Lack of Functional Trehalase Activity in Candida parapsilosis Increases Susceptibility to Itraconazole
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Preparation of Cell-Free Extracts
2.3. Enzymatic Assays
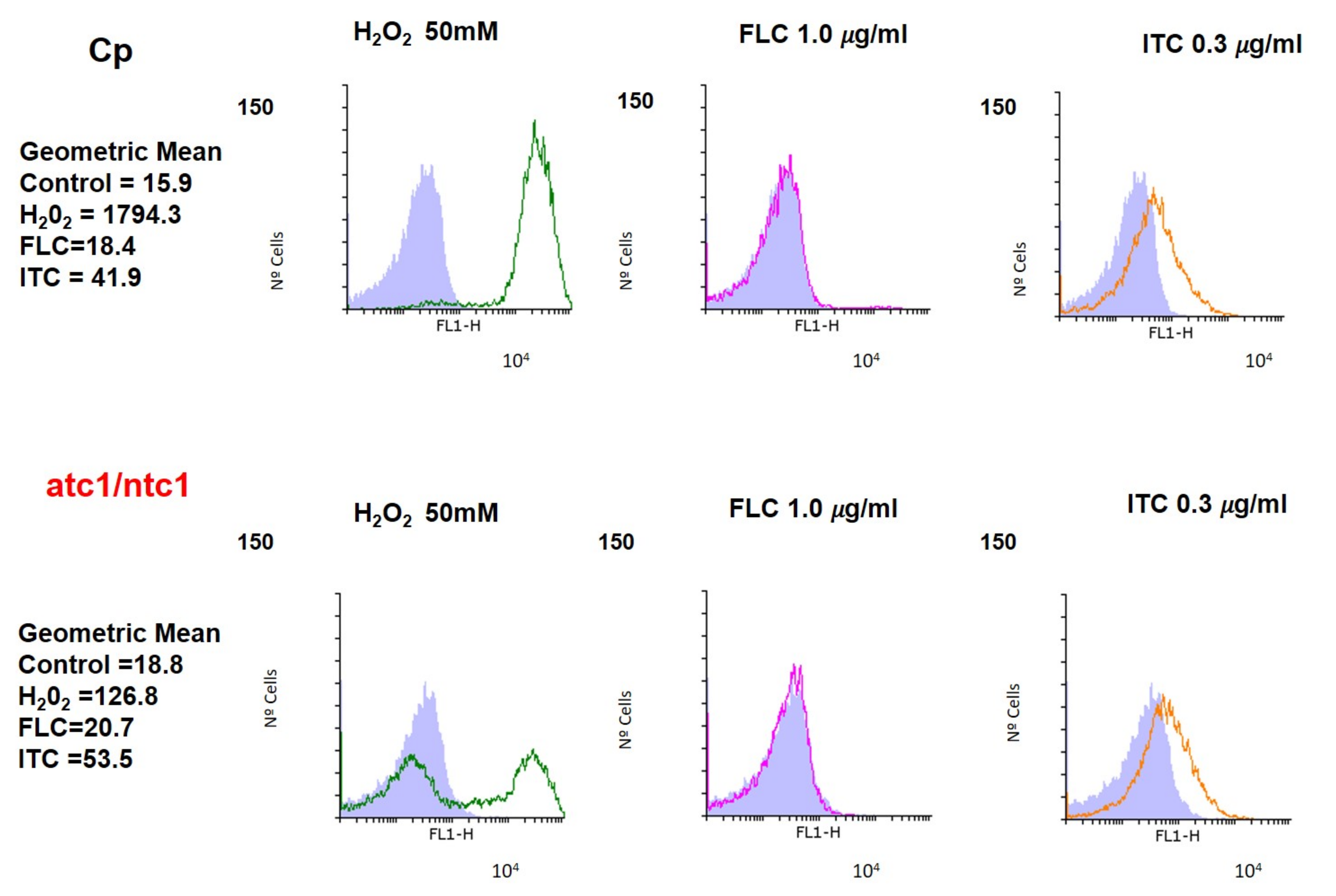
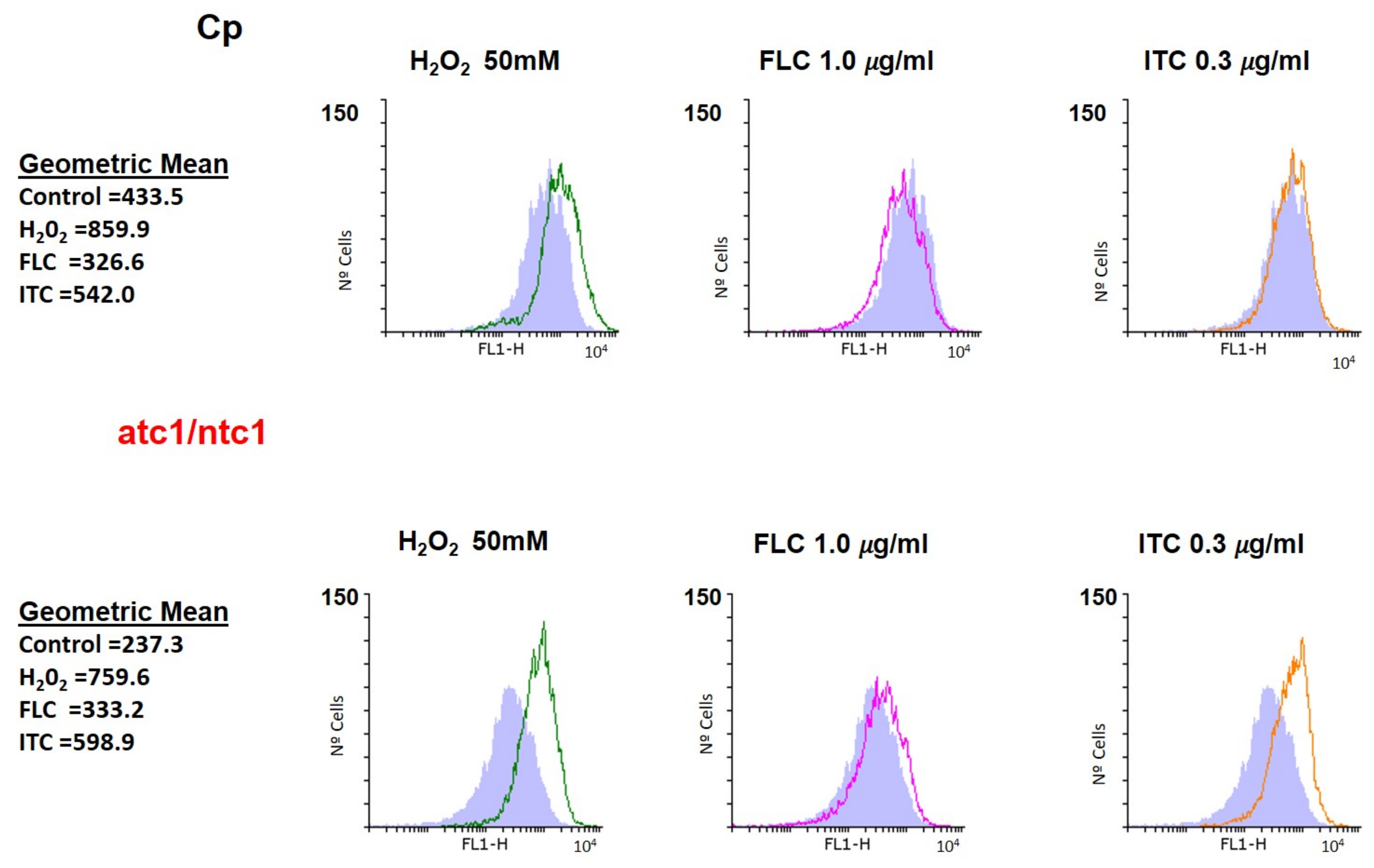
2.4. ROS and Membrane Potential Determination by Flow Cytometry
2.5. Biofilm Formation
3. Results
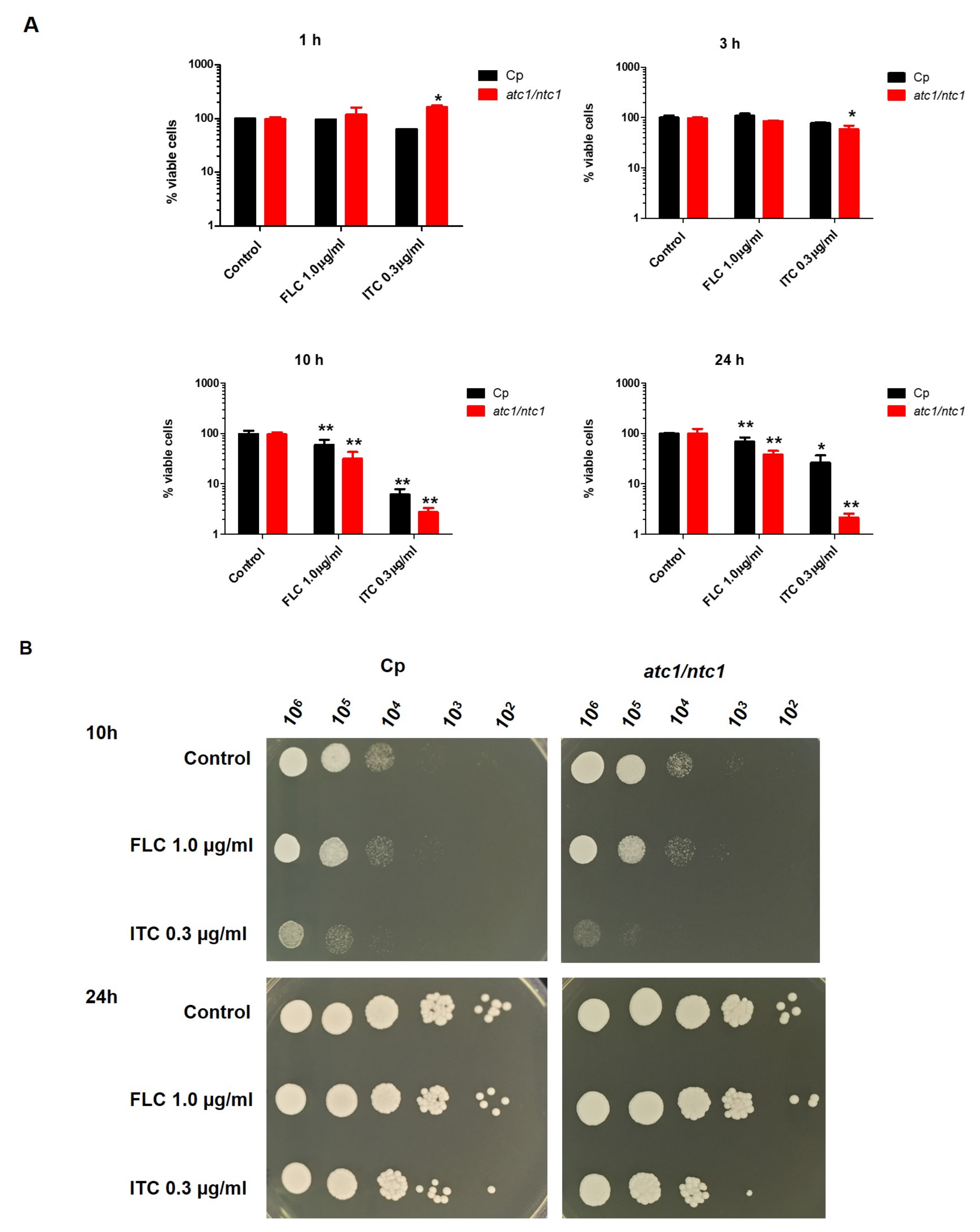
3.1. Cell Survival Rate of C. parapsilosis after Treatment with Azoles
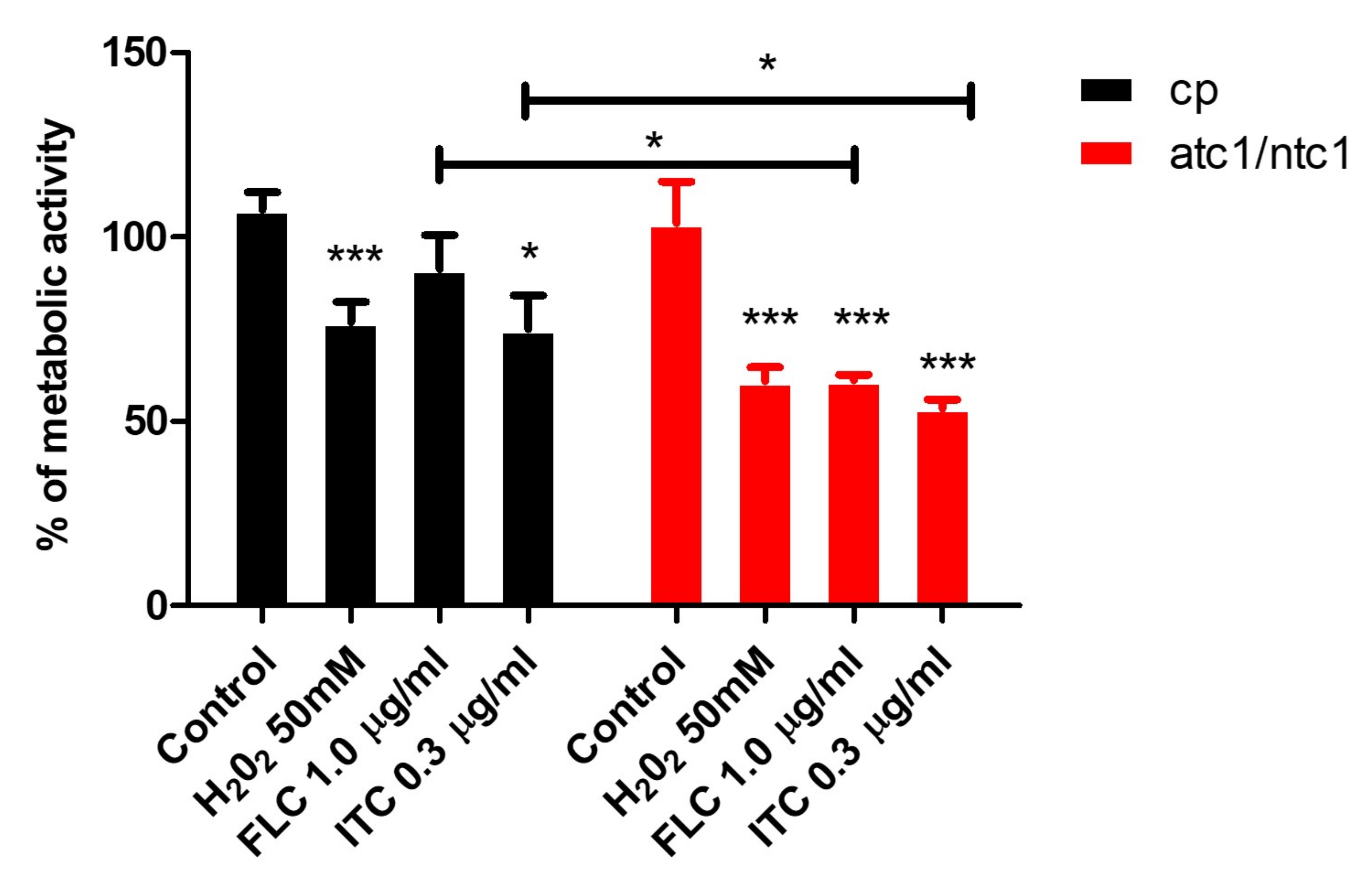
3.2. Level of ROS Formation and Mitochondrial Activity after Addition of Fluconazole and Itraconazole
3.3. Effect of Antifungal Treatment on Trehalose Synthesis
3.4. Level of Biofilm Formation in Trehalase-Deficient Mutant of C. parapsilosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AmB | Amphotericin B |
| FLC | Fluconazole |
| ITC | Itraconazole |
| PBS | Phosphate-Buffered Saline |
| ROS | Reactive Oxygen Species |
References
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Mycoses in North America. Crit. Rev. Microbiol. 2010, 36, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Yapar, N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014, 10, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Andes, D.R.; Diekema, D.J.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Franks, B.; Azie, N.E. Epidemiology and Outcomes of Invasive Candidiasis Due to Non-albicans Species of Candida in 2496 Patients: Data from the Prospective Antifungal Therapy (PATH) Registry 2004–2008. PLoS ONE 2014, 9, e101510. [Google Scholar] [CrossRef] [PubMed]
- Trofa, D.; Gácser, A.; Nosanchuk, J.D. Candida parapsilosis, an Emerging Fungal Pathogen. Clin. Microbiol. Rev. 2008, 21, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Toth, R.; Nosek, J.; Mora-Montes, H.; Gabaldón, T.; Bliss, J.; Nosanchuck, J.; Turner, S.B.; Butler, G.; Vágvölgyi, C.; Gácser, A. Candida parapsilosis: From Genes to the Bedside. Clin. Microbiol. Rev. 2019, 32, e00111-18. [Google Scholar] [CrossRef] [PubMed]
- Pál, S.; Toth, R.; Nosanchuk, J.; Cacsaba, A.; Vágvölgyi, C.; Németh, T.; Gácser, A. A Candida parapsilosis Overexpression Collection Reveals Genes Required for Pathogenesis. J. Fungi 2021, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Holland, L.M.; Schroder, M.S.; Turner, S.A.; Taff, H.; Andes, D.; Grozer, Z.; Gacser, A.; Ames, L.; Haynes, K.; Higgins, D.G.; et al. Comparative Phenotypic Analysis of the Major Fungal Pathogens Candida parapsilosis and Candida albicans. PLoS Pathog. 2014, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Papp, C.; Kocsis, K.; Tóth, R.; Bodai, L.; Willis, J.R.; Ksiezopolska, E.; Lozoya-Pérez, N.E.; Vágvölgyi, C.; Mora Montes, H.; Gabaldón, T.; et al. Echinocandin-Induced Microevolution of Candida parapsilosis Influences Virulence and Abiotic Stress Tolerance. mSphere 2018, 3, e00547-18. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fresneda, R.; Martínez-Esparza, M.; Maicas, S.; Argüelles, J.C.; Valentín, E. In Candida parapsilosis the ATC1 Gene Encodes for an Acid Trehalase Involved in Trehalose Hydrolysis, Stress Resistance and Virulence. PLoS ONE 2014, 9, e99113. [Google Scholar] [CrossRef] [PubMed]
- Salci, T.P.; Negri, M.; Abadio, A.K.R.; Svidzinski, T.I.E.; Kioshima, E.S. Targeting Candida spp. to develop antifungal agents. Drug Discov. Today 2018, 23, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Z.; Chen, Z.; Li, Y.; Su, S.; Sun, S. Potential Antifungal Targets Based on Glucose Metabolism Pathways of Candida albicans. Front. Microbiol. 2020, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Wijnants, S.; Vreys, J.; Van Dijck, P. Interesting antifungal drug targets in the central metabolism of Candida albicans. Trends Pharmacol. Sci. 2022, 43, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R.; Tenor, J.L.; Miao, Y.; Brennan, R.G. Trehalose pathway as an antifungal target. Virulence 2017, 8, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Argüelles, J.C. Trehalose as antifungal target: The picture is still incomplete. Virulence 2017, 8, 237–238. [Google Scholar] [CrossRef]
- Thammahong, A.; Puttikamonkul, S.; Perfect, J.R.; Brennan, R.G.; Cramer, R.A. Central Role of the Trehalose Biosynthesis Pathway in the Pathogenesis of Human Fungal Infections: Opportunities and Challenges for Therapeutic Development. Microbiol. Mol. Biol. Rev. 2017, 81, e00053-16. [Google Scholar] [CrossRef]
- Zaragoza, O.; Blazquez, M.A.; Gancedo, C. Disruption of the Candida albicans TPS1 gene encoding trehalose-6P-synthase impairs formation of hyphae and decreases infectivity. J. Bacteriol. 1998, 180, 3809–3815. [Google Scholar] [CrossRef]
- Van Dijck, P.; De Rop, L.; Szlufcik, K.; Van Ael, E.; Thevelein, J.M. Disruption of the Candida albicans TPS2 Gene Encoding Trehalose-6-Phosphate Phosphatase Decreases Infectivity without Affecting Hypha Formation. Infect. Immun. 2002, 70, 1772–1782. [Google Scholar] [CrossRef]
- Martínez-Esparza, M.; Aguinaga, A.; González-Párraga, P.; García-Peñarrubia, P.; Jouault, T.; Argüelles, J.C. Role of trehalose in resistance to macrophage killing: Study with a tps1/tps1 trehalose-deficient mutant of Candida albicans. Clin. Microbiol. Infect. 2007, 13, 384–394. [Google Scholar] [CrossRef]
- Pedreño, Y.; González-Párraga, P.; Martínez-Esparza, M.; Sentandreu, R.; Valentín, E.; Argüelles, J.C. Disruption of the Candida albicans ATC1 gene encoding a cell-linked acid trehalase decreases hypha formation and infectivity without affecting resistance to oxidative stress. Microbiology 2007, 153, 1372–1381. [Google Scholar] [CrossRef][Green Version]
- Lopes, R.G.; Muñoz, J.E.; Barros, L. The secreted acid trehalase encoded by the CgATH1 gene is involved in virulence. Mem. Inst. Oswaldo Cruz 2020, 115, e200401. [Google Scholar] [CrossRef] [PubMed]
- Van Ende, M.; Timmermans, B.; Vanreppelen, G.; Siscar-Lewin, S.; Fischer, D.; Wijnants, S.; Lobo-Romero, C.; Yazdani, S.; Rogiers, O.; Demuyser, L.; et al. The involvement of the Candida glabrata trehalase enzymes in stress resistance and gut colonization. Virulence 2021, 12, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fresneda, R.; Guirao-Abad, J.P.; Martinez-Esparza, M.; Maicas, S.; Valentín, E.; Argüelles, J.C. Homozygous deletion of ATC1 and NTC1 genes in Candida parapsilosis abolishes trehalase activity and affects cell growth, sugar metabolism, stress resistance, infectivity and biofilm formation. Fungal Genet. Biol. 2015, 85, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Sangalli-Leite, F.; Scorzoni, L.; Mesa-Arango, A.C.; Casas, C.; Herrero, E.; Soares, M.J.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M.; Zaragoza, O. Amphotericin B mediates killing in Cryptococcus neoformans through the induction of a strong oxidative burst. Microbes Infect. 2011, 13, 457–467. [Google Scholar] [CrossRef]
- Pierce, C.G.; Uppuluri, P.; Tummala, S.; Lopez-Ribot, J.L. A 96 Well Microtiter Plate-based Method for Monitoring Formation and Antifungal Susceptibility Testing of Candida albicans Biofilms. J. Vis. Exp. 2010, 21, e2287. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Román, E.; Sánchez-Fresneda, R.; Casas, C.; Herrero, E.; Argüelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The Production of Reactive Oxygen Species Is a Universal Action Mechanism of Amphotericin B against Pathogenic Yeasts and Contributes to the Fungicidal Effect of This Drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef]
- Guirao-Abad, J.P.; Sánchez-Fresneda, R.; Román, E.; Plá, J.; Argüelles, J.C.; Alonso-Monge, R. The MAPK Hog1 mediates the response to amphotericin B in Candida albicans. Fungal Genet. Biol. 2020, 136, 103302. [Google Scholar] [CrossRef]
- Belenky, P.; Camacho, D.; Collins, J.J. Fungicidal Drugs Induce a Common Oxidative-Damage Cellular Death Pathway. Cell Rep. 2013, 3, 350–358. [Google Scholar] [CrossRef]
- Shekhova, E.; Kniemeyer, O.; Brakhage, A.A. Induction of Mitochondrial Reactive Oxygen Species Production by Itraconazole, Terbinafine, and Amphotericin B as a Mode of Action against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2017, 61, e00978-17. [Google Scholar] [CrossRef]
- Tournu, H.; Fiori, A.; van Dijck, P. Relevance of Trehalose in Pathogenicity: Some General Rules, Yet Many Exceptions. PLOS Pathogens 2013, 9, e1003447. [Google Scholar] [CrossRef]
- González-Párraga, P.; Sánchez-Fresneda, R.; Zaragoza, O.; Argüelles, J.C. Amphotericin B induces trehalose synthesis and simultaneously activates an antioxidant enzymatic response in Candida albicans. Biochim. Biophys. Acta (BBA) Gen. Subj. 2011, 1810, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 3397–3409. [Google Scholar] [CrossRef]
- Van Acker, H.; van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Mukherjee, P.K. Candida Biofilms: Development, Architecture, and Resistance. Microbiol. Spectr. 2015, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Zambrano, L.J.; Escribano, P.; Bouza, E.; Guinea, J. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: Comparison of biomass production and metabolic activity and development of cut-off points. Int. J. Med. Microbiol. 2014, 304, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Vanaporn, M.; Titball, R.W. Trehalose and bacterial virulence. Virulence 2020, 11, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; Chatzimoschou, A.; Simitsopoulou, M.; Dalakiouridou, M.; Diza-Mataftsi, E.; Tsantali, C.; Roilides, E. Differential Activities of Newer Antifungal Agents against Candida albicans and Candida parapsilosis Biofilms. Antimicrob. Agents Chemother. 2008, 52, 357–360. [Google Scholar] [CrossRef]
| Time (h) | Neutral Trehalase (Ntc1p) a | Acid Trehalase (Atc1p) a | ||
|---|---|---|---|---|
| Cp | atc1Δ/ntc1Δ | Cp | atc1Δ/ntc1Δ | |
| 1 | 22.5 ± 0.9 | <0.3 | 2.7 ± 0.3 | <0.3 |
| 5 | 18.3 ± 0.7 | <0.3 | 3.9 ± 0.2 | <0.3 |
| 10 | 12.7 ± 0.3 | <0.3 | 8.1 ± 0.5 | <0.3 |
| 24 | 6.8 ± 0.2 | <0.3 | 9.7 ± 0.7 | <0.3 |
| Treatment | Trehalose (nmol (mg wet wt)−1) | |
|---|---|---|
| Cp | atc1Δ/ntc1Δ | |
| Control | 4.8 ± 0.5 | 3.3 ± 0.2 |
| Fluconazole (1.0 μg/mL) | 6.3 ± 0.2 *** | 4.9 ± 0.2 *** |
| Itraconazole (0.3 μg/mL) | 9.6 ± 0.3 *** | 7.2 ± 0.3 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Fresneda, R.; Muñoz-Megías, M.L.; Yagüe, G.; Solano, F.; Maicas, S.; Argüelles, J.C. Lack of Functional Trehalase Activity in Candida parapsilosis Increases Susceptibility to Itraconazole. J. Fungi 2022, 8, 371. https://doi.org/10.3390/jof8040371
Sánchez-Fresneda R, Muñoz-Megías ML, Yagüe G, Solano F, Maicas S, Argüelles JC. Lack of Functional Trehalase Activity in Candida parapsilosis Increases Susceptibility to Itraconazole. Journal of Fungi. 2022; 8(4):371. https://doi.org/10.3390/jof8040371
Chicago/Turabian StyleSánchez-Fresneda, Ruth, María Luz Muñoz-Megías, Genoveva Yagüe, Francisco Solano, Sergi Maicas, and Juan Carlos Argüelles. 2022. "Lack of Functional Trehalase Activity in Candida parapsilosis Increases Susceptibility to Itraconazole" Journal of Fungi 8, no. 4: 371. https://doi.org/10.3390/jof8040371
APA StyleSánchez-Fresneda, R., Muñoz-Megías, M. L., Yagüe, G., Solano, F., Maicas, S., & Argüelles, J. C. (2022). Lack of Functional Trehalase Activity in Candida parapsilosis Increases Susceptibility to Itraconazole. Journal of Fungi, 8(4), 371. https://doi.org/10.3390/jof8040371









