H3K4 Methyltransferase CfSet1 Is Required for Development and Pathogenesis in Colletotrichum fructicola
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Strain
2.2. CfSet1 Sequence Analysis
2.3. Generation of CfSET1 Deletion and Complemented Strains
2.3.1. Obtaining of CfSET1 Deletion Strains
2.3.2. Obtaining Complemented Strains of CfSET1 Deletion Mutant
2.4. Phenotypic Assays of the CfSet1 Deletion Mutants
2.4.1. Growth Rate Determination
2.4.2. Asexual Conidia Assays
2.4.3. Appressorium Formation and Penetration Assays
2.4.4. Pathogenicity Assays
3. Results and Discussion
3.1. Identification and Phylogenetic Tree Analysis of CfSet1
3.2. CfSet1 Is Involved in Vegetative Growth and Asexual Reproduction of C. fructicola
3.3. CfSet1 Is Required for Appressorium Formation and Penetration of C. fructicola
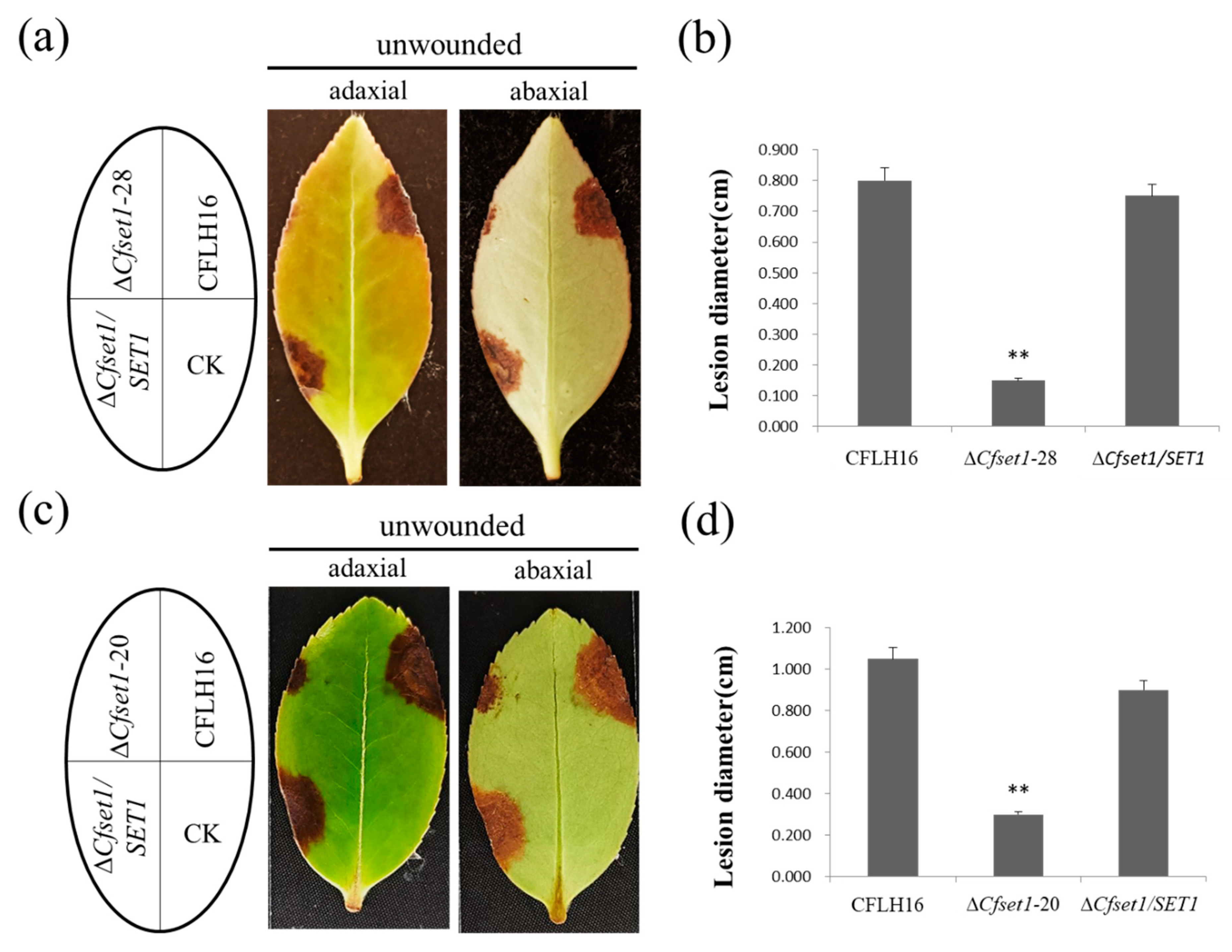
3.4. CfSet1 Is Important for Pathogenicity of C. fructicola
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Guo, Y.; Li, S.; Zhou, G.; Liu, J.; Xu, J.; Li, H. Functional analysis of CfSnf1 in the development and pathogenicity of anthracnose fungus Colletotrichum fructicola on tea-oil tree. BMC Genet. 2019, 20, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zhou, G.-Y.; Liu, J.-A.; Xu, J. Population Genetic Analyses of the Fungal Pathogen Colletotrichum fructicola on Tea-Oil Trees in China. PLoS ONE 2016, 11, e0156841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Guo, Y.; Chen, S.; Li, H. The Histone Acetyltransferase CfGcn5 Regulates Growth, Development, and Pathogenicity in the Anthracnose Fungus Colletotrichum fructicola on the Tea-Oil Tree. Front. Microbiol. 2021, 12, 680415. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; Krogan, N.J.; Dover, J.; Erdjument-Bromage, H.; Tempst, P.; Johnston, M.; Greenblatt, J.F.; Shilatifard, A. COMPASS: A complex of proteins associated with a trithorax-related SET domain protein. Proc. Natl. Acad. Sci. USA 2001, 98, 12902–12907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallery, J.F.; Adelin, É.; Le Goff, G.; Pigné, S.; Auger, A.; Ouazzani, J.; O’Connell, R.J. H3K4 trimethylation by CclA regulates pathogenicity and the production of three families of terpenoid secondary metabolites in Colletotrichum higginsianum. Mol. Plant Pathol. 2019, 20, 831–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallery, J.F.; Le Goff, G.; Adelin, E.; Iorga, B.I.; Pigné, S.; O’Connell, R.J.; Ouazzani, J. Deleting a chromatin remodeling gene increases the diversity of secondary me-tabolites produced by Colletotrichum higginsianum. J. Nat. Prod. 2019, 82, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, G.M.; Tanaka, A.; Eaton, C.J.; Scott, B. Formation of arthroconidia during regeneration and selection of transformed Epichloë festucae protoplasts. Fungal Biol. 2014, 118, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Fukada, F.; Kodama, S.; Nishiuchi, T.; Kajikawa, N.; Kubo, Y. Plant pathogenic fungi Colletotrichum and Magnaporthe share a common G1phase monitoring strategy for proper appressorium development. New Phytol. 2019, 222, 1909–1923. [Google Scholar] [CrossRef] [PubMed]
- Nislow, C.; Ray, E.; Pillus, L. SET1, A Yeast Member of the Trithorax Family, Functions in Transcriptional Silencing and Diverse Cellular Processes. Mol. Biol. Cell 1997, 8, 2421–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, R.; Mou, Y.N.; Tong, S.M.; Ying, S.H.; Feng, M.G. SET1/KMT2-governed histone H3K4 methylation coordinates the lifecycle in vivo and in vitro of the fungal insect pathogen Beauveria bassiana. Environ. Microbiol. 2021, 23, 5541–5554. [Google Scholar]
- Pham, K.T.; Inoue, Y.; Vu, B.V.; Nguyen, H.H.; Nakayashiki, T.; Ikeda, K.; Naka-yashiki, H. MoSET1 (histone H3K4 methyltransferase in Magnaporthe oryzae) regulates global gene expression during infection-related morphogenesis. PLoS Genet. 2015, 11, e1005385. [Google Scholar]
- Richard, A.W.; Robert, P.G.; Cristian, F.Q.; Jennifer, A.L.; Nicholas, J.T. An NADPH-dependent genetic switch regulates plant infection by the rice blast fungus. Proc. Natl. Acad. Sci. USA 2010, 107, 21902–21907. [Google Scholar]

| Primer | Primer Sequence (5′→3′) | Purpose |
|---|---|---|
| Set1-1F | GCAGCCAAGGCTTGTATGAA | amplify CfSET1 5′ flank sequence |
| Set1-2R | TTGACCTCCACTAGCTCCAGCCAAGCCCGTGAGGTGTATCTGTCTCT | amplify CfSET1 5′ flank sequence |
| Set1-3F | CAAAGGAATAGAGTAGATGCCGACCGTCCTGATCGCTTCTTTCCGG | amplify CfSET1 3′ flank sequence |
| Set1-4R | GCTATCAGATAAAGTCCCGT | amplify CfSET1 3′ flank sequence |
| Set1-5F | TGAGCCTACAATATCACGAC | validation of CfSET1 gene deletion |
| H855R | GCTGATCTGACCAGTTGC | validation of CfSET1 gene deletion |
| Set1-7F | TGCCTACCGACTTCAAGCTG | amplify CfSET1 gene sequence |
| Set1-8R | GGTCCAAACTGCCGATCTCA | amplify CfSET1 gene sequence |
| Set1-9F | ACTCACTATAGGGCGAATTGGGTACTCAAATTGGTTACTAGGCCTGCCAGAGCAGC | amplify complemented sequence |
| Set1-10R | CCTCGCCCTTGCTCACCATCGTGAGGTGTATCTGTCTCT | amplify complemented sequence |
| Set1-11F | GCATGGACGAGCTGTACAAGATGACCCGCCAACCGTCGGC | amplify complemented sequence |
| Set1-12R | CACCACCCCGGTGAACAGCTCCTCGCCCTTGCTCACTTAGTTGAGGAATCCCTTGC | amplify complemented sequence |
| GFPF | ATGGTGAGCAAGGGCGAGG | amplify GFP gene sequence |
| GFPR | CTTGTACAGCTCGTCCATGC | amplify GFP gene sequence |
| Hyg-F | CTCTATTCCTTTGCCCTCG | amplify HPH gene sequence |
| Hyg-R | GCTGATCTGACCAGTTGC | amplify HPH gene sequence |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zhang, S.; Li, H. H3K4 Methyltransferase CfSet1 Is Required for Development and Pathogenesis in Colletotrichum fructicola. J. Fungi 2022, 8, 363. https://doi.org/10.3390/jof8040363
Gao Y, Zhang S, Li H. H3K4 Methyltransferase CfSet1 Is Required for Development and Pathogenesis in Colletotrichum fructicola. Journal of Fungi. 2022; 8(4):363. https://doi.org/10.3390/jof8040363
Chicago/Turabian StyleGao, Yalan, Shengpei Zhang, and He Li. 2022. "H3K4 Methyltransferase CfSet1 Is Required for Development and Pathogenesis in Colletotrichum fructicola" Journal of Fungi 8, no. 4: 363. https://doi.org/10.3390/jof8040363





