Biosynthesis of Fungal Natural Products Involving Two Separate Pathway Crosstalk
Abstract
:1. Introduction
2. Fungal Polyketide Biosynthesis Involving Two Separate Pathway Crosstalk
2.1. The Biosynthesis of Penilactones A and B
2.2. The Biosynthesis of Dalmanol A and Acetodalmanol A
2.3. The Biosynthesis of Azasperpyranone A
3. Fungal Meroterpenoids Biosynthesis Involving Two Separate Pathway Crosstalk
The Biosynthesis of Austinol
4. Fungal Non-Ribosomal Peptide Biosynthesis Involving Two Separate Pathway Crosstalk
4.1. The Biosynthesis of Spirotryprostatin A
4.2. The Biosynthesis of Echinocandin B
5. Representative Fungal NPs Might Be Biosynthesized by Two Separate Pathways Crosstalk
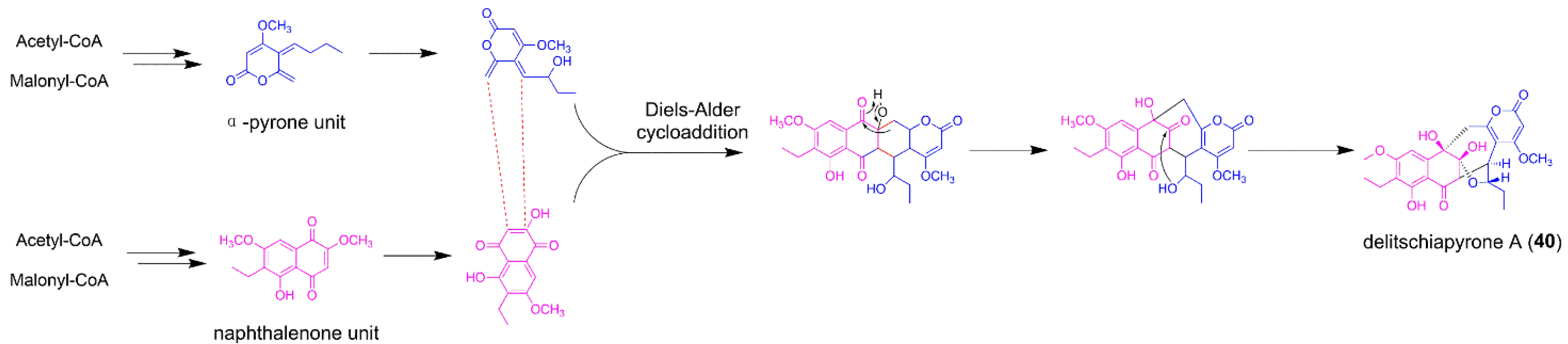
5.1. Delitschiapyrone A
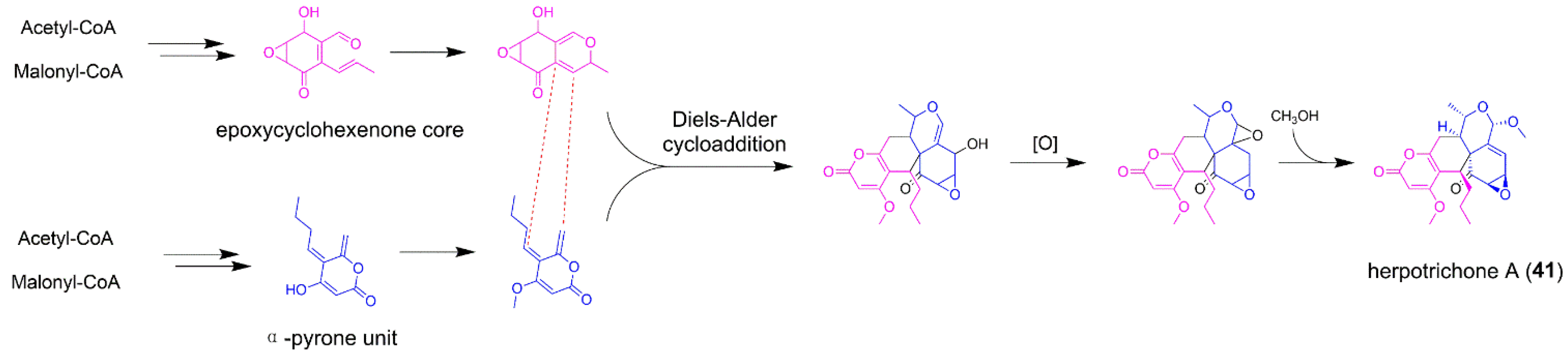
5.2. Herpotrichone A
5.3. Citrifuran A
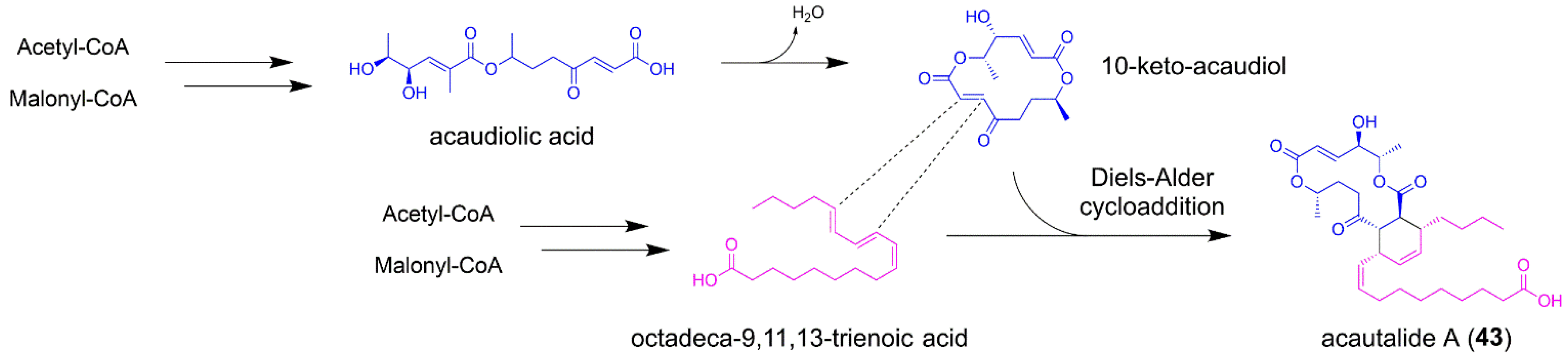
5.4. Acautalide A
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NP | natural product |
| BGC | biosynthetic gene cluster |
| PKS | polyketide synthase |
| NR-PKS | non-reducing polyketide synthase |
| HR-PKS | highly reducing polyketide synthase |
| NRPS | non-ribosomal peptide synthetases |
| RiPP | post-translationally modified peptide |
| FMO | FAD-dependent monooxygenase |
| ER | enoyl reductase |
| AHA | alkyl 5-hydroxylanthranilate |
| IPMS | isopropyl-malate synthase |
| DMOA | 3,5-dimethylorsellinic acid |
| LPS | lipopolysaccharide |
| PBEO | 1-(2,6-dihydroxyphenyl)but-2-en-1-one |
| DFT | density functional theory |
References
- González-Medina, M.; Owen, J.R.; El-Elimat, T.; Pearce, C.J.; Oberlies, N.H.; Figueroa, M.; Medina-Franco, J.L. Scaffold diversity of fungal metabolites. Front. Pharmacol. 2017, 8, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.M.; Li, J.W.; Choi, J.W.; Zhou, H.; Lee, K.K.; Moorthie, V.A.; Xie, X.; Kealey, J.T.; Da Silva, N.A.; Vederas, J.C.; et al. Complete reconstitution of a highly reducing iterative polyketide synthase. Science 2009, 326, 589–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Du, L.; Qu, Z.; Zhang, X.; Li, F.; Li, Z.; Qi, F.; Wang, X.; Jiang, Y.; Men, P.; et al. Compartmentalized biosynthesis of mycophenolic acid. Proc. Natl. Acad. Sci. USA 2019, 116, 13305–13310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, T.; Tokunaga, K.; Matsuda, Y.; Fujii, I.; Abe, I.; Ebizuka, Y.; Kushiro, T. Reconstitution of a fungal meroterpenoid biosynthesis reveals the involvement of a novel family of terpene cyclases. Nat. Chem. 2010, 2, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Karageorgis, G.; Foley, D.J.; Laraia, L.; Waldmann, H. Principle and design of pseudo-natural products. Nat. Chem. 2020, 12, 227–235. [Google Scholar] [CrossRef]
- Osbourn, A. Secondary metabolic gene clusters: Evolutionary toolkits for chemical innovation. Trends Genet. 2010, 26, 449–457. [Google Scholar] [CrossRef]
- Smanski, M.J.; Zhou, H.; Claesen, J.; Shen, B.; Fischbach, M.A.; Voigt, C.A. Synthetic biology to access and expand nature’s chemical diversity. Nat. Rev. Microbiol. 2016, 14, 135–149. [Google Scholar] [CrossRef] [Green Version]
- van Santen, J.A.; Kautsar, S.A.; Medema, M.H.; Linington, R.G. Microbial natural product databases: Moving forward in the multi-omics era. Nat. Prod. Rep. 2021, 38, 264–278. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Li, Y.; Zhuo, L.; Li, X.; Zhu, Y.; Wu, S.; Shen, T.; Hu, W.; Li, Y.Z.; Wu, C. Myxadazoles, Myxobacterium-derived isoxazole-benzimidazole hybrids with cardiovascular activities. Angew. Chem. Int. Ed. Engl. 2021, 60, 21679–21684. [Google Scholar] [CrossRef]
- Ma, G.L.; Candra, H.; Pang, L.M.; Xiong, J.; Ding, Y.; Tran, H.T.; Low, Z.J.; Ye, H.; Liu, M.; Zheng, J.; et al. Biosynthesis of tasikamides via pathway coupling and diazonium-mediated hydrazone formation. J. Am. Chem. Soc. 2022, 144, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.W.; Ma, H.Y.; Zhu, T.J.; Li, J.; Gu, Q.Q.; Li, D.H. Penilactones A and B, two novel polyketides from Antarctic deep-sea derived fungus Penicillium crustosum PRB-2. Tetrahedron 2012, 68, 9745–9749. [Google Scholar] [CrossRef]
- Spence, J.T.; George, J.H. Biomimetic total synthesis of ent-penilactone A and penilactone B. Org. Lett. 2013, 15, 3891–3893. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liao, G.; Kindinger, F.; Ludwig-Radtke, L.; Yin, W.B.; Li, S.M. Peniphenone and penilactone formation in Penicillium crustosum via 1,4-Michael additions of ortho-quinone methide from hydroxyclavatol to γ-butyrolactones from crustosic acid. J. Am. Chem. Soc. 2019, 141, 4225–4229. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liao, G.; Ludwig-Radtke, L.; Yin, W.B.; Li, S.M. Formation of terrestric acid in Penicillium crustosum requires redox-assisted decarboxylation and stereoisomerization. Org. Lett. 2020, 22, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zhang, J.; Jiang, N.; Lu, Y.H.; Wang, L.; Xu, S.H.; Wang, W.; Zhang, G.F.; Xu, Q.; Ge, H.M.; et al. Immunosuppressive polyketides from mantis-associated Daldinia eschscholzii. J. Am. Chem. Soc. 2011, 133, 5931–5940. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Ge, H.M.; Zhao, W.; Dong, H.; Xu, Q.; Li, S.H.; Li, J.; Zhang, J.; Song, Y.C.; Tan, R.X. Unprecedented immunosuppressive polyketides from Daldinia eschscholzii, a mantis-associated fungus. Angew. Chem. Int. Ed. Engl. 2008, 47, 5823–5826. [Google Scholar] [CrossRef]
- Zhang, A.H.; Tan, R.; Jiang, N.; Yusupu, K.; Wang, G.; Wang, X.L.; Tan, R.X. Selesconol, a fungal polyketide that induces stem cell differentiation. Org. Lett. 2016, 18, 5488–5491. [Google Scholar] [CrossRef]
- Zhou, Z.Z.; Zhu, H.J.; Lin, L.P.; Zhang, X.; Ge, H.M.; Jiao, R.H.; Tan, R.X. Dalmanol biosyntheses require coupling of two separate polyketide gene clusters. Chem. Sci. 2018, 10, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. Engl. 2009, 48, 4688–4716. [Google Scholar] [CrossRef]
- Abe, I.; Oguro, S.; Utsumi, Y.; Sano, Y.; Noguchi, H. Engineered biosynthesis of plant polyketides: Chain length control in an octaketide-producing plant type III polyketide synthase. J. Am. Chem. Soc. 2005, 127, 12709–12716. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Ji, S.; Jiang, N.; Wang, W.; Zhao, G.Y.; Zhang, S.; Ge, H.M.; Xu, Q.; Zhang, A.H.; Zhang, Y.L.; et al. Naphthol radical couplings determine structural features and enantiomeric excess of dalesconols in Daldinia eschscholzii. Nat. Commun. 2012, 3, 1039. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.I.; Wang, L.; Crump, M.P.; Willis, C.L. Core steps to the azaphilone family of fungal natural products. ChemBioChem 2021, 22, 3027–3036. [Google Scholar]
- Huang, X.; Zhang, W.; Tang, S.; Wei, S.; Lu, X. Collaborative biosynthesis of a class of bioactive azaphilones by two separate gene clusters containing four PKS/NRPSs with transcriptional crosstalk in fungi. Angew. Chem. Int. Ed. Engl. 2020, 59, 4349–4353. [Google Scholar] [CrossRef]
- Wang, M.; Beissner, M.; Zhao, H. Aryl-aldehyde formation in fungal polyketides: Discovery and characterization of a distinct biosynthetic mechanism. Chem. Biol. 2014, 21, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, Y.M.; Szewczyk, E.; Davidson, A.D.; Keller, N.; Oakley, B.R.; Wang, C.C. A gene cluster containing two fungal polyketide synthases encodes the biosynthetic pathway for a polyketide, asperfuranone, in Aspergillus nidulans. J. Am. Chem. Soc. 2009, 131, 2965–2970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, Y.M.; Oakley, C.E.; Ahuja, M.; Entwistle, R.; Schultz, A.; Chang, S.L.; Sung, C.T.; Wang, C.C.; Oakley, B.R. An efficient system for heterologous expression of secondary metabolite genes in Aspergillus nidulans. J. Am. Chem. Soc. 2013, 135, 7720–7731. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Tang, Y.; Xie, J.; Zhao, Z.; Cui, H. Meroterpenoids produced by fungi: Occurrence, structural diversity, biological activities, and their molecular targets. Eur. J. Med. Chem. 2021, 209, 112860. [Google Scholar] [CrossRef]
- Moodie, L.W.K.; Sepčić, K.; Turk, T.; FrangeŽ, R.; Svenson, J. Natural cholinesterase inhibitors from marine organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef]
- Omura, S.; Tomoda, H.; Kim, Y.K.; Nishida, H. Pyripyropenes, highly potent inhibitors of acyl-CoA:cholesterol acyltransferase produced by Aspergillus fumigatus. J. Antibiot. 1993, 46, 1168–1169. [Google Scholar] [CrossRef] [Green Version]
- Barra, L.; Abe, I. Chemistry of fungal meroterpenoid cyclases. Nat. Prod. Rep. 2021, 38, 566–585. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, E.; Chiang, Y.M.; Oakley, C.E.; Davidson, A.D.; Wang, C.C.; Oakley, B.R. Identification and characterization of the asperthecin gene cluster of Aspergillus nidulans. Appl. Environ. Microbiol. 2008, 74, 7607–7612. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.L.; Nielsen, J.B.; Rank, C.; Klejnstrup, M.L.; Holm, D.K.; Brogaard, K.H.; Hansen, B.G.; Frisvad, J.C.; Larsen, T.O.; Mortensen, U.H. A genome-wide polyketide synthase deletion library uncovers novel genetic links to polyketides and meroterpenoids in Aspergillus nidulans. FEMS Microbiol. Lett. 2011, 321, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.C.; Entwistle, R.; Guo, C.J.; Ahuja, M.; Szewczyk, E.; Hung, J.H.; Chiang, Y.M.; Oakley, B.R.; Wang, C.C. Two separate gene clusters encode the biosynthetic pathway for the meroterpenoids austinol and dehydroaustinol in Aspergillus nidulans. J. Am. Chem. Soc. 2012, 134, 4709–4720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, Y.; Awakawa, T.; Wakimoto, T.; Abe, I. Spiro-ring formation is catalyzed by a multifunctional dioxygenase in austinol biosynthesis. J. Am. Chem. Soc. 2013, 135, 10962–10965. [Google Scholar] [CrossRef]
- Matsuda, Y.; Wakimoto, T.; Mori, T.; Awakawa, T.; Abe, I. Complete biosynthetic pathway of anditomin: Nature’s sophisticated synthetic route to a complex fungal meroterpenoid. J. Am. Chem. Soc. 2014, 136, 15326–15336. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.T.; Xu, Q.L.; Xiong, Y.; Zhang, L.; Han, H.; Xu, K.; Guo, W.J.; Xu, Q.; Tan, R.X.; et al. Genome mining and comparative biosynthesis of meroterpenoids from two phylogenetically distinct fungi. Angew. Chem. Int. Ed. Engl. 2018, 57, 8184–8188. [Google Scholar] [CrossRef]
- Matsuda, Y.; Quan, Z.; Mitsuhashi, T.; Li, C.; Abe, I. Cytochrome P450 for citreohybridonol synthesis: Oxidative derivatization of the andrastin scaffold. Org. Lett. 2016, 18, 296–299. [Google Scholar] [CrossRef]
- Xu, P.W.; Yu, J.S.; Chen, C.; Cao, Z.Y.; Zhou, F.; Zhou, J. Catalytic enantioselective construction of spiro quaternary carbon stereocenters. ACS Catalysis 2019, 9, 1820–1882. [Google Scholar] [CrossRef]
- Zi, W.; Zuo, Z.; Ma, D. Intramolecular dearomative oxidative coupling of indoles: A unified strategy for the total synthesis of indoline alkaloids. Acc. Chem. Res. 2015, 48, 702–711. [Google Scholar] [CrossRef]
- Cui, C.B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 1996, 52, 12651–12666. [Google Scholar] [CrossRef]
- Tsunematsu, Y.; Ishikawa, N.; Wakana, D.; Goda, Y.; Noguchi, H.; Moriya, H.; Hotta, K.; Watanabe, K. Distinct mechanisms for spiro-carbon formation reveal biosynthetic pathway crosstalk. Nat. Chem. Biol. 2013, 9, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.B.; Baccile, J.A.; Bok, J.W.; Chen, Y.; Keller, N.P.; Schroeder, F.C. A nonribosomal peptide synthetase-derived iron(III) complex from the pathogenic fungus Aspergillus fumigatus. J. Am. Chem. Soc. 2013, 135, 2064–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, D.; Chen, Q.; Gao, J.; Bai, J.; Liu, B.; Zhang, Y.; Zhang, L.; Zhang, C.; Zou, Y.; Hu, Y. Complexity and diversity generation in the biosynthesis of fumiquinazoline-related peptidyl alkaloids. Org. Lett. 2019, 21, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chooi, Y.H.; Ames, B.D.; Wang, P.; Walsh, C.T.; Tang, Y. Fungal indole alkaloid biosynthesis: Genetic and biochemical investigation of the tryptoquialanine pathway in Penicillium aethiopicum. J. Am. Chem. Soc. 2011, 133, 2729–2741. [Google Scholar] [CrossRef] [Green Version]
- Haynes, S.W.; Ames, B.D.; Gao, X.; Tang, Y.; Walsh, C.T. Unraveling terminal C-domain-mediated condensation in fungal biosynthesis of imidazoindolone metabolites. Biochemistry 2011, 50, 5668–5679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Finefield, J.M.; Sunderhaus, J.D.; McAfoos, T.J.; Williams, R.M.; Sherman, D.H. Biochemical characterization of NotB as an FAD-dependent oxidase in the biosynthesis of notoamide indole alkaloids. J. Am. Chem. Soc. 2012, 134, 788–791. [Google Scholar] [CrossRef] [Green Version]
- Cacho, R.A.; Jiang, W.; Chooi, Y.H.; Walsh, C.T.; Tang, Y. Identification and characterization of the echinocandin B biosynthetic gene cluster from Emericella rugulosa NRRL 11440. J. Am. Chem. Soc. 2012, 134, 16781–16790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L.; Diao, X.; Zhang, N.; Li, F.; Zhou, H.; Chen, H.; Bai, X.; Ren, X.; Zhang, Y.; Wu, D.; et al. Engineering and elucidation of the lipoinitiation process in nonribosomal peptide biosynthesis. Nat. Commun. 2021, 12, 296. [Google Scholar] [CrossRef]
- Kegler, C.; Bode, H.B. Artificial splitting of a non-ribosomal peptide synthetase by inserting natural docking domains. Angew. Chem. Int. Ed. Engl. 2020, 59, 13463–13467. [Google Scholar] [CrossRef]
- Bozhueyuek, K.A.J.; Watzel, J.; Abbood, N.; Bode, H.B. Synthetic zippers as an enabling tool for engineering of non-ribosomal peptide synthetases. Angew. Chem. Int. Ed. Engl. 2021, 60, 17531–17538. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.G.; Wang, X.B.; Xu, Y.M.; U’Ren, J.M.; Arnold, A.E.; Kong, L.Y.; Gunatilaka, A.A. Delitschiapyrone A, a pyrone-naphthalenone adduct bearing a new pentacyclic ring system from the leaf-associated fungus Delitschia sp. FL1581. Org. Lett. 2014, 16, 5944–5947. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Houk, K.N. Mechanisms and dynamics of synthetic and biosynthetic formation of delitschiapyrones: Solvent control of ambimodal periselectivity. J. Am. Chem. Soc. 2021, 143, 11734–11740. [Google Scholar] [CrossRef] [PubMed]
- Han, W.B.; Wang, G.Y.; Tang, J.J.; Wang, W.J.; Liu, H.; Gil, R.R.; Navarro-Vázquez, A.; Lei, X.; Gao, J.M. Herpotrichones A and B, two intermolecular [4 + 2] adducts with anti-neuroinflammatory activity from a Herpotrichia species. Org. Lett. 2020, 22, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ying, Y.; Hung, Y.S.; Tang, Y. Genome mining reveals Neurospora crassa can produce the salicylaldehyde sordarial. J. Nat. Prod. 2019, 82, 1029–1033. [Google Scholar] [CrossRef]
- Nies, J.; Ran, H.; Wohlgemuth, V.; Yin, W.B.; Li, S.M. Biosynthesis of the prenylated salicylaldehyde flavoglaucin requires temporary reduction to salicyl alcohol for decoration before reoxidation to the final product. Org. Lett. 2020, 22, 2256–2260. [Google Scholar] [CrossRef]
- Liu, L.; Tang, M.C.; Tang, Y. Fungal highly reducing polyketide synthases biosynthesize salicylaldehydes that are precursors to epoxycyclohexenol natural products. J. Am. Chem. Soc. 2019, 141, 19538–19541. [Google Scholar] [CrossRef]
- Lin, T.S.; Chiang, Y.M.; Wang, C.C. Biosynthetic pathway of the reduced polyketide product citreoviridin in Aspergillus terreus var. aureus revealed by heterologous expression in Aspergillus nidulans. Org. Lett. 2016, 18, 1366–1369. [Google Scholar] [CrossRef]
- Li, H.; Hu, J.; Wei, H.; Solomon, P.S.; Vuong, D.; Lacey, E.; Stubbs, K.A.; Piggott, A.M.; Chooi, Y.H. Chemical ecogenomics-guided discovery of phytotoxic α-pyrones from the fungal wheat pathogen Parastagonospora nodorum. Org. Lett. 2018, 20, 6148–6152. [Google Scholar] [CrossRef]
- Yin, G.P.; Wu, Y.R.; Yang, M.H.; Li, T.X.; Wang, X.B.; Zhou, M.M.; Lei, J.L.; Kong, L.Y. Citrifurans A-D, four dimeric aromatic polyketides with new carbon skeletons from the fungus Aspergillus sp. Org. Lett. 2017, 19, 4058–4061. [Google Scholar] [CrossRef]
- Wang, W.G.; Wang, H.; Du, L.Q.; Li, M.; Chen, L.; Yu, J.; Cheng, G.G.; Zhan, M.T.; Hu, Q.F.; Zhang, L.; et al. Molecular Basis for the Biosynthesis of an Unusual Chain-Fused Polyketide, Gregatin A. J. Am. Chem. Soc. 2020, 142, 8464–8472. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cox, R.J. The molecular steps of citrinin biosynthesis in fungi. Chem. Sci. 2016, 7, 2119–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Z.W.; Xie, X.H.; Wang, T.T.; Lu, M.; Jiao, R.H.; Ge, H.M.; Hu, G.; Tan, R.X. Acautalides A-C, neuroprotective Diels-Alder adducts from solid-state cultivated Acaulium sp. H-JQSF. Org. Lett. 2021, 23, 5587–5591. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Wei, Y.J.; Ge, H.M.; Jiao, R.H.; Tan, R.X. Acaulide, an osteogenic macrodiolide from Acaulium sp. H-JQSF, an isopod-associated fungus. Org. Lett. 2018, 20, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Wei, Y.J.; Ge, H.M.; Jiao, R.H.; Tan, R.X. Acaulins A and B, trimeric macrodiolides from Acaulium sp. H-JQSF. Org. Lett. 2018, 20, 2490–2493. [Google Scholar] [CrossRef]
- Jensen, P.R. Natural products and the gene cluster revolution. Trends Microbiol. 2016, 24, 968–977. [Google Scholar] [CrossRef] [Green Version]
- Pradeepraj, D.; Li, S.Y. Functional expression and regulation of eukaryotic cytochrome P450 enzymes in surrogate microbial cell factories. Eng. Microbiol. 2022, 2, 100011. [Google Scholar] [CrossRef]
- Kahlert, L.; Bassiony, E.F.; Cox, R.J.; Skellam, E.J. Diels-Alder reactions during the biosynthesis of sorbicillinoids. Angew. Chem. Int. Ed. Engl. 2020, 59, 5816–5822. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.B.; Bai, W.; Ding, C.X.; Liang, J.; Wu, S.H.; Tan, R.X. Intertwined biosynthesis of skyrin and rugulosin A underlies the formation of cage-structured bisanthraquinones. J. Am. Chem. Soc. 2021, 143, 14218–14226. [Google Scholar] [CrossRef]
- Hu, J.; Li, H.; Chooi, Y.H. Fungal dirigent protein controls the stereoselectivity of multicopper oxidase-catalyzed phenol coupling in viriditoxin biosynthesis. J. Am. Chem. Soc. 2019, 141, 8068–8072. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, G.; Shen, Q.; Zhang, Y.; Bian, X. Biosynthesis of Fungal Natural Products Involving Two Separate Pathway Crosstalk. J. Fungi 2022, 8, 320. https://doi.org/10.3390/jof8030320
Dai G, Shen Q, Zhang Y, Bian X. Biosynthesis of Fungal Natural Products Involving Two Separate Pathway Crosstalk. Journal of Fungi. 2022; 8(3):320. https://doi.org/10.3390/jof8030320
Chicago/Turabian StyleDai, Guangzhi, Qiyao Shen, Youming Zhang, and Xiaoying Bian. 2022. "Biosynthesis of Fungal Natural Products Involving Two Separate Pathway Crosstalk" Journal of Fungi 8, no. 3: 320. https://doi.org/10.3390/jof8030320
APA StyleDai, G., Shen, Q., Zhang, Y., & Bian, X. (2022). Biosynthesis of Fungal Natural Products Involving Two Separate Pathway Crosstalk. Journal of Fungi, 8(3), 320. https://doi.org/10.3390/jof8030320





