, P.; Alonso, R.; Valerio, M.; Padilla, B.; Bouza, E.; Muñoz, P. Incidence of Candidemia Is Higher in COVID-19 versus Non-COVID-19 Patients, but Not Driven by Intrahospital Transmission. J. Fungi 2022, 8, 305. https://doi.org/10.3390/jof8030305
Machado M, Estévez A, Sánchez-Carrillo C, Guinea J, Escribano P, Alonso R, Valerio M, Padilla B, Bouza E, Muñoz P. Incidence of Candidemia Is Higher in COVID-19 versus Non-COVID-19 Patients, but Not Driven by Intrahospital Transmission. Journal of Fungi. 2022; 8(3):305. https://doi.org/10.3390/jof8030305
Chicago/Turabian StyleMachado, Marina, Agustín Estévez, Carlos Sánchez-Carrillo, Jesús Guinea, Pilar Escribano, Roberto Alonso, Maricela Valerio, Belén Padilla, Emilio Bouza, and Patricia Muñoz. 2022. "Incidence of Candidemia Is Higher in COVID-19 versus Non-COVID-19 Patients, but Not Driven by Intrahospital Transmission" Journal of Fungi 8, no. 3: 305. https://doi.org/10.3390/jof8030305
APA StyleMachado, M., Estévez, A., Sánchez-Carrillo, C., Guinea, J., Escribano, P., Alonso, R., Valerio, M., Padilla, B., Bouza, E., & Muñoz, P. (2022). Incidence of Candidemia Is Higher in COVID-19 versus Non-COVID-19 Patients, but Not Driven by Intrahospital Transmission. Journal of Fungi, 8(3), 305. https://doi.org/10.3390/jof8030305









