A Rapid Detection Method for Fungal Spores from Greenhouse Crops Based on CMOS Image Sensors and Diffraction Fingerprint Feature Processing
Abstract
:1. Introduction
2. Materials and Methods
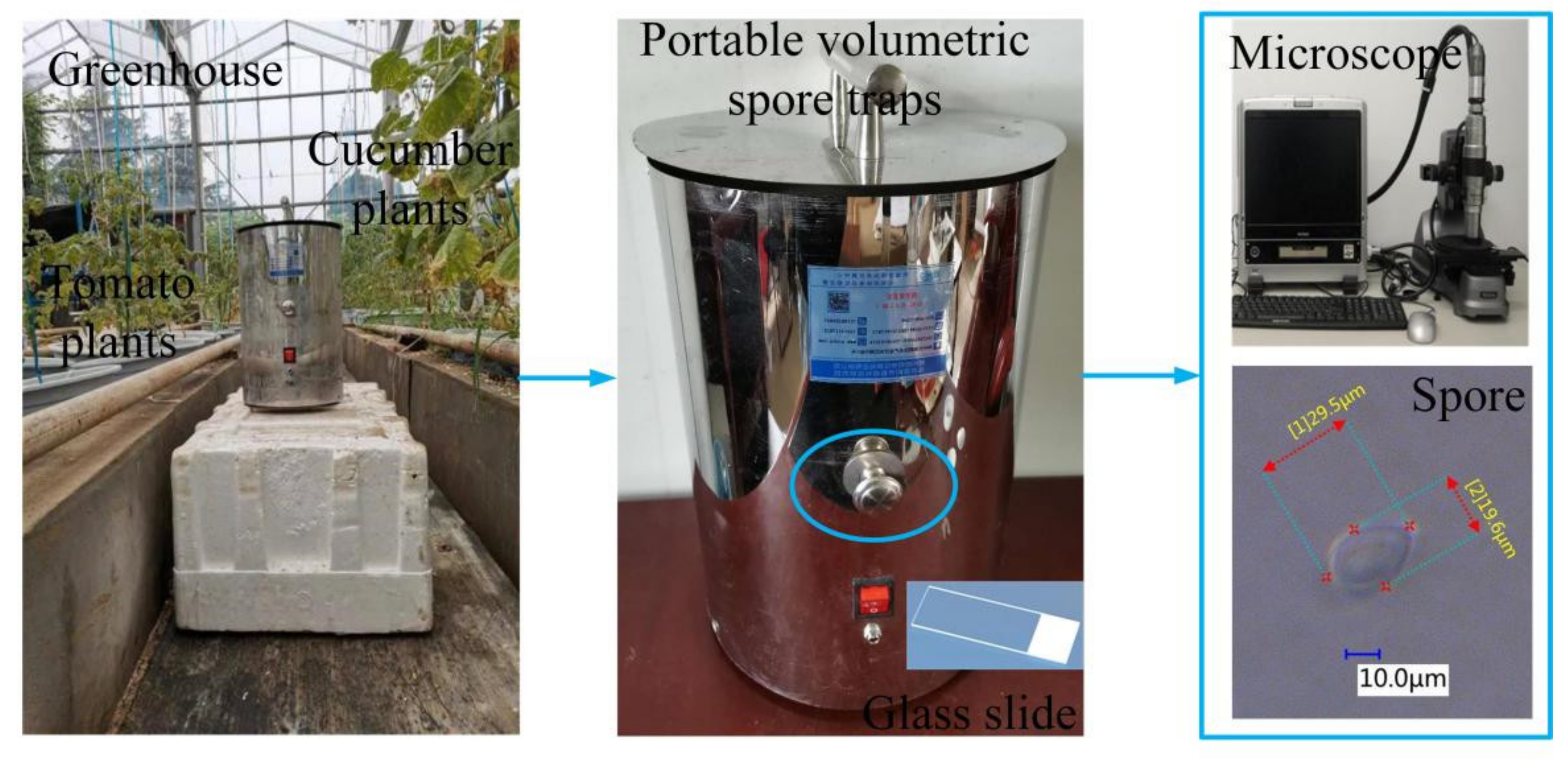
2.1. Fungal Spore Sample Collection and Parameter Measurement
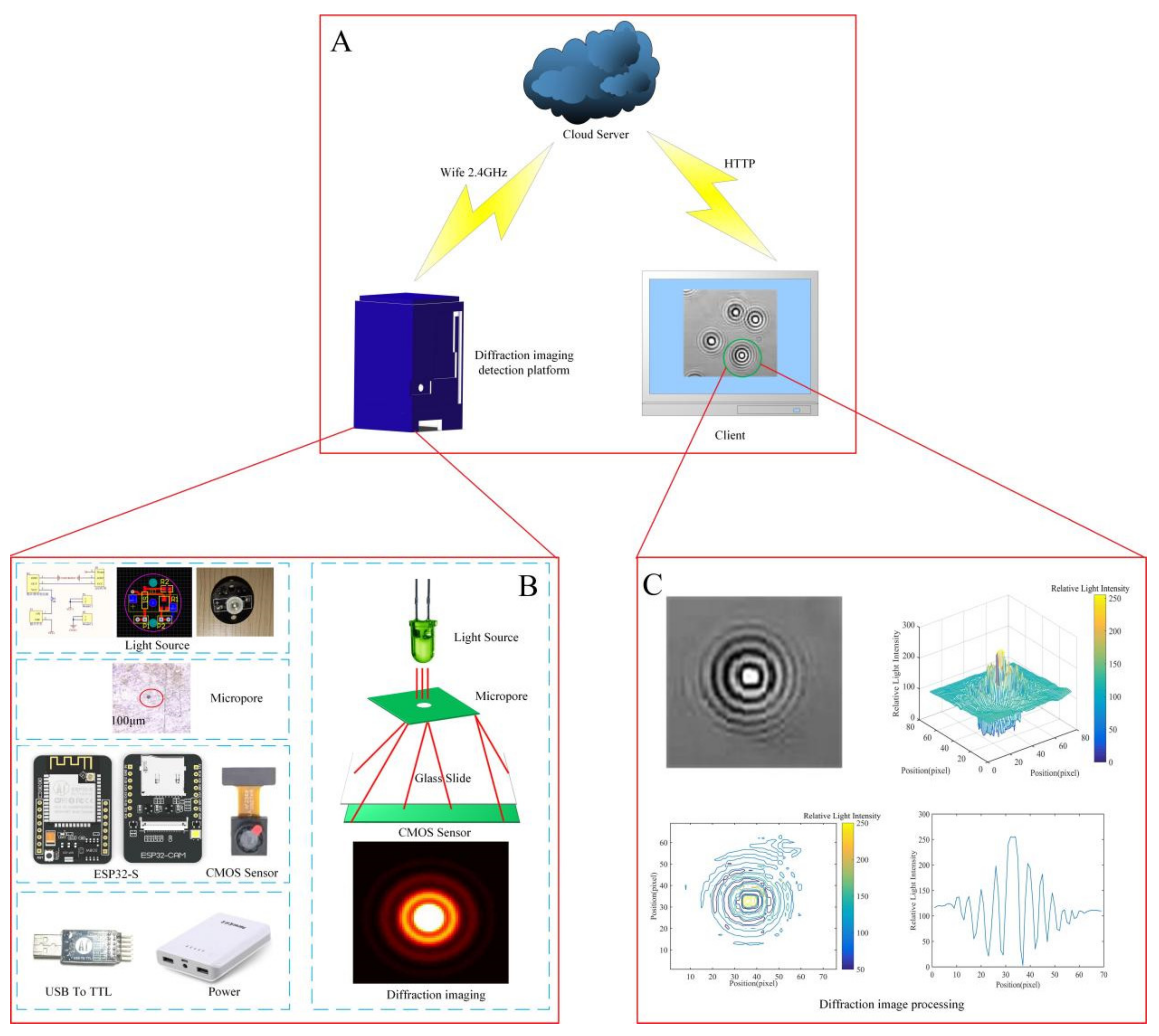
2.2. Construction of the Fungal Spore Diffraction Fingerprint Collection System
2.3. Fungal Spore Diffraction Fingerprint Image Processing
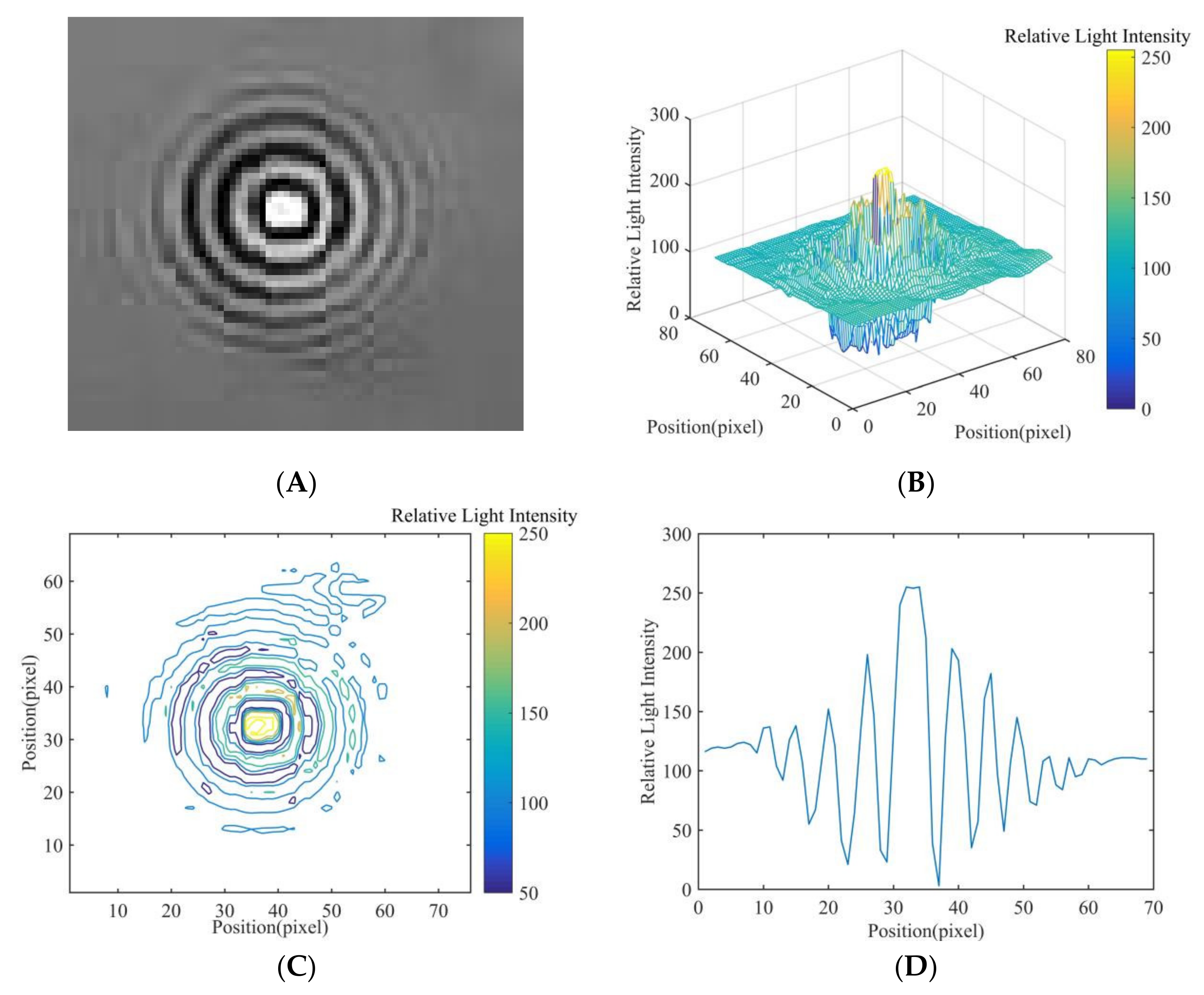
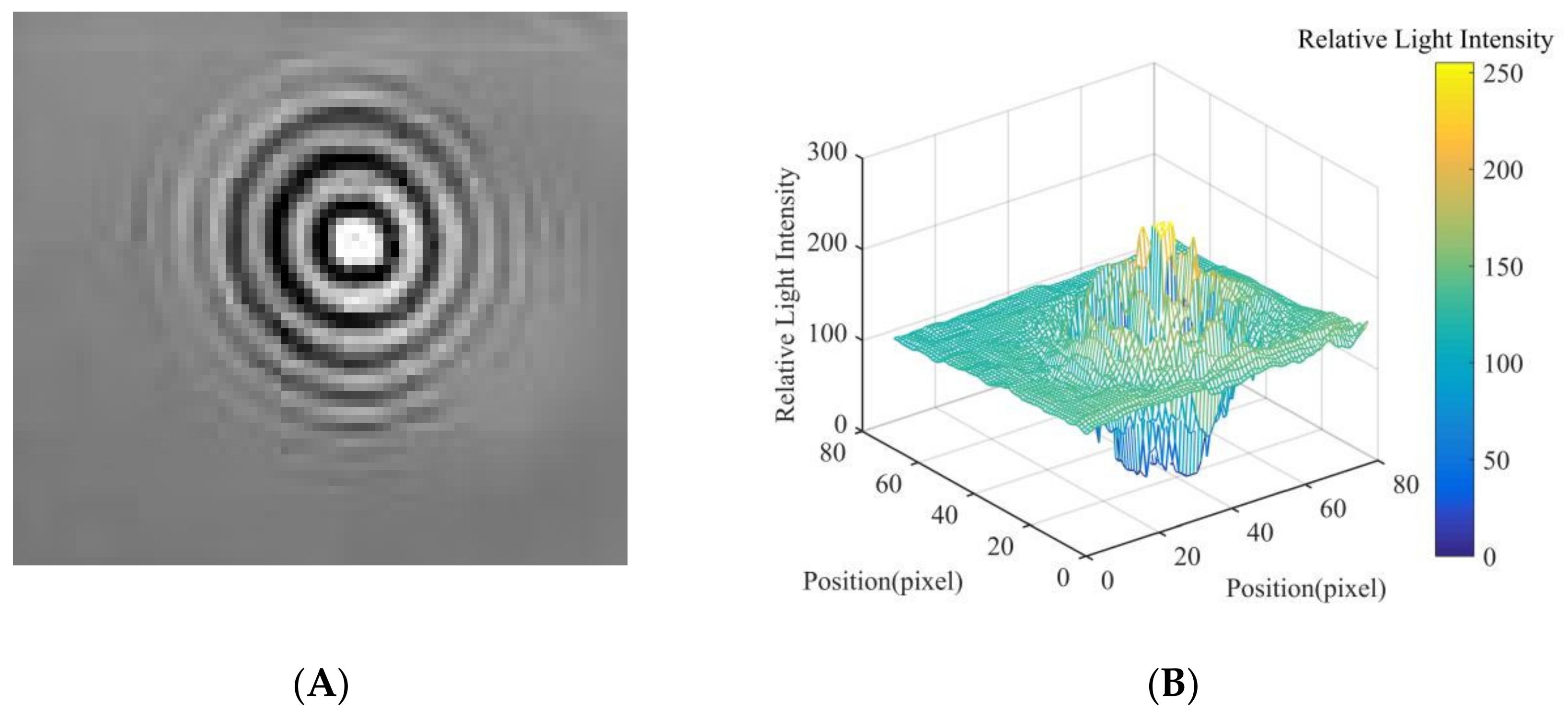
2.4. Relative Light Intensity Distribution of Diffraction Fingerprint Image
2.5. Feature Extraction of Fungal Spore Diffraction Fingerprint
2.6. Sample Division and Test Platform
2.7. Evaluation Index of Classification Results
2.8. Statistical Analysis Software
3. Results
3.1. Results and Analysis of Fungal Spore Diffraction Fingerprint Image
3.2. Analysis of Classification Results
3.2.1. Evaluation Index
3.2.2. Classification Results of Different Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Zhang, Y.; Liu, W.; Zhao, J.; Yu, S.; Jia, H.; Zhang, C.; Li, Y. Incorporating in vitro bioaccessibility into human health risk assessment of heavy metals and metalloid (As) in soil and pak choi (Brassica chinensis L.) from greenhouse vegetable production fields in a megacity in Northwest China. Food Chem. 2021, 373, 131488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, X.; Yan, H.; Ullah, I.; Zuo, Z.; Li, L.; Yu, J. Effects of irrigation quantity and biochar on soil physical properties, growth characteristics, yield and quality of greenhouse tomato. Agric. Water Manag. 2020, 241, 106263. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Yang, N.; Ma, G.; Du, X.; Mao, H. Separation-enrichment method for airborne disease spores based on microfluidic chip. Int. J. Agric. Biol. Eng. 2021, 14, 199–205. [Google Scholar] [CrossRef]
- Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Bensy, A.D.V.; Rajaselvam, J. Bioactive potential of Albizia lebbeck extract against phytopathogens and protective properties on tomato plant against speck disease in greenhouse. Physiol. Mol. Plant Pathol. 2021, 117, 101750. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, G.; Du, X.; Liu, Y.; Wang, B.; Xu, G.; Mao, H. Effects of Nutrient Solution Irrigation Quantity and Downy Mildew Infection on Growth and Physiological Traits of Greenhouse Cucumber. Agronomy 2020, 10, 1921. [Google Scholar] [CrossRef]
- Hafez, Y.M.; Attia, K.A.; Kamel, S.; Alamery, S.F.; El-Gendy, S.; Al-Doss, A.A.; Mehiar, F.; Ghazy, A.I.; Ibrahim, E.I.; Abdelaal, K.A. Bacillus subtilis as a bio-agent combined with nano molecules can control powdery mildew disease through histochemical and physiobiochemical changes in cucumber plants. Physiol. Mol. Plant Pathol. 2020, 111, 101489. [Google Scholar] [CrossRef]
- Tanaka, K.; Fukuda, M.; Amaki, Y.; Sakaguchi, T.; Inai, K.; Ishihara, A.; Nakajima, H. Importance of prumycin produced byBacillus amyloliquefaciensSD-32 in biocontrol against cucumber powdery mildew disease. Pest Manag. Sci. 2017, 73, 2419–2428. [Google Scholar] [CrossRef]
- Wallace, E.C.; D’Arcangelo, K.N.; Quesada-Ocampo, L.M. Population Analyses Reveal Two Host-Adapted Clades of Pseudoperonospora cubensis, the Causal Agent of Cucurbit Downy Mildew, on Commercial and Wild Cucurbits. Phytopathology 2020, 110, 1578–1587. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, G.; Chen, A.; Yi, J.; Zhang, W.; Hu, Y. Identification of tomato leaf diseases based on combination of ABCK-BWTR and B-ARNet. Comput. Electron. Agric. 2020, 178, 105730. [Google Scholar] [CrossRef]
- Abbas, A.; Jain, S.; Gour, M.; Vankudothu, S. Tomato plant disease detection using transfer learning with C-GAN synthetic images. Comput. Electron. Agric. 2021, 187, 106279. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Zhang, C.; Wang, X.; Shi, Y. Cucumber leaf disease identification with global pooling dilated convolutional neural network. Comput. Electron. Agric. 2019, 162, 422–430. [Google Scholar] [CrossRef]
- Sireesha, Y.; Velazhahan, R. Rapid and specific detection of Peronosclerospora sorghi in maize seeds by conventional and real-time PCR. Eur. J. Plant Pathol. 2017, 150, 521–526. [Google Scholar] [CrossRef]
- Bandamaravuri, K.B.; Nayak, A.K.; Bandamaravuri, A.S.; Samad, A. Simultaneous detection of downy mildew and powdery mildew pathogens on Cucumis sativus and other cucurbits using duplex-qPCR and HRM analysis. AMB Express 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Chen, J.J.; Li, K.T. Analysis of PCR Kinetics inside a Microfluidic DNA Amplification System. Micromachines 2018, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Ramasamy, R.P. Current and Prospective Methods for Plant Disease Detection. Biosensors 2015, 5, 537–561. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Wang, R.; Feng, L.; Li, Q.; Wu, H. Dual-branch, efficient, channel attention-based crop disease identification. Comput. Electron. Agric. 2021, 190, 106410. [Google Scholar] [CrossRef]
- Agarwal, M.; Gupta, S.; Biswas, K. A new Conv2D model with modified ReLU activation function for identification of disease type and severity in cucumber plant. Sustain. Comput. Inform. Syst. 2020, 30, 100473. [Google Scholar] [CrossRef]
- Wen, D.; Ren, A.; Ji, T.; Flores-Parra, I.M.; Yang, X.; Li, M. Segmentation of thermal infrared images of cucumber leaves using K-means clustering for estimating leaf wetness duration. Int. J. Agric. Biol. Eng. 2020, 13, 161–167. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, Y.; Man, C.; Jiang, Z.; Li, S. Identification of cucumber leaf diseases using deep learning and small sample size for agricultural Internet of Things. Int. J. Distrib. Sens. Netw. 2021, 17, 15501477211007407. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, L.; Li, D. EfficientNet-B4-Ranger: A novel method for greenhouse cucumber disease recognition under natural complex environment. Comput. Electron. Agric. 2020, 176, 105652. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, H.; Guo, W.; Han, X.; Chen, C.; Wu, H. EFDet: An efficient detection method for cucumber disease under natural complex environments. Comput. Electron. Agric. 2021, 189, 106378. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Wang, C.; Wu, H.; Zhao, C.; Teng, G. Crop disease identification and interpretation method based on multimodal deep learning. Comput. Electron. Agric. 2021, 189, 106408. [Google Scholar] [CrossRef]
- Xie, C.; Wang, H.; Shao, Y.; He, Y. Different Algorithms for Detection of Malondialdehyde Content in Eggplant Leaves Stressed by Grey Mold Based on Hyperspectral Imaging Technique. Intell. Autom. Soft Comput. 2015, 21, 395–407. [Google Scholar] [CrossRef]
- Gu, Q.; Sheng, L.; Zhang, T.; Lu, Y.; Zhang, Z.; Zheng, K.; Hu, H.; Zhou, H. Early detection of tomato spotted wilt virus infection in tobacco using the hyperspectral imaging technique and machine learning algorithms. Comput. Electron. Agric. 2019, 167, 105066. [Google Scholar] [CrossRef]
- Akhmadeev, A.A.; Salakhov, M.K. A new approach of recognition of ellipsoidal micro- and nanoparticles on AFM images and determination of their sizes. Meas. Sci. Technol. 2016, 27, 105402. [Google Scholar] [CrossRef]
- Abdulridha, J.; Ampatzidis, Y.; Kakarla, S.C.; Roberts, P. Detection of target spot and bacterial spot diseases in tomato using UAV-based and benchtop-based hyperspectral imaging techniques. Precis. Agric. 2019, 21, 955–978. [Google Scholar] [CrossRef]
- Lei, Y.; Yao, Z.; He, D. Automatic detection and counting of urediniospores of Puccinia striiformis f. sp. tritici using spore traps and image processing. Sci. Rep. 2018, 8, 13647. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Du, X.; Ma, G.; Liu, Y.; Wang, B.; Mao, H. Classification Methods for Airborne Disease Spores from Greenhouse Crops Based on Multifeature Fusion. Appl. Sci. 2020, 10, 7850. [Google Scholar] [CrossRef]
- Li, X.L.; Ma, Z.H.; Sun, Z.Y.; Wang, H.G. Automatic counting for trapped urediospores of Puccinia striiformis f. sp. tritici based on image processing. Trans. Chin. Soc. Agric. Eng. 2013, 29, 199–206, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Qi, L.; Jiang, Y.; Li, Z.H.; Ma, X.; Zheng, Z.X.; Wang, W.J. Automatic detection and counting method for spores of rice blast based on micro image processing. Trans. Chin. Soc. Agric. Eng. 2015, 31, 186–193, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wang, Y.; Mao, H.; Zhang, X.; Liu, Y.; Du, X. A Rapid Detection Method for Tomato Gray Mold Spores in Greenhouse Based on Microfluidic Chip Enrichment and Lens-Less Diffraction Image Processing. Foods 2021, 10, 3011. [Google Scholar] [CrossRef]
- Yang, N.; Yu, J.; Wang, A.; Tang, J.; Zhang, R.; Xie, L.; Shu, F.; Kwabena, O.P. A rapid rice blast detection and identification method based on crop disease spores’ diffraction fingerprint texture. J. Sci. Food Agric. 2020, 100, 3608–3621. [Google Scholar] [CrossRef]
- Woyzichovski, J.; Shchepin, O.; Dagamac, N.H.; Schnittler, M. A workflow for low-cost automated image analysis of myxomycete spore numbers, size and shape. PeerJ 2021, 9, e12471. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Ma, G.; Du, X.; Shaheen, N.; Mao, H. Recognition of weeds at asparagus fields using multi-feature fusion and backpropagation neural network. Int. J. Agric. Biol. Eng. 2021, 14, 190–198. [Google Scholar] [CrossRef]
- Indu, V.T.; Priyadharsini, S.S. Crossover-based wind-driven optimized convolutional neural network model for tomato leaf disease classification. J. Plant Dis. Prot. 2021, 128, 1–20. [Google Scholar] [CrossRef]
- Cengil, E.; Çınar, A. Hybrid convolutional neural network based classification of bacterial, viral, and fungal diseases on tomato leaf images. Concurr. Comput. Pract. Exp. 2021, 34, e6617. [Google Scholar] [CrossRef]
- Wen, D.-M.; Chen, M.-X.; Zhao, L.; Ji, T.; Li, M.; Yang, X.-T. Use of thermal imaging and Fourier transform infrared spectroscopy for the pre-symptomatic detection of cucumber downy mildew. Eur. J. Plant Pathol. 2019, 155, 405–416. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Z.; Wang, F.; Wang, Y. Hyperspectral Leaf Image-Based Cucumber Disease Recognition Using the Extended Collaborative Representation Model. Sensors 2020, 20, 4045. [Google Scholar] [CrossRef]
- Olorocisimo, J.P.; Briones, J.; Sasagawa, K.; Haruta, M.; Takehara, H.; Tashiro, H.; Ishida-Kitagawa, N.; Bessho, Y.; Ohta, J. Ultrasmall compact CMOS imaging system for bioluminescence reporter-based live gene expression analysis. J. Biomed. Opt. 2021, 26, 116002. [Google Scholar] [CrossRef]
- Woo, C.; An, C.; Xu, S.; Yi, S.-M.; Yamamoto, N. Taxonomic diversity of fungi deposited from the atmosphere. ISME J. 2018, 12, 2051–2060. [Google Scholar] [CrossRef] [Green Version]
- Chai, A.-L.; Wang, Y.-K.; Zhu, F.-D.; Shi, Y.-X.; Xie, X.-W.; Li, B.-J. Identification of Plant-Pathogenic Fungi Using Fourier Transform Infrared Spectroscopy Combined with Chemometric Analyses. Guang Pu Xue Yu Guang Pu Fen Xi = Guang Pu 2016, 36, 3764–3771. [Google Scholar]
- Jeong, Y.-S.; Choi, S.; Chong, E.; Kim, J.; Kim, S.-J. Rapid detection of Bacillus spore aerosol particles by direct in situ analysis using MALDI-TOF mass spectrometry. Lett. Appl. Microbiol. 2014, 59, 177–183. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, W.; Huang, M.; Ni, X.; Chu, X.; Li, C. Evaluation and classification of five cereal fungi on culture medium using Visible/Near-Infrared (Vis/NIR) hyperspectral imaging. Infrared Phys. Technol. 2020, 105, 103206. [Google Scholar] [CrossRef]
- Li, Y.X.; Hu, X.T.; Zhang, F.; Shi, J.Y.; Qiu, B.J. Colony image segmentation and counting based on hyperspectral technology. Trans. Chin. Soc. Agric. Eng. 2020, 36, 326–332. [Google Scholar] [CrossRef]
- Chesmore, D.; Bernard, T.; Inman, A.J.; Bowyer, R.J. Image analysis for the identification of the quarantine pest Tilletia indica. EPPO Bull. 2003, 33, 495–499. [Google Scholar] [CrossRef]
- Yang, N.; Qian, Y.; El-Mesery, H.S.; Zhang, R.; Wang, A.; Tang, J. Rapid detection of rice disease using microscopy image identification based on the synergistic judgment of texture and shape features and decision tree–confusion matrix method. J. Sci. Food Agric. 2019, 99, 6589–6600. [Google Scholar] [CrossRef]
- Xiaolong, L.; Zhanhong, M.; Bienvenido, F.; Feng, Q.; Haiguang, W. Development of automatic counting system for urediospores of wheat stripe rust based on image processing. Int. J. Agric. Biol. Eng. 2017, 10, 134–143. [Google Scholar] [CrossRef] [Green Version]


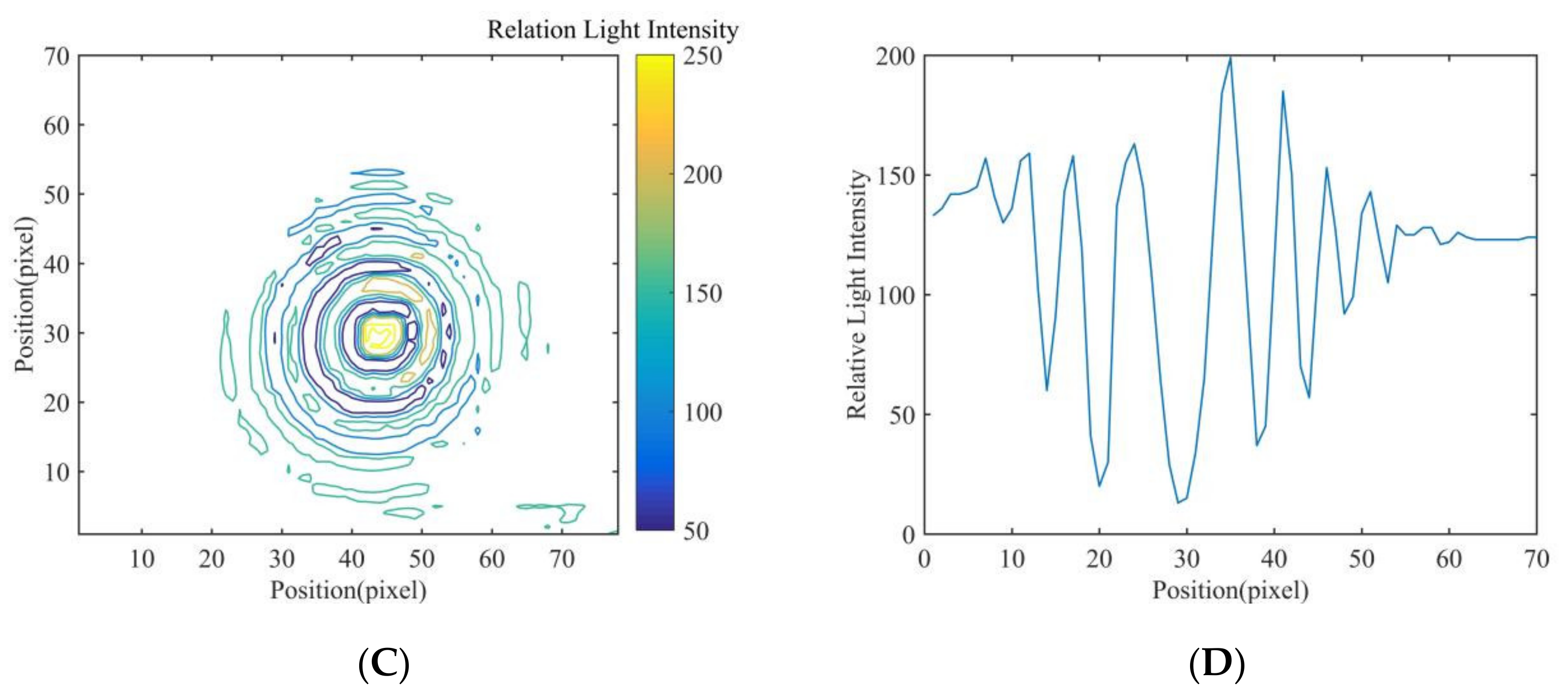
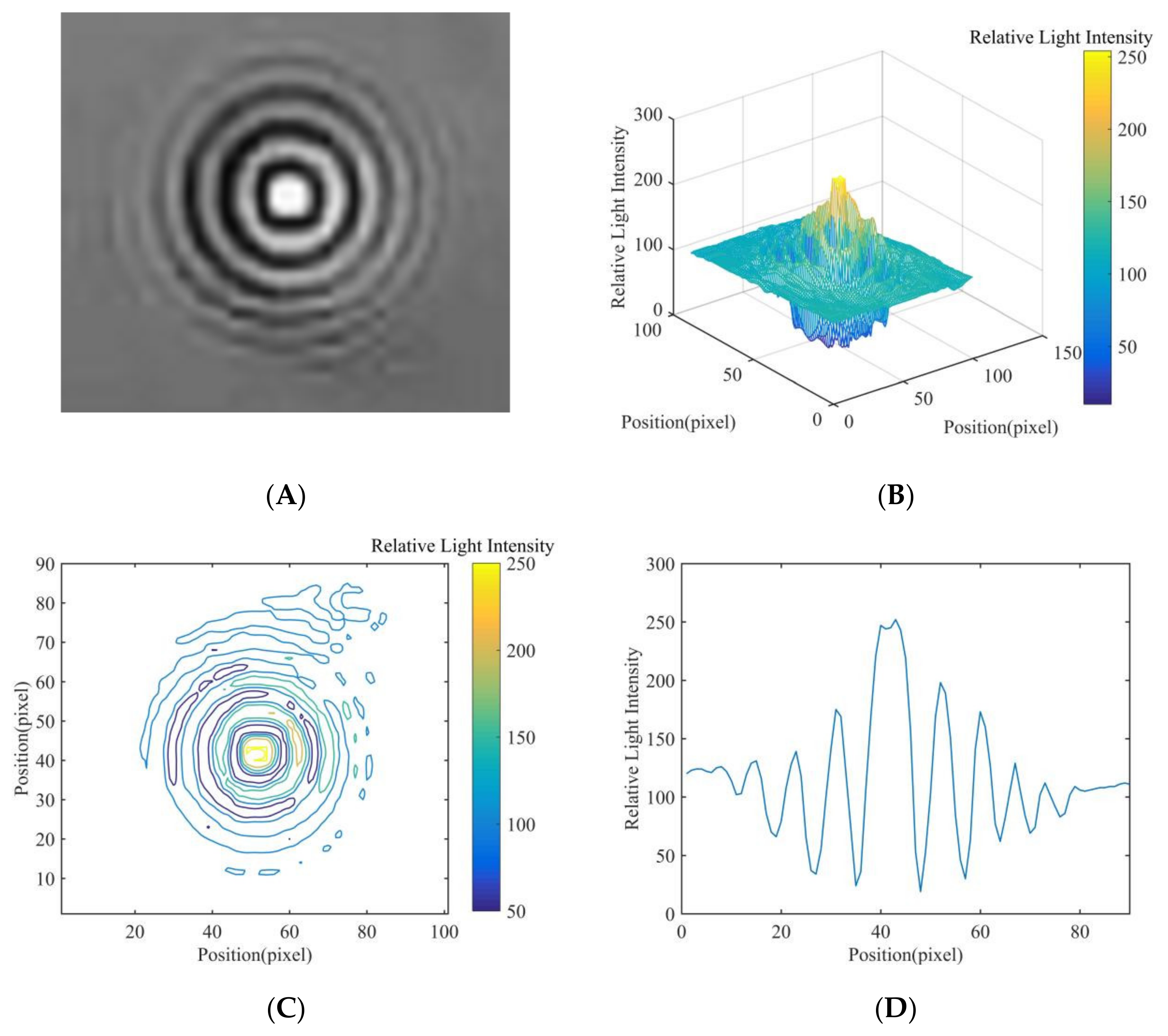

| Feature | Relative Light Intensity Distribution Value | |||
|---|---|---|---|---|
| P. cubensis Spore | P. Xanthii Spore | B. cinerea Spore | ||
| Center | C | 243–275 | 182–224 | 253–286 |
| Main bright fringe | P1 and P6 | 102–154 | 131–164 | 104–153 |
| P2 and P5 | 150–183 | 141–173 | 133–206 | |
| P3 and P4 | 184–223 | 152–195 | 172–215 | |
| Main dark fringe | V1 and V6 | 53–106 | 49–107 | 61–83 |
| V2 and V5 | 26–51 | 23–63 | 21–52 | |
| V3 and V4 | 8–33 | 12–39 | 14–37 | |
| Species | Basic Indicators | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LR | KNN | RF | SVM | |||||||||||||
| TP | TN | FP | FN | TP | TN | FP | FN | TP | TN | FP | FN | TP | TN | FP | FN | |
| B. cinerea spores | 46 | 95 | 13 | 14 | 49 | 99 | 7 | 11 | 51 | 100 | 7 | 9 | 53 | 108 | 4 | 7 |
| P. cubensis spores | 44 | 97 | 15 | 16 | 47 | 101 | 13 | 13 | 46 | 105 | 12 | 14 | 51 | 110 | 7 | 9 |
| P. xanthii spores | 51 | 90 | 11 | 9 | 52 | 96 | 12 | 8 | 54 | 97 | 10 | 6 | 57 | 104 | 8 | 3 |
| Species | Classification Results | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy | Precision | Recall | F1-Score | |||||||||||||
| LR | KNN | RF | SVM | LR | KNN | RF | SVM | LR | KNN | RF | SVM | LR | KNN | RF | SVM | |
| B. cinerea spores | 83.93 | 89.16 | 90.42 | 93.60 | 77.97 | 87.50 | 87.93 | 92.98 | 77.67 | 81.67 | 85.00 | 88.33 | 77.31 | 84.48 | 86.44 | 90.60 |
| P. cubensis spores | 81.98 | 85.06 | 85.31 | 90.96 | 74.58 | 78.33 | 79.31 | 87.93 | 73.33 | 78.33 | 76.67 | 85.00 | 73.95 | 78.33 | 77.97 | 86.44 |
| P. xanthii spores | 87.58 | 88.10 | 90.42 | 93.60 | 82.26 | 81.25 | 84.38 | 87.69 | 85.00 | 86.67 | 90.00 | 95.00 | 83.61 | 83.87 | 87.10 | 91.20 |
| Indexes | Classification Model | |||
|---|---|---|---|---|
| LR | KNN | RF | SVM | |
| Accuracy | 84.97 | 87.44 | 88.72 | 92.72 |
| Precision | 78.27 | 82.36 | 83.87 | 89.53 |
| Recall | 78.67 | 82.22 | 83.89 | 89.44 |
| F1-Score | 78.29 | 82.23 | 83.84 | 89.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Mao, H.; Xu, G.; Zhang, X.; Zhang, Y. A Rapid Detection Method for Fungal Spores from Greenhouse Crops Based on CMOS Image Sensors and Diffraction Fingerprint Feature Processing. J. Fungi 2022, 8, 374. https://doi.org/10.3390/jof8040374
Wang Y, Mao H, Xu G, Zhang X, Zhang Y. A Rapid Detection Method for Fungal Spores from Greenhouse Crops Based on CMOS Image Sensors and Diffraction Fingerprint Feature Processing. Journal of Fungi. 2022; 8(4):374. https://doi.org/10.3390/jof8040374
Chicago/Turabian StyleWang, Yafei, Hanping Mao, Guilin Xu, Xiaodong Zhang, and Yakun Zhang. 2022. "A Rapid Detection Method for Fungal Spores from Greenhouse Crops Based on CMOS Image Sensors and Diffraction Fingerprint Feature Processing" Journal of Fungi 8, no. 4: 374. https://doi.org/10.3390/jof8040374
APA StyleWang, Y., Mao, H., Xu, G., Zhang, X., & Zhang, Y. (2022). A Rapid Detection Method for Fungal Spores from Greenhouse Crops Based on CMOS Image Sensors and Diffraction Fingerprint Feature Processing. Journal of Fungi, 8(4), 374. https://doi.org/10.3390/jof8040374







