The Effects of Nitrogen and Phosphorus on Colony Growth and Zoospore Characteristics of Soil Chytridiomycota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection and Maintenance of Isolates
2.2. Treatment Media for Experimental Testing
2.3. Soil Extract Medium
2.4. Dry Weight Biomass
2.5. Protein Quantification
2.6. Chytrid Zoospore Quantification
2.7. Microscopic Analysis of Zoospore Motility and Lipid Quantification
2.8. Attachment to Substrates
2.9. Statistical Analysis
3. Results
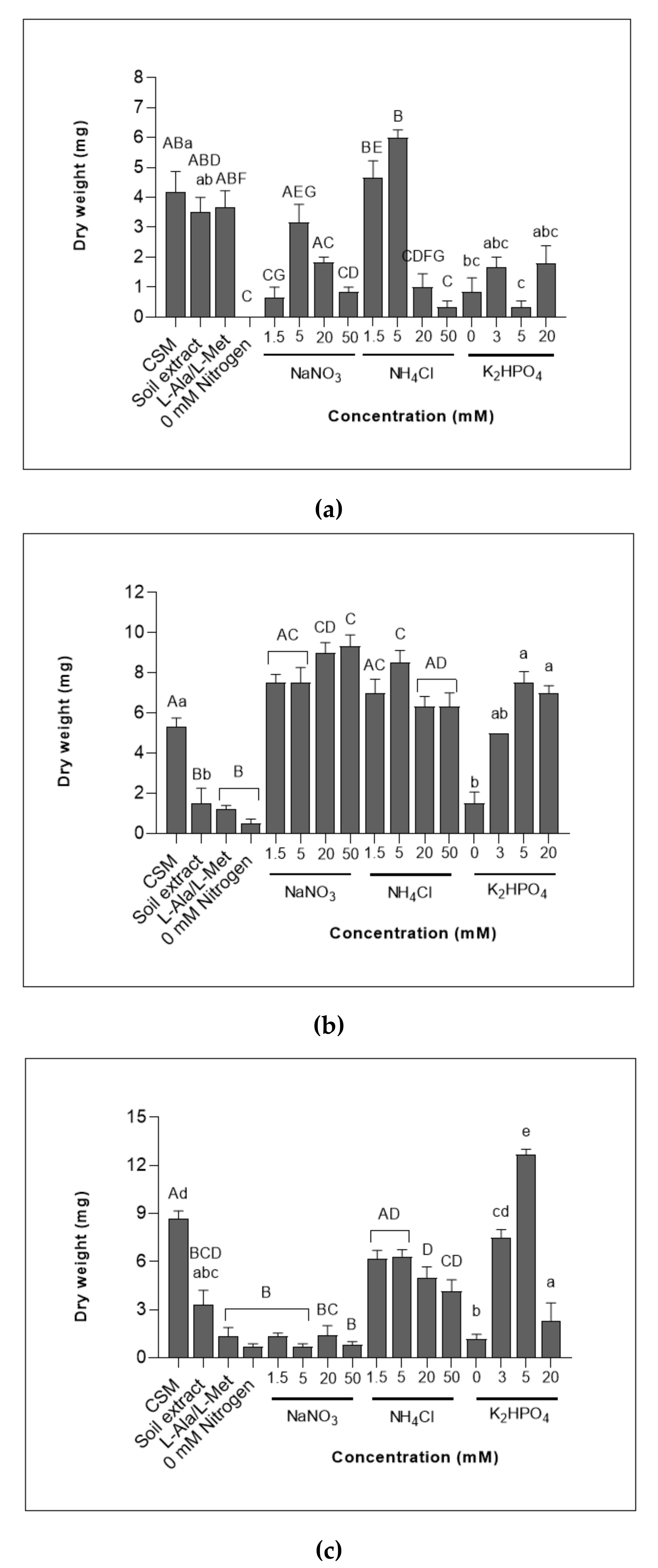
3.1. Chytrid Biomass Measured as Dry Weight (mg)
3.1.1. Cell Yields Varied between Chytrid Species When Exposed to Different Sources and Concentrations of Nitrogen
3.1.2. All Chytrids Increased in Dry Weight When Grown in Various Concentrations of Phosphate
3.2. Total Protein (µg mL−1 BSA Equivalents) Is a Reliable Method for the Measurement of Chytrid Biomass
3.2.1. Chytrids Showed Diverse Abilities to Grow in the Presence of Different Nitrogen Treatments as Measured by Total Protein
3.2.2. The Maximum Yield of Total Protein Varied between Chytrids Depending on Phosphorus Concentrations
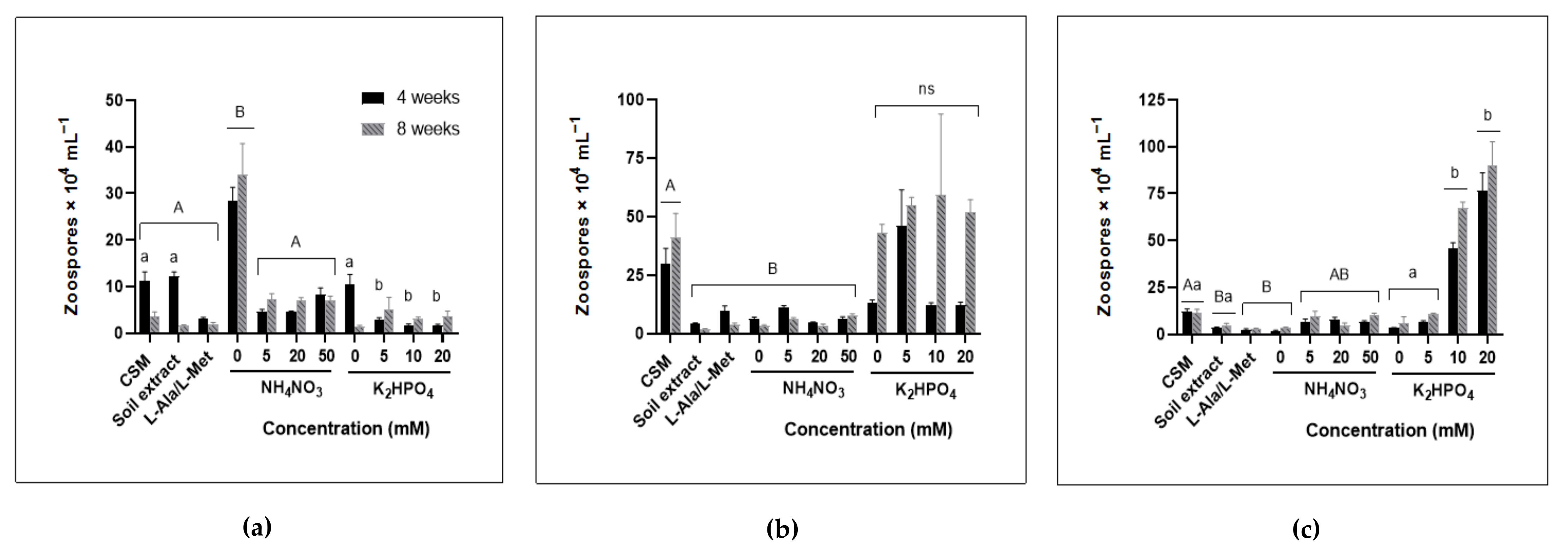
3.3. Zoospore Production Increased under Nutrient Deprivation for Some Chytrids Whilst Others Benefited from Increased Nutrient Availability
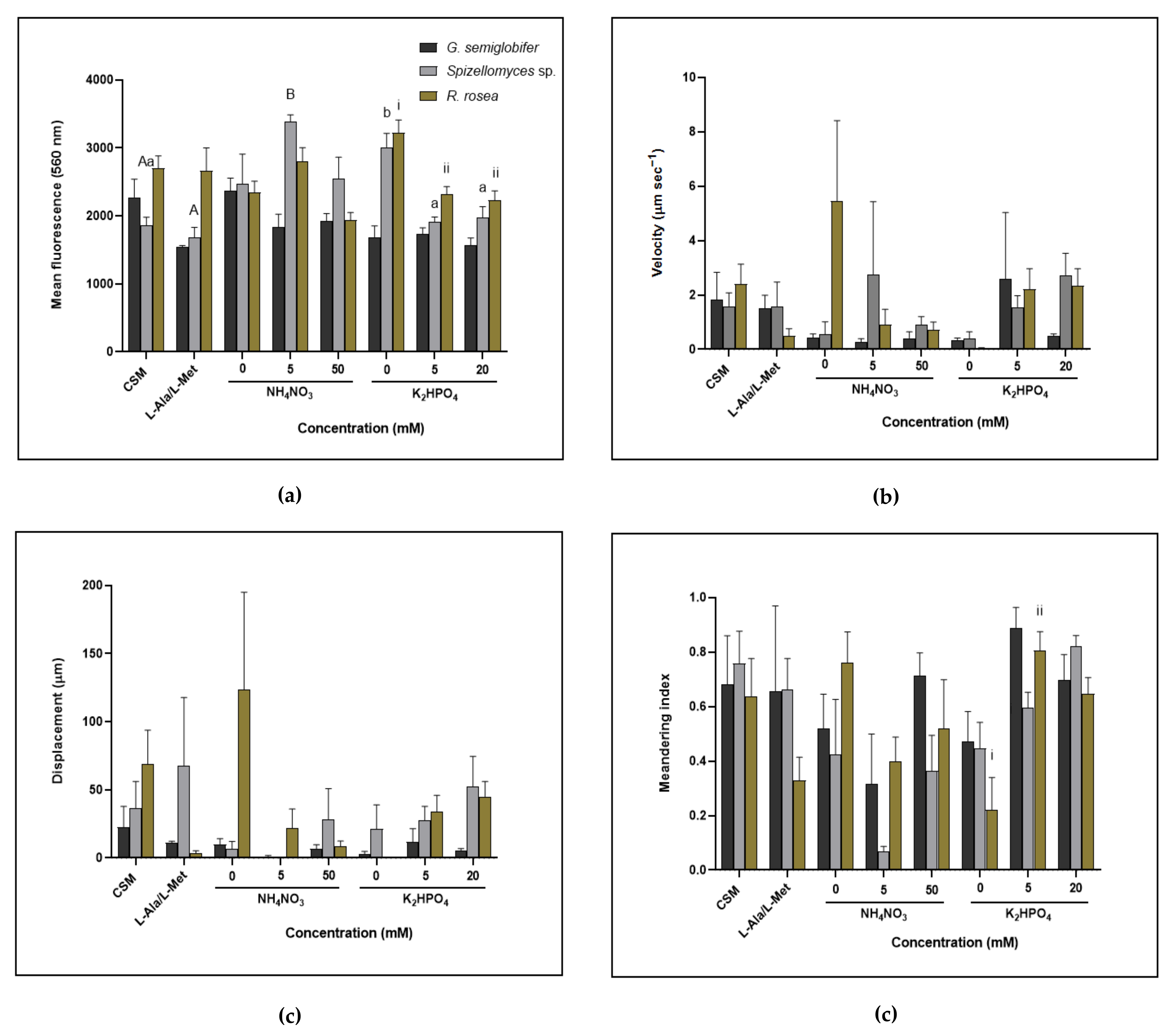
3.4. Zoospore Motility and Lipid Production Differed between Chytrids Depending on Nutrient Source and Concentration
3.5. Attachment to an Organic Substrate Changed Significantly in Response to Different Nitrogen Sources
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hicks, L.C.; Meir, P.; Nottingham, A.T.; Reay, D.S.; Stott, A.W.; Salinas, N.; Whitaker, J. Carbon and nitrogen inputs differentially affect priming of soil organic matter in tropical lowland and montane soils. Soil Biol. Biochem. 2019, 129, 212–222. [Google Scholar]
- Paungfoo-Lonhienne, C.; Yeoh, Y.K.; Kasinadhuni, N.R.; Lonhienne, T.G.; Robinson, N.; Hugenholtz, P.; Ragan, M.A.; Schmidt, S. Nitrogen fertilizer dose alters fungal communities in sugarcane soil and rhizosphere. Sci. Rep. 2015, 5, 8678. [Google Scholar] [PubMed] [Green Version]
- Digby, A.L.; Gleason, F.H.; McGee, P.A. Some fungi in the Chytridiomycota can assimilate both inorganic and organic sources of nitrogen. Fungal Ecol. 2010, 3, 261–266. [Google Scholar]
- Midgley, D.J.; Letcher, P.M.; McGee, P.A. Access to organic and insoluble sources of phosphorus varies among soil Chytridiomycota. Arch Microbiol. 2006, 186, 211–217. [Google Scholar]
- Powell, M.J. Looking at mycology with a Janus face: A glimpse at Chytridiomycetes active in the environment. Mycologia 1993, 83, 1–20. [Google Scholar]
- Gleason, F.H.; Kagami, M.; Lefevre, E.; Sime-Ngando, T. The ecology of chytrids in aquatic ecosystems: Roles in food web dynamics. Fungal Biol. Rev. 2008, 22, 17–25. [Google Scholar]
- Commandeur, Z.; Letcher, P.M.; McGee, P.A. Diversity of Chytridiaceous fungi in a cropping soil. Australas. Mycol. 2005, 24, 1–6. [Google Scholar]
- Gleason, F.H.; Letcher, P.M.; McGee, P.A. Some Chytridiomycota in soil recover from drying and high temperatures. Mycol. Res. 2004, 108, 583–589. [Google Scholar]
- Letcher, P.M.; McGee, P.A.; Powell, M.J. Distribution and diversity of zoosporic fungi from soils of four vegetation types in New South Wales, Australia. Can. J. Bot. 2004, 82, 1490–1500. [Google Scholar]
- Nikolcheva, L.G.; Bärlocher, F. Taxon-specific fungal primers reveal unexpectedly high diversity during leaf decomposition in a stream. Mycol. Prog. 2004, 3, 41–49. [Google Scholar]
- Zhao, B.; Xing, P.; Wu, Q.L. Microbes participated in macrophyte leaf litters decomposition in freshwater habitat. FEMS Microbiol. Ecol. 2017, 93, fix108. [Google Scholar]
- Kagami, M.; Motoki, Y.; Masclaux, H.; Bec, A. Carbon and nutrients of indigestible pollen are transferred to zooplankton by chytrid fungi. Freshw. Biol. 2017, 62, 954–964. [Google Scholar]
- Powell, M.J. Chytridiomycota. In Handbook of the Protists, 2nd ed.; Archibald, J., Simpson, A., Slamovits, C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1523–1558. [Google Scholar]
- Song, N.; Xu, H.; Yan, Z.; Yang, T.; Wang, C.; Jiang, H.-L. Improved lignin degradation through distinct microbial community in subsurface sediments of one eutrophic lake. Renew. Energy 2019, 138, 861–869. [Google Scholar]
- Haskins, R.H. Cellulose as a substratum for saprophytic chytrids. Am. J. Bot. 1939, 26, 635–639. [Google Scholar]
- Pessier, A.P.; Nichols, D.K.; Longcore, J.E.; Fuller, M.S. Cutaneous Chytridiomycosis in Poison Dart Frogs (Dendrobates spp.) and White’s Tree Frogs (Litoria Caerulea). J. Vet. Diagn. Investig. 1999, 11, 194–199. [Google Scholar]
- Henderson, L.E. The Effect of Metals on Growth, Reproduction and Attachment of Zoosporic True Fungi. Ph.D. Thesis, University of Sydney, Camperdown, NSW, Australia, 2018. [Google Scholar]
- Letcher, P.M.; Powell, M.J.; Barr, D.J.; Churchill, P.F.; Wakefield, W.S.; Picard, K.T. Rhizophlyctidales—A new order in Chytridiomycota. Mycol. Res. 2008, 112, 1031–1048. [Google Scholar]
- Powell, M.J. Ultrastructure and isolation of glyoxysomes (microbodies) in zoospores of the fungus Entophlyctis sp. Protoplasma 1976, 89, 1–27. [Google Scholar]
- Frenken, T.; Wierenga, J.; Gsell, A.S.; van Donk, E.; Rohrlack, T.; Van de Waal, D.B. Changes in N:P Supply Ratios Affect the Ecological Stoichiometry of a Toxic Cyanobacterium and Its Fungal Parasite. Front Microbiol 2017, 8, 1015. [Google Scholar]
- Canter, H.M.; Lund, J.W.G. Studies on plankton parasites III. Examples of the interaction between parasitism and other factors determining the growth of diatoms. Ann. Bot. 1951, 15, 359–371. [Google Scholar]
- Ibelings, B.W.; De Bruin, A.; Kagami, M.; Rijkeboer, M.; Brehm, M.; Donk, E.V. Host parasite interactions between freshwater phytoplankton and chytrid fungi (Chytridiomycota). J. Phycol. 2004, 40, 437–453. [Google Scholar]
- Kagami, M.; Van Donk, E.; De Bruin, A.; Rijkeboer, M.; Ibelings, B.W. Daphnia can protect diatoms from fungal parasitism. Limnol. Oceanogr. 2004, 49, 680–685. [Google Scholar]
- Kagami, M.; Elert, E.; Ibelings, B.; de Bruin, A.; Donk, E. The parasitic chytrid, Zygorhizidium, facilitates the growth of the cladoceran zooplankter, Daphnia, in cultures of the inedible alga, Asterionella. Proc. Biol. Sci./R. Soc. 2007, 274, 1561–1566. [Google Scholar]
- Gleason, F.H.; Crawford, J.W.; Neuhauser, S.; Henderson, L.E.; Lilje, O. Resource seeking strategies of zoosporic true fungi in heterogeneous soil habitats at the microscale level. Soil Biol. Biochem. 2012, 45, 79–88. [Google Scholar] [PubMed] [Green Version]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [PubMed] [Green Version]
- Hernandez, M.M.; Menéndez, C.M. Influence of seasonality and management practices on diversity and composition of fungal communities in vineyard soils. Appl. Soil Ecol. 2019, 135, 113–119. [Google Scholar]
- Song, G.; Chen, R.; Xiang, W.; Yang, F.; Zheng, S.; Zhang, J.; Lin, X. Contrasting effects of long-term fertilization on the community of saprotrophic fungi and arbuscular mycorrhizal fungi in a sandy loam soil. Plant Soil Environ. 2015, 61, 127–136. [Google Scholar]
- Lin, X.; Feng, Y.; Zhang, H.; Chen, R.; Wang, J.; Zhang, J.; Chu, H. Long-Term Balanced Fertilization Decreases Arbuscular Mycorrhizal Fungal Diversity in an Arable Soil in North China Revealed by 454 Pyrosequencing. Environ. Sci. Technol. 2012, 46, 5764–5771. [Google Scholar]
- Jiao, S.; Wang, J.; Wei, G.; Chen, W.; Lu, Y. Dominant role of abundant rather than rare bacterial taxa in maintaining agro-soil microbiomes under environmental disturbances. Chemosphere 2019, 235, 248–259. [Google Scholar]
- Freeman, K.R.; Martin, A.P.; Karki, D.; Lynch, R.C.; Mitter, M.S.; Meyer, A.F.; Longcore, J.E.; Simmons, D.R.; Schmidt, S.K. Evidence That Chytrids Dominate Fungal Communities in High-Elevation Soils. Proc. Natl. Acad. Sci. USA 2009, 106, 18315–18320. [Google Scholar]
- Smit, E.; Leeflang, P.; Glandorf, B.; van Elsas, J.D.; Wernars, K. Analysis of fungal diversity in the wheat rhizosphere by sequencing of cloned PCR-amplified genes encoding 18S rRNA and temperature gradient gel electrophoresis. Appl. Environ. Microbiol. 1999, 65, 2614–2621. [Google Scholar]
- Lange, L.; Pilgaard, B.; Herbst, F.-A.; Busk, P.K.; Gleason, F.; Pedersen, A.G. Origin of fungal biomass degrading enzymes: Evolution, diversity and function of enzymes of early lineage fungi. Fungal Biol. Rev. 2019, 33, 82–97. [Google Scholar]
- Pugh, G.J.F.; Boddy, L. A view of disturbance and life strategies in fungi. Proc. R. Soc. Edinburgh. Sect. B. Biol. Sci. 1988, 94, 3–11. [Google Scholar]
- DSMZ-German Collection of Microorganisms and Cell Cultures, Soil Extract Medium. 2007. Available online: https://www.dsmz.de/microorganisms/medium/pdf/DSMZ_Medium12.pdf (accessed on 23 March 2022).
- Kennedy, S.I.; Fewson, C.A. Enzymes of the mandelate pathway in Bacterium N.C.I.B. 8250. Biochem. J. 1968, 107, 497–506. [Google Scholar] [PubMed]
- Strittmatter, M.; Guerra, T.; Silva, J.; Gachon, C.M.M. A new flagellated dispersion stage in Paraphysoderma sedebokerense, a pathogen of Haematococcus pluvialis. J. Appl. Phycol. 2016, 28, 1553–1558. [Google Scholar] [PubMed] [Green Version]
- Nittayajarn, A.; Baker, D.D. Methods for the quantification of Frankia cell biomass. Plant Soil 1989, 118, 199–204. [Google Scholar]
- Ruzicka, S.; Edgerton, D.; Norman, M.; Hill, T. The utility of ergosterol as a bioindicator of fungi in temperate soils. Soil Biol. Biochem. 2000, 32, 989–1005. [Google Scholar]
- Weete, J.D.; Laseter, J.L. Distribution of sterols in the fungi I. Fungal spores. Lipids 1974, 9, 575–581. [Google Scholar]
- Lin, C.; Wang, Y.; Liu, M.; Li, Q.; Xiao, W.; Song, X. Effects of nitrogen deposition and phosphorus addition on arbuscular mycorrhizal fungi of Chinese fir (Cunninghamia lanceolata). Sci. Rep. 2020, 10, 12260. [Google Scholar]
- McGee, P.A.; Daynes, C.N.; Gleason, F.H.; Marano, A.V.; Barrera, M.D.; Steciow, M.M. Rhizophlyctis rosea (Rhizophlyctidales, Chytridiomycota) in soil: Frequency, abundance and density of colonization of lens paper baits. Nova Hedwig. 2011, 93, 73–84. [Google Scholar]
- Trinci, A.J.P.; Collinge, A.J. Spore formation in nitrogen and carbon starved cultures of Geotrichum candidum and Mucor racemosus. Trans. Br. Mycol. Soc. 1974, 62, 351–358. [Google Scholar]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L.E. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 2017, 93, fix006. [Google Scholar]
- Tudzynski, B. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014, 5, 656. [Google Scholar] [PubMed] [Green Version]
- Cove, D.J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Et Biophys. Acta. Enzymol. Biol. Oxid. 1966, 113, 51–56. [Google Scholar]
- Pfannmüller, A.; Boysen, J.M.; Tudzynski, B. Nitrate Assimilation in Fusarium fujikuroi is Controlled by Multiple Levels of Regulation. Front. Microbiol. 2017, 8, 381. [Google Scholar] [PubMed] [Green Version]
- Marzluf, G.A. Genetic regulation of nitrogen metabolism in the fungi. Microbiol. Mol. Biol. Rev. 1997, 61, 17–32. [Google Scholar]
- Wong, K.H.; Hynes, M.J.; Davis, M.A. Recent advances in nitrogen regulation: A comparison between Saccharomyces cerevisiae and filamentous fungi. Eukaryot. Cell 2008, 7, 917–925. [Google Scholar]
- Slot, J.C.; Hibbett, D.S. Horizontal transfer of a nitrate assimilation gene cluster and ecological transitions in fungi: A phylogenetic study. PLoS ONE 2007, 2, e1097. [Google Scholar]
- Slot, J.C.; Hallstrom, K.N.; Matheny, P.B.; Hibbett, D.S. Diversification of NRT2 and the Origin of Its Fungal Homolog. Mol. Biol. Evol. 2007, 24, 1731–1743. [Google Scholar]
- Eldridge, D.J.; Maestre, F.T.; Koen, T.B.; Delgado-Baquerizo, M. Australian dryland soils are acidic and nutrient-depleted, and have unique microbial communities compared with other drylands. J. Biogeogr. 2018, 45, 2803–2814. [Google Scholar]
- Goodwin, T. Fungal carotenoids. Bot. Rev. 1952, 18, 291–316. [Google Scholar]
- Haskins, R.H.; Weston, W.H., Jr. Studies in the lower Chytridiales. I. Factors affecting pigmentation, growth, and metabolism of a strain of Karlingia (Rhizophlyctis) rosea. Am. J. Bot. 1950, 37, 739–750. [Google Scholar]
- Avalos, J.; Carmen Limón, M. Biological roles of fungal carotenoids. Curr. Genet. 2015, 61, 309–324. [Google Scholar] [PubMed]
- Gleason, F.H.; Larkum, A.W.D.; Raven, J.A.; Manohar, C.S.; Lilje, O. Ecological implications of recently discovered and poorly studied sources of energy for the growth of true fungi especially in extreme environments. Fungal Ecol. 2019, 39, 380–387. [Google Scholar]
- Aguilar-Trigueros, C.A.; Hempel, S.; Powell, J.R.; Cornwell, W.K.; Rillig, M.C. Bridging reproductive and microbial ecology: A case study in arbuscular mycorrhizal fungi. ISME J. 2019, 13, 873–884. [Google Scholar] [PubMed]
- Brzezinski, M.A.; Reed, D.C.; Amsler, C.D. Neutral Lipids As Major Storage Products In Zoospores Of The Giant Kelp Macrocystis Pyrifera (Phaeophyceae). J. Phycol. 1993, 29, 16–23. [Google Scholar]
- Steinhoff, F.S.; Graeve, M.; Wiencke, C.; Wulff, A.; Bischof, K. Lipid content and fatty acid consumption in zoospores/developing gametophytes of Saccharina latissima (Laminariales, Phaeophyceae) as potential precursors for secondary metabolites as phlorotannins. Polar Biol. 2011, 34, 1011–1018. [Google Scholar]
- Dellero, Y.; Rose, S.; Metton, C.; Morabito, C.; Lupette, J.; Jouhet, J.; Maréchal, E.; Rébeillé, F.; Amato, A. Ecophysiology and lipid dynamics of a eukaryotic mangrove decomposer: Autecology and ecophysiology of Aurantiochytrium limacinum. Environ. Microbiol. 2018, 20, 3057–3068. [Google Scholar]
- Bruning, K. Effects of phosphorus limitation on the epidemiology of a chytrid phytoplankton parasite. Freshw. Biol. 1991, 25, 409–417. [Google Scholar]
- Iwamoto, Y.; Inoue, K.; Nishiguchi, S.; Matsuura, K.; Aino, M.; Nakayashiki, H.; Ikeda, K. Acidic soil conditions suppress zoospore release from zoosporangia in Olpidium virulentus. J. Gen. Plant Pathol. JGPP 2017, 83, 240–243. [Google Scholar]
- Kagami, M.; Miki, T.; Takimoto, G. Mycoloop: Chytrids in aquatic food webs. Front. Microbiol. 2014, 5, 166. [Google Scholar]
- Rad-Menéndez, C.; Gerphagnon, M.; Garvetto, A.; Arce, P.; Badis, Y.; Sime-Ngando, T.; Gachon, C.M.M. Rediscovering Zygorhizidium affluens Canter: Molecular Taxonomy, Infectious Cycle, and Cryopreservation of a Chytrid Infecting the Bloom-Forming Diatom Asterionella formosa. Appl. Environ. Microbiol. 2018, 84, e01826-18. [Google Scholar]
- Gousterova, A.; Braikova, D.; Goshev, I.; Christov, P. Degradation of keratin and collagen containing wastes by newly isolated thermoactinomycetes or by alkaline hydrolysis. J. Appl. Microbiol. 2005, 40, 335–340. [Google Scholar]
- Gupta, R.; Ramnani, P. Microbial keratinases and their prospective applications: An overview. Appl. Microbiol. Biotechnol. 2006, 70, 21–33. [Google Scholar] [PubMed]
- McKerns, K.W.; Rittersporn, E. The Nutritional Significance of Processed Keratin in Poultry Feeding. Poult. Sci. 1958, 37, 433–436. [Google Scholar]
- Kuhad, R.C.; Singh, A. Lignocellulose biotechnology: Current and future prospects. Crit. Rev. Biotechnol. 1993, 13, 151–172. [Google Scholar]



| Isolate | Order | Accession Number | Collection Site | Bait Substrate | Collector |
|---|---|---|---|---|---|
| Gaertneriomyces semiglobifer Mar C/C2 | Spizellomycetales | FJ827645 FJ827701 FJ827738 | Narrabri— cotton crop (−30°16′47.4″, 149°47′43.2″) | Pine pollen | Commandeur, Z. |
| Spizellomyces sp. Dec CC 4-10Z | Spizellomycetales | AB586075 AB586080 | Narrabri— cotton fallow crop (−30°16′47.4″, 149°47′43.2″) | Pine pollen | Commandeur, Z. |
| Rhizophlyctis rosea AUS 13 | Rhizophlyctidales | EU379156 EU379199 | University of Sydney— garden soil (−33°53′23.8″, 151°11′31.4″) | Filter paper (cellulose) | Letcher, P. |
| CSM | Starvation CSM | Organic Nitrogen (L-Ala/L-Met) | 0 mM Nitrogen | NH4Cl Concentration Series | NaNO3 Concentration Series | 0 mM K2HPO4 | K2HPO4 Concentration Series | |
|---|---|---|---|---|---|---|---|---|
| MgSO4·7H2O (mM) | 1.66 | 1.66 | 1.66 | 1.66 | 1.66 | 1.66 | 1.66 | 1.66 |
| CaCl2 (mM) | 0.033 | 0.033 | 0.033 | 0.033 | 0.033 | 0.033 | 0.033 | 0.033 |
| NH4NO3 (mM) | 2.5 | 0 | 0 | 0 | 0 | 0 | 2.5 | 2.5 |
| NH4Cl (mM) | 0 | 0 | 0 | 0 | 1.5–50 1 | 0 | 0 | 0 |
| NaNO3 (mM) | 0 | 0 | 0 | 0 | 0 | 1.5–50 1 | 0 | 0 |
| D-glucose (mM) | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 |
| L-alanine (mM) | 10.10 | 0 | 10.10 | 0 | 0 | 0 | 10.10 | 10.10 |
| L-methionine (mM) | 0.67 | 0 | 0.67 | 0 | 0 | 0 | 0.67 | 0.67 |
| K2HPO4 (mM) | 3.44 | 0 | 3.44 | 3.44 | 3.44 | 3.44 | 0 | 1.5–20 2 |
| FeEDTA (μM) | 1.36 | 1.36 | 1.36 | 1.36 | 1.36 | 1.36 | 1.36 | 1.36 |
| Trace elements (mL) 3 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| DI H2O 4 (mL) | 997.5 | 997.5 | 997.5 | 997.5 | 997.5 | 997.5 | 997.5 | 997.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanrahan-Tan, D.G.; Henderson, L.; Kertesz, M.A.; Lilje, O. The Effects of Nitrogen and Phosphorus on Colony Growth and Zoospore Characteristics of Soil Chytridiomycota. J. Fungi 2022, 8, 341. https://doi.org/10.3390/jof8040341
Hanrahan-Tan DG, Henderson L, Kertesz MA, Lilje O. The Effects of Nitrogen and Phosphorus on Colony Growth and Zoospore Characteristics of Soil Chytridiomycota. Journal of Fungi. 2022; 8(4):341. https://doi.org/10.3390/jof8040341
Chicago/Turabian StyleHanrahan-Tan, Deirdre G., Linda Henderson, Michael A. Kertesz, and Osu Lilje. 2022. "The Effects of Nitrogen and Phosphorus on Colony Growth and Zoospore Characteristics of Soil Chytridiomycota" Journal of Fungi 8, no. 4: 341. https://doi.org/10.3390/jof8040341
APA StyleHanrahan-Tan, D. G., Henderson, L., Kertesz, M. A., & Lilje, O. (2022). The Effects of Nitrogen and Phosphorus on Colony Growth and Zoospore Characteristics of Soil Chytridiomycota. Journal of Fungi, 8(4), 341. https://doi.org/10.3390/jof8040341






