Abstract
Lespedeza davurica (Laxm.) is highly important for reducing soil erosion and maintaining the distinctive natural scenery of semiarid grasslands in northwest China. In this study, a pot experiment was conducted to investigate the effects of drought (20% water-holding capacity) on biomass and its allocation, root characteristics, plant hormones, and soil microbial communities and nutrients after L. davurica was grown in a greenhouse. Drought reduced the total biomass of L. davurica but increased the root:shoot biomass ratio. In addition, drought altered the composition and structure of microbial communities by limiting the mobility of nutrients in non-rhizosphere soils. In particular, drought increased the relative abundances of Basidiomycota, Acidobacteria, Actinobacteria, Coprinellus, Humicola and Rubrobacter, which were closely positively related to the soil organic carbon, pH, available phosphorus, ammonia nitrogen (N) and nitrate N under drought conditions. Furthermore, soil fungi could play a more potentially significant role than that of bacteria in the response of L. davurica to drought. Consequently, our study uncovered the effects of drought on the growth of L. davurica by altering soil microbial communities and/or soil nutrients, thus providing new insights for forage production and natural grassland restoration on the Loess Plateau of China.
1. Introduction
A drought event is a recurring phenomenon in many ecosystems under global climate change, and it is predicted to occur more frequently in the upcoming decades [1,2,3,4]. Drought significantly threatens the structure and function of ecosystems, production and many other aspects of agriculture and human society [5,6,7,8]. Semiarid grasslands occupy vast areas in northwest China and are sensitive to drought [9]. However, water availability is considered a key driver for plant composition and productivity in semiarid grasslands [2,10,11,12,13,14]. In addition, drought is a primary limiting factor for agricultural productivity, plant growth and species distribution in the semiarid regions of northwest China [15]. Moreover, root morphological characteristics have become more important to evaluate the environmental impacts on agriculture in semiarid areas and play a crucial role for the uptake of nutrients and affect the ability of plants to compete in the natural community [16].
In addition, drought and nutrient deficiencies are the essential environmental factors that limit plant growth, interactions and aboveground productivity in semiarid grasslands [8,17]. Drought may have a strong impact on ecosystem processes by affecting the chemical properties of soil and availability of water [18,19,20]. On the one hand, drought has substantial considerable effects on plant growth directly through alterations in the availability of water in the soil and its chemical properties [11,21,22,23]. Alternatively, drought affects the activity, abundance, diversity and community structure of soil microbes [24,25,26,27,28,29,30,31]. It also restrains the uptake of nutrients, which, in turn, indirectly influences the performance of plants [29,32,33]; in addition, soil microbes, as major components of soil ecosystems, which have an amazing amount of variety and abundance [34], participating in many key biogeochemical cycling processes terrestrial ecosystems [35,36,37,38]. Many soil microorganisms benefit plants via reducing pathogen incursions and assisting in the acquisition of nutrients from soil [39]. Drought has impacts on the function of soil microbial communities and their ability to interact with plant roots through a reduction in water availability and carbon and nutrient diffusion in soils, leading to changes in nutrient uptake and enzyme activities [40]. For example, drought can lead to the extinction of less resistant microbial populations, a reduction in microbial biomass, and changes in the composition of microbial communities [41,42,43]. During droughts, soil microbes can also synthesize extracellular polymeric compounds to protect their cells and the local environment [44]. As well, a previous study indicated that drought led to a shift in fungal but not bacterial community composition [45]. The differences in the composition of soil microbial communities, in turn, can impact plant performance [46]. A recent study also reported that drought can alter the soil microbial communities, producing strong legacy effects on the competitive interactions of plants [32]. For example, changes in the soil microbial communities can influence the drought tolerance of subsequent generations of plants [47,48]. However, how drought influences semiarid grassland ecosystems remains poorly understood [49,50].
Lespedeza davurica (Laxm.) is a C3 perennial leguminous subshrub species, which is primarily distributed in temperate regions of the world [51]. It is one of the dominant species in the natural communities of semiarid grasslands in northwest China [52] and is an excellent natural pasture species owing to its high quality and adaptability [51,53]. The severe soil environment in the semiarid areas of northwest China and the decreased abundance of L. davurica caused by grazing [15,54] hamper its ability to reduce water and soil loss and maintain forage production in the Loess Plateau region of northwest China [51,55]. Understanding the growth characteristics of the response of L. davurica to drought would help to complete the knowledge of its mechanisms of drought tolerance and help to understand its potential role in the production of forage and recovery of natural grassland in semiarid regions of northwest China.
In addition, drought is expected to alter plant physiology and metabolic pathways [56], and plants are likely to alter the level of their hormones to adapt to resource-limited environments. For example, salicylic acid (SA) and indole-3-acetic acid (IAA) are interacted with jasmonic acid (JA), thus regulating the adaptation of plant to its surroundings [57] and the involvement of chitinase in the drought tolerance of tomato (Solanum lycopersicum) when subjected to drought [58]. To date, there have been few studies on the response of L. davurica to drought stress. One study found that drought decreased the relative growth rate and relative leaf water content of L. davurica but increased the content of proline and malondialdehyde, while the activities of catalase, superoxide dismutase and peroxidase increased first and then decreased with the time of drought stress [59]. However, whether the hormones (such as IAA, SA, JA) and the activity of chitinase in the L. davurica roots would relate to the growth and development of L. davurica under drought conditions was unknown. Besides, drought decreased the total biomass and the total length, surface, and average diameter of the L. davurica roots [8,53]. Recently, a study indicated that drought clearly decreased the photosynthesis and concentration of N in L. davurica leaves as well [60]. However, studies on L. davurica have primarily focused on growth parameters and have rarely examined the indirect effects of drought on the growth of L. davurica by alteration of the soil nutrients and soil microbial communities.
The purpose of this study was to illuminate the effects of drought on the growth of L. davurica owing to changes in the microbial communities and/or nutrients in soils that were from non-rhizosphere. To achieve this goal, we tested the following hypotheses: (1) drought would increase the allocation of root biomass by L. davurica; (2) drought would reduce the mobility of soil nutrients; and (3) drought would have negative effects on the soil microbial communities.
2. Materials and methods
2.1. Soil Sampling, Processing and Seed Collection
The soils used in the study were collected from the Grassland Research Station of Lanzhou University (LZUGRS), Huan County, Gansu Province, northwestern China (37.12° N, 106.82° E). This region is a typical semiarid monsoon climate at an elevation of 1650 m asl. The mean annual temperature is approximately 7.1 °C, and the average annual precipitation is approximately 360 mm. The soil in this area is classified as Cambisol [61], and water is the main limiting factor for plant growth in this area. Approximately 240 kg of fresh topsoil (10 cm depth) were sampled in April 2018. The soil samples were instantly taken to the laboratory where they were sieved to 5 mm to eliminate large roots and plant residues. All the soils were stored at 4 °C before the greenhouse experiment. The maximum water-holding capacity (WHC) of the soils was determined as previously described [62]. We also measured the soil basic concentrations before the seeds were sown in the greenhouse (Table S1).
Lespedeza davurica is one of the predominant species in this grassland and covers an average of 7% of the soil [52]. It is a C3 perennial herbaceous semi-shrub of the genus Lespedeza of the Leguminosae family and is highly palatable [63]. It also has some excellent characteristics, such as tolerance to drought and barren soil. Lespedeza davurica seeds were collected from plants that were growing on roadsides (1–2 km from LZUGRS) in early October 2017 when they were ripe and taken to the laboratory where they were air-dried, cleaned, and stored at 4 °C.
2.2. Experimental Design
To test whether and how drought modifies the growth of L. davurica by altering the soil microbial communities and/or soil nutrients, we performed a pot experiment in which L. davurica seeds that had been pre-germinated for 3 days were sown approximately 1 cm deep. Two different soil water treatments were established, including normal soil moisture (60% water-holding capacity (WHC)) and drought treatment (20% WHC), The soil water treatments were used to match the soil water content of natural grasslands in the Loess Plateau of China [64,65].
Lespedeza davurica seeds were surface-sterilized with 70% ethanol for 1 min followed by 1% NaClO for 2 min, rinsed three times with ddH2O and germinated on sterile glass beads on 10 May 2018. All the pots were maintained at the normal soil moisture before sowing. On 13 May 2018, the 3-day-old, germinated seeds were carefully sown in plastic pots (16 cm in diameter and 18 cm deep) that contained approximately 4 kg of dry soil. Five seeds were sown in each pot. To reduce the edge effect, the germinated seeds were sown 4 cm from the edge of the pot. For the water treatment per soil water, 12 pots (namely 12 replicates) were established. The greenhouse experiment was duplicated to enable us to independently determine the plant biomass of L. davurica after 16 days of growth to calculate the relative growth ratio (RGR) of the plants. Thus, this trial included two soil water treatments × 12 replicates × 2 duplicates for a total of 48 pots. The pots were randomized on greenhouse tables at 60% relative humidity, a day/night cycle of 16/8 h, and a day/night temperature regime of 21/16 °C. All the pots were watered every two days to maintain the appropriate soil moisture content at 20% and 60% WHC by weighing at 18:00. Seedlings that died during the first week were immediately replaced by seedlings that had grown for the same number of days. No fertilizers were used throughout the growth period of L. davurica. The pots were randomly repositioned weekly to minimize possible variation from the effects of a microclimate in the greenhouse. The plant RGR in g day−1 was calculated as shown in Equation (1) [66]:
where two consecutive harvests at times t1 (plants grown 16 days) and t2 (plants grown 106 days) in this study yielded the plant biomass M1 and M2, respectively.
The first set of pots was destructively sampled after 16 days. The shoots of each pot were clipped at the soil surface, and the roots were carefully rinsed with tap water in a 60-mesh sieve. The shoots along with the roots were then oven-dried at 70 °C for 48 h to a constant weight and weighed.
The second set of pots was collected for destructive sampling at the end of the experiment, which was 106 days after sowing. The plant height was first measured using a flexible ruler. All the plant aboveground parts of each pot were harvested by clipping the shoots at soil surface. Soil samples were obtained destructively in each pot before the roots were harvested and passed through a 2.0-mm sieve to remove large roots and residue. They were then merged uniformly to obtain a pooled soil sample. Soil samples from the pots of each treatment were randomly divided into three portions. The first portion was used to determine the soil water content. The second portion was stored at 4 °C to analyze the soil chemical properties and microbial biomass carbon. The third portion was transported to the laboratory in an icebox and stored at −80 °C until the soil DNA was extracted. This entailed the mixture of four pots of subsamples in each treatment to obtain a pooled soil sample. Thus, three subsamples in each treatment were used to extract DNA. Finally, the L. davurica roots were carefully washed away from the soil with tap water, and the length of primary root was measured with a ruler. A subset of fresh root samples from each replicate was rinsed three times with ddH2O, quickly frozen in liquid nitrogen and stored at −80 °C before the contents of indole-3-acetic acid (IAA), salicylic acid (SA), jasmonic acid (JA) and chitinase were measured in the roots. The shoots and remaining roots were placed in paper bags, respectively, and oven-dried at 70 °C for 48 h to a constant weight and weighed.
2.3. Soil Chemical Properties and Microbial Biomass
The soil organic carbon was determined by the Walkley–Black method, as described by Nelson and Sommer (1982). Total P and total N were determined by adding 1.65 g of catalyst (CuSO4 and K2SO4 at a 1:10 ratio (w/w)) and 5 mL of concentrated sulfuric acid to a 0.5 g soil sample. The sample was then digested for 1.5 h at 420 °C, analyzed on a Smartchem 450 Discrete Auto Analyzer (AMS-Alliance, Rome, Italy). Soil ammonia N (NH4+-N) and nitrate N (NO3−-N) were analyzed on a Smartchem 450 Discrete Auto Analyzer (AMS-Alliance) by KCl extraction. The soil available phosphorus was determined by NaHCO3 extraction—molybdenum antimony colorimetry as previously described [67]. The soil pH was measured as a soil/water mixture at a ratio of 1:2.5 (w/v) on a PHS-3C acidometer (Shanghai Yoke Instrument Co., Shanghai, China). The soil microbial biomass carbon was measured by chloroform fumigation-K2SO4 soil extraction, as previously described [68].
2.4. Plant Hormone Measurements
Approximately 0.2 g samples of fresh roots were used to measure the contents of IAA [69], SA [70], JA [71] and the activity of chitinase [72] in the L. davurica roots.
2.5. DNA Extraction, PCR Amplification and High-Throughput Sequencing
A Magnetic Soil and Stool DNA Kit (Tiangen Biotech, Beijing, China) was used to extract DNA from 0.5 g soil samples, following the manufacturer’s instructions. The primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2R (5′-GCTGCGTTCTTCATCGATGC-3′) were used to amplify the ITS1 region of the fungal rRNA. The V3-V4 regions of 16S rRNA were amplified using the 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) primer set. Each sample was amplified in triplicate using an ABI Gene Amp®9700 PCR system (Applied Biosystems, Foster City, CA, USA) in a 20 μL reaction system. PCR reactions of the DNA from soil fungi were performed in a final volume of 20 μL, in which 10 ng template DNA, 0.8 μL of forward primer (5 μM), 0.8 μL of reverse primer (5 μM), 2 μL buffer (10×), 2 μL dNTPs (2.5 mM), 0.2 μL rTaq polymerase (TaKaRa, Dalian, China), 0.2 μL bovine serum albumin (BSA), and ddH2O had been added to a final volume of 20 μL. The following thermal cycling conditions were used: an initial denaturation at 95 °C for 3 min, followed by 37 cycles of denaturation at 95 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s, and a final extension at 72 °C for 10 min. The PCR reactions of the DNA of soil bacteria were performed in the same final volume of 20 μL with 10 ng template DNA, 0.8 μL of forward primer (5 μM), 0.8 μL of reverse primer (5 μM), 4 μL FastPfu Buffer (5×), 2 μL dNTPs (2.5 mM), 0.4 μL TransStart FastPfu DNA Polymerase (Transgen Biotech Co., Ltd. Beijing, China), 0.2 μL BSA, and ddH2O to 20 μL. The same thermal cycling conditions were used as described above for the fungi, except that the 27 cycles of denaturation were used for the 16S rRNA. After amplification, 3 μL PCR of product was detected on a 2% agarose gel. The PCR products were purified using an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). The samples were sequenced on a 300PE Illumina MiSeq platform (Illumina, Inc., San Diego, CA, USA) by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
2.6. Bioinformatics Analysis
Low-quality raw sequences (average phred quality score Q < 20 or sequence lengths < 200 bp) were removed, and the chimeras were filtered. After removing the chimeras, all the effective tags of all the samples were clustered by Uparse (v. 7.0.1090), and high-quality sequences were clustered into operational taxonomic units (OTUs) with a 97% similarity [73]. The representative sequence for each OTU was screened for further annotation. For each ITS representative sequence, the UNITE database [74] was used on the basis of the BLAST algorithm that was calculated by QIIME (v. 1.9.1) to annotate the taxonomic information. In contrast, the Silva Database [75] was used on each 16S rRNA representative sequence based on the RDP classifier (v. 2.11). All the sequence numbers of each sample were normalized to an even number of sequences per sample (52,778 and 28,260 sequences per sample for ITS and 16S rRNA, respectively) for further analysis. α-Diversity, including the Chao 1 and Shannon indices, and β-diversity were computed with Mothur (v. 1.30.2) and QIIME (v. 1.9.1), respectively. A principal co-ordinates analysis (PCoA) was used to visualize the similarity of fungal and bacterial compositions among sampling groups at the OTU level using Bray–Curtis dissimilarities by the cmdscale function via the “vegan” package in R. The variance inflation factor (VIF value < 10) was analyzed to screen the environmental factors used for the subsequent redundancy analysis (RDA) owing to the limited number of samples. The RDA was used to reveal the relationships between soil microbial communities and soil properties, which was conducted by the rda function via the “vegan” package in R. To further discern the relative importance of drought and the soil property variables for the soil fungal community assembly, a variation partitioning analysis (VPA) was conducted using the “varpart” function of the “vegan” package in R, and the adjusted R2 values were used to determine the proportion of variation in soil fungal community explained by the fitted model [76]. The bioinformatics analysis described above was performed through an in-house pipeline that was built on the online Majorbio I-Sanger Cloud Platform (http://www.majorbio.com, accessed on 7 September 2021).
2.7. Statistical Analysis
All the statistical analyses were performed using R v. 3.5.1 [77]. The normal distribution of data and homogeneity of variance were checked by Shapiro–Wilk and Levene’s tests, respectively. All the data were log-transformed as necessary to meet the standards for normality. Differences between the control and drought were determined by a Tukey’s HSD test (p < 0.05).
3. Results
3.1. Plant Biomass
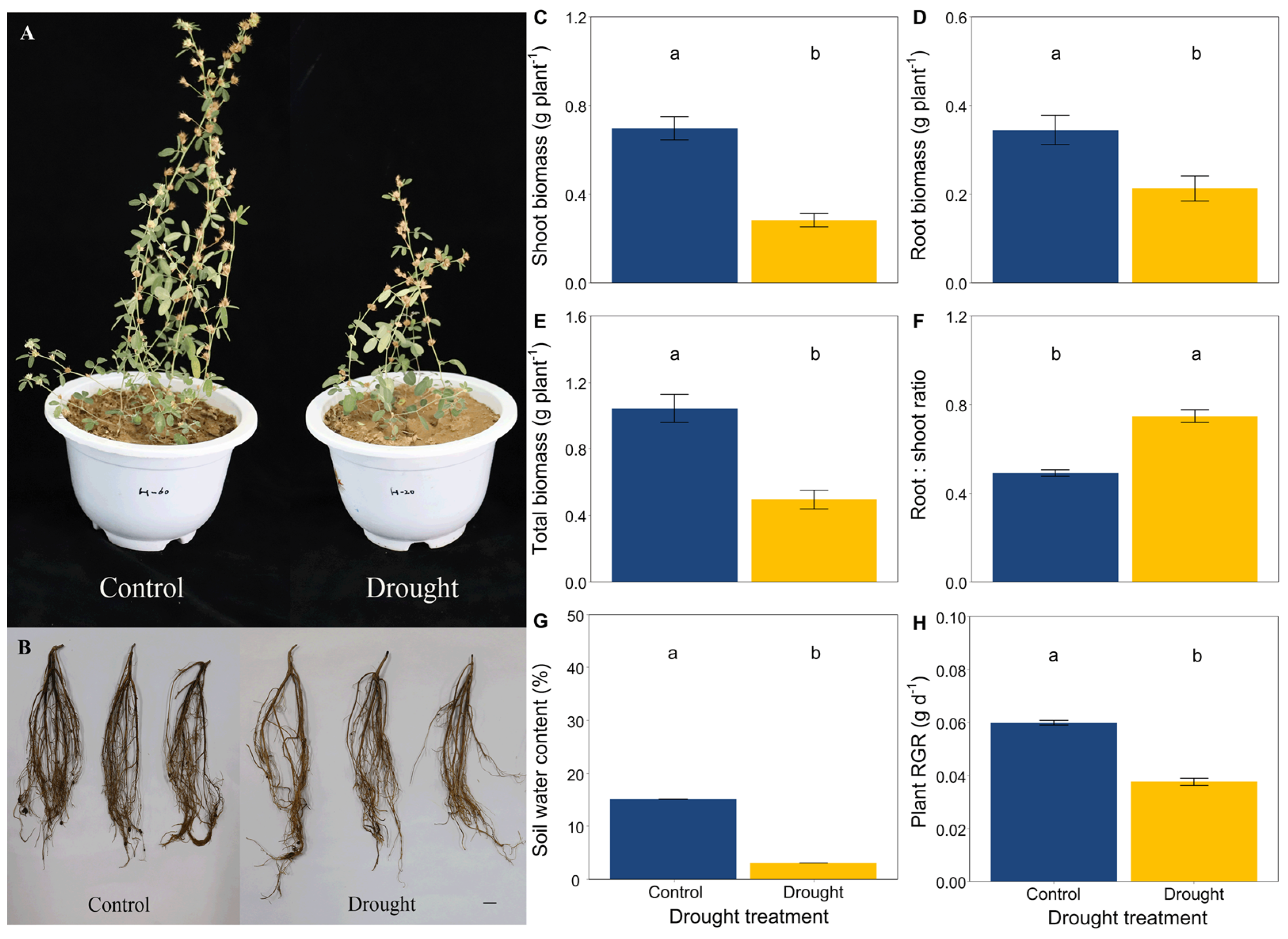
Drought significantly limited the growth and development of L. davurica by the end of this trial (Figure 1A,B). Drought significantly decreased the height of L. davurica but increased the length of its primary root (Figure S1). The soil water content and plant RGR were significantly lower in drought conditions compared with the control (Figure 1G,H). Drought significantly decreased the shoot biomass, root biomass and total biomass of L. davurica but increased the root:shoot ratio (Figure 1C–F).
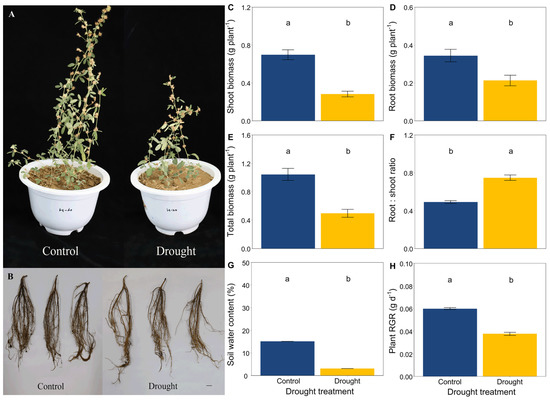
Figure 1.
Effects of drought on (A) aboveground, (B) roots, (C) shoot biomass, (D) root biomass, (E) total biomass, (F) the root:shoot biomass ratio of L. davurica, (G) soil water content, and (H) plant relative growth rate (RGR). Black bar = 2 cm in plot B. Mean ± SE are shown (n = 12). Within each panel, different lowercase letters indicate significant differences at p < 0.05 (Tukey’s HSD).
3.2. Plant Hormones
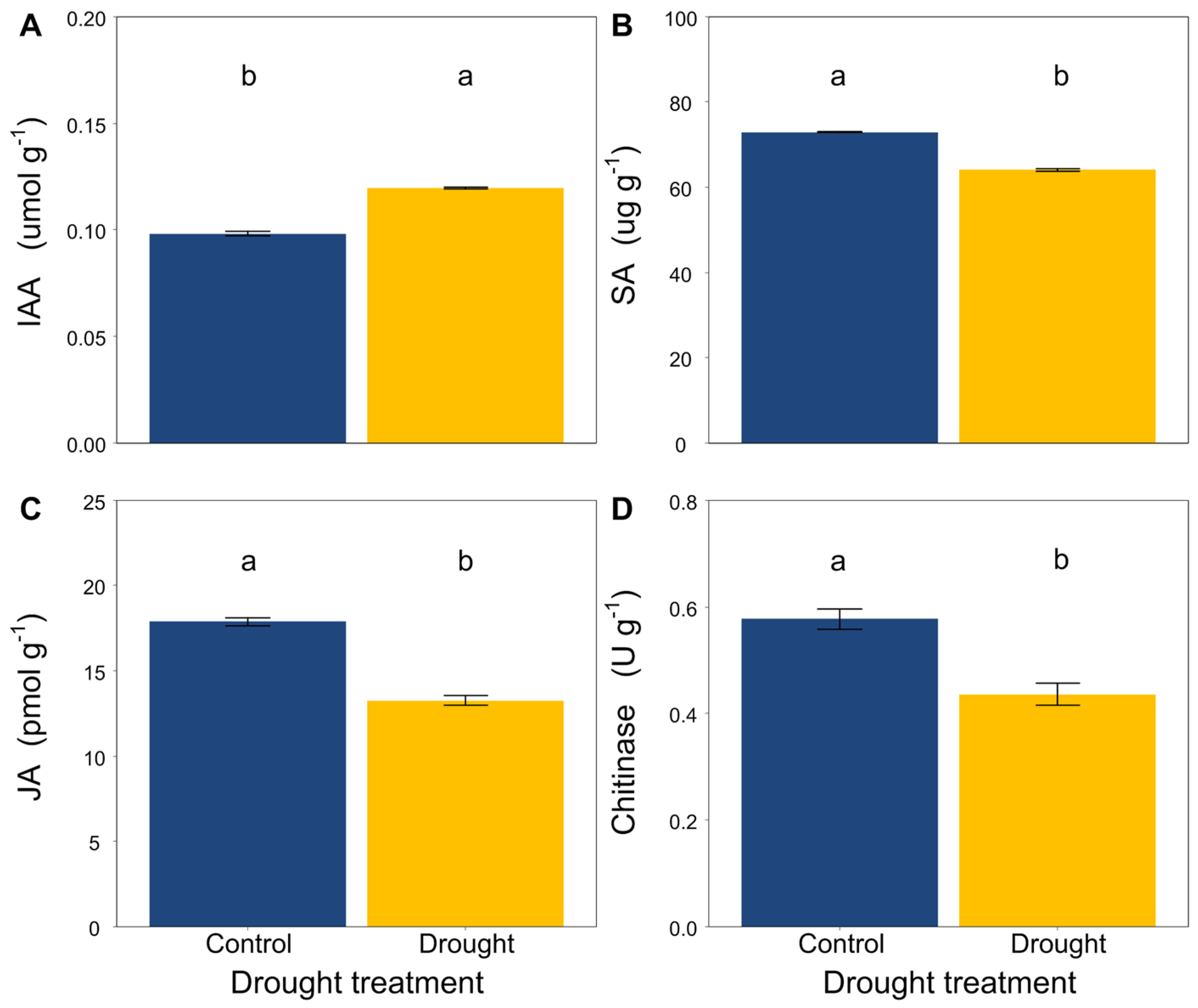
Drought significantly increased the contents of IAA in the L. davurica roots (Figure 2A), while it decreased the contents of SA and JA and the activity of chitinase (Figure 2B–D).
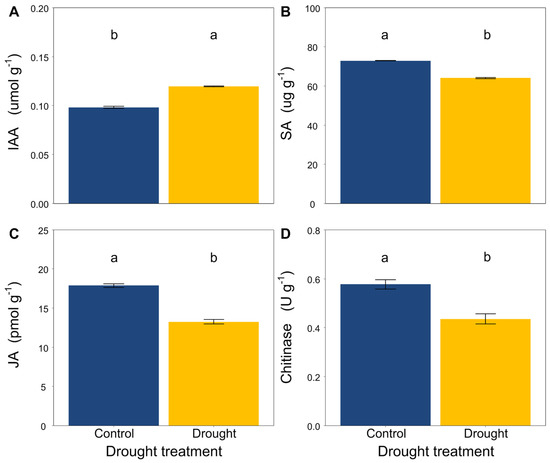
Figure 2.
Effects of drought on (A) IAA, (B) SA, (C) JA content and (D) chitinase activity in roots of L. davurica. Mean ± SE are shown (n = 12). Within each panel, different lowercase letters indicate significant differences at p < 0.05 (Tukey’s HSD).
3.3. Soil Nutrients and Microbial Biomass
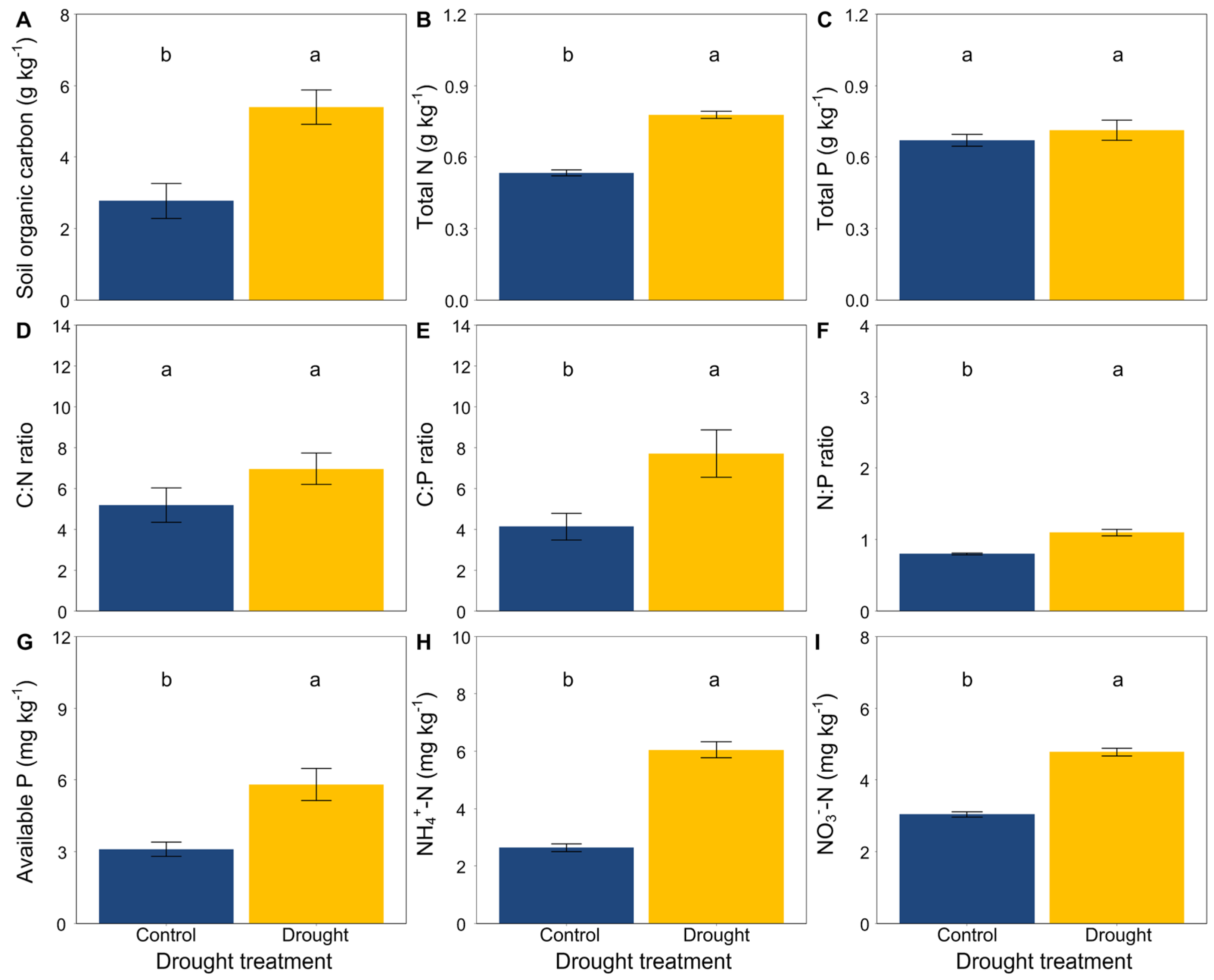
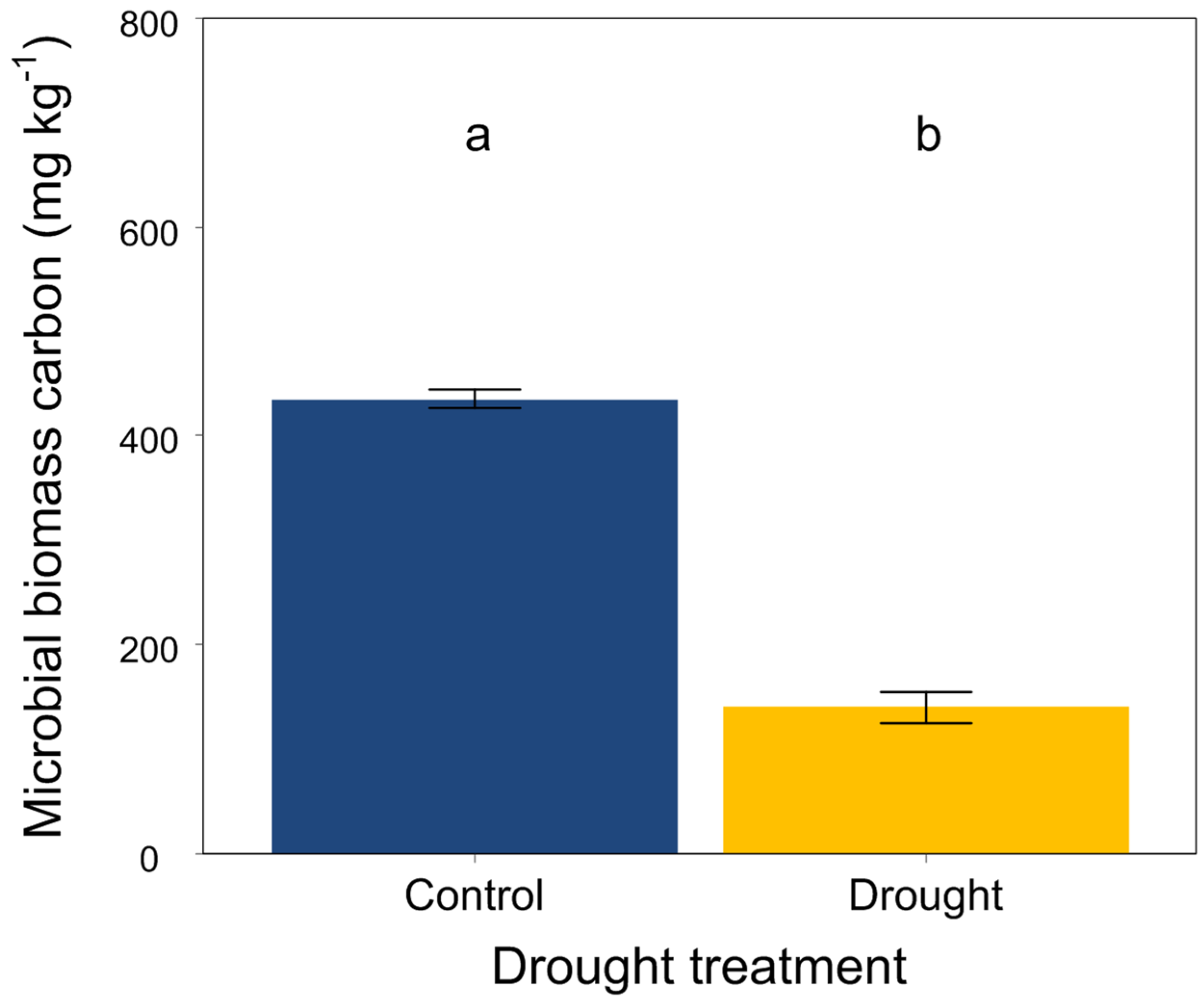
At the end of the experiment, drought significantly increased the soil organic carbon, total N, available P, NH4+-N and NO3−-N (Figure 3A,B,G–I) but did not affect the soil total P (Figure 3C). Moreover, drought had an obvious effect on the soil stoichiometric characteristics. The ratios of C:N, C:P and N:P were significantly higher in drought conditions than that in the control (Figure 3D–F). In addition, drought significantly decreased the soil microbial biomass carbon (Figure 4).
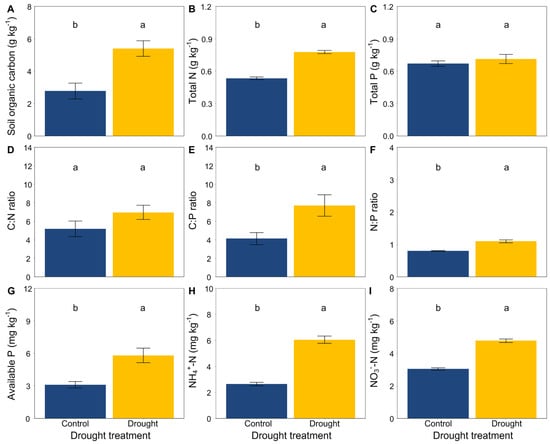
Figure 3.
Effects of drought on (A) soil organic carbon, (B) total N, (C) total P, (D) the C:N ratio, (E) the C:P ratio, (F) the N:P ratio, (G) available P, and (H,I) inorganic N (NH4+-N and NO3−-N). Mean ± SE are shown (n = 12). Within each panel, different lowercase letters indicate significant differences at p < 0.05 (Tukey’s HSD).
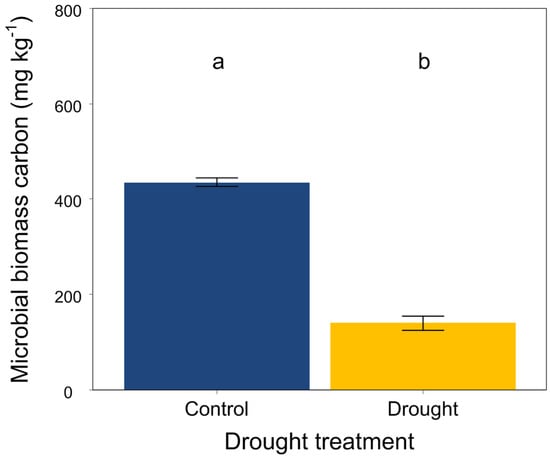
Figure 4.
Effects of drought on soil microbial biomass carbon. Mean ± SE are shown (n = 12). Different lowercase letters indicate significant differences at p < 0.05 (Tukey’s HSD).
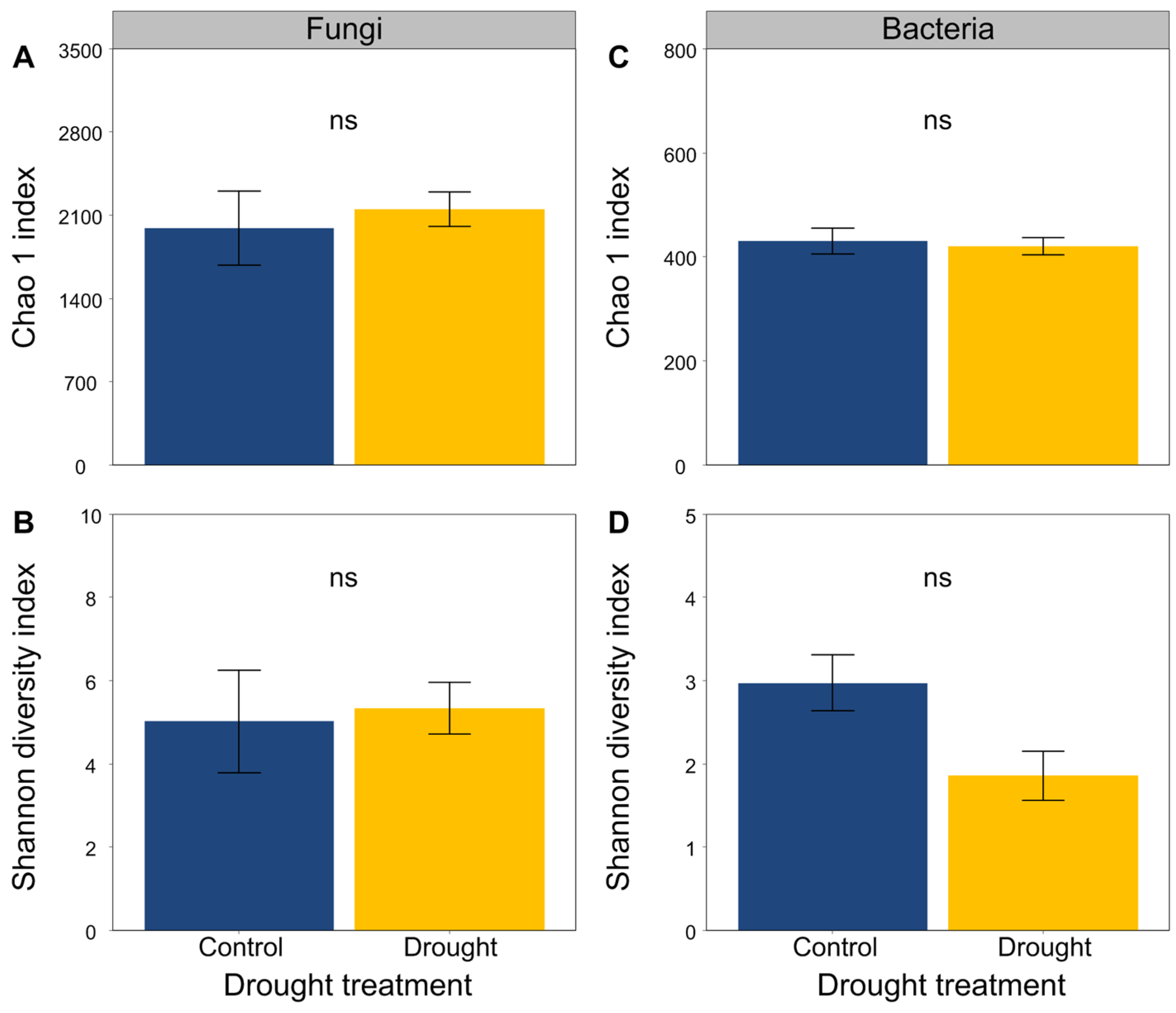
3.4. The Chao1 and Shannon Diversity Indices of Soil Microbial Communities
The soil fungal and bacterial sequences clustered into a total of 772 and 2961 OTUs, respectively. Drought did not obviously alter the Chao1 and Shannon diversity indices of the soil fungal and bacterial communities (Figure 5).
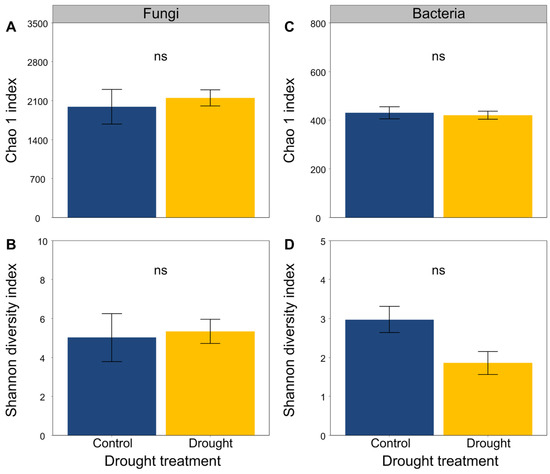
Figure 5.
Effects of drought on (A,B) fungal and (C,D) bacterial community diversity. Mean ± SE are shown (n = 3). ns indicates no significant differences.
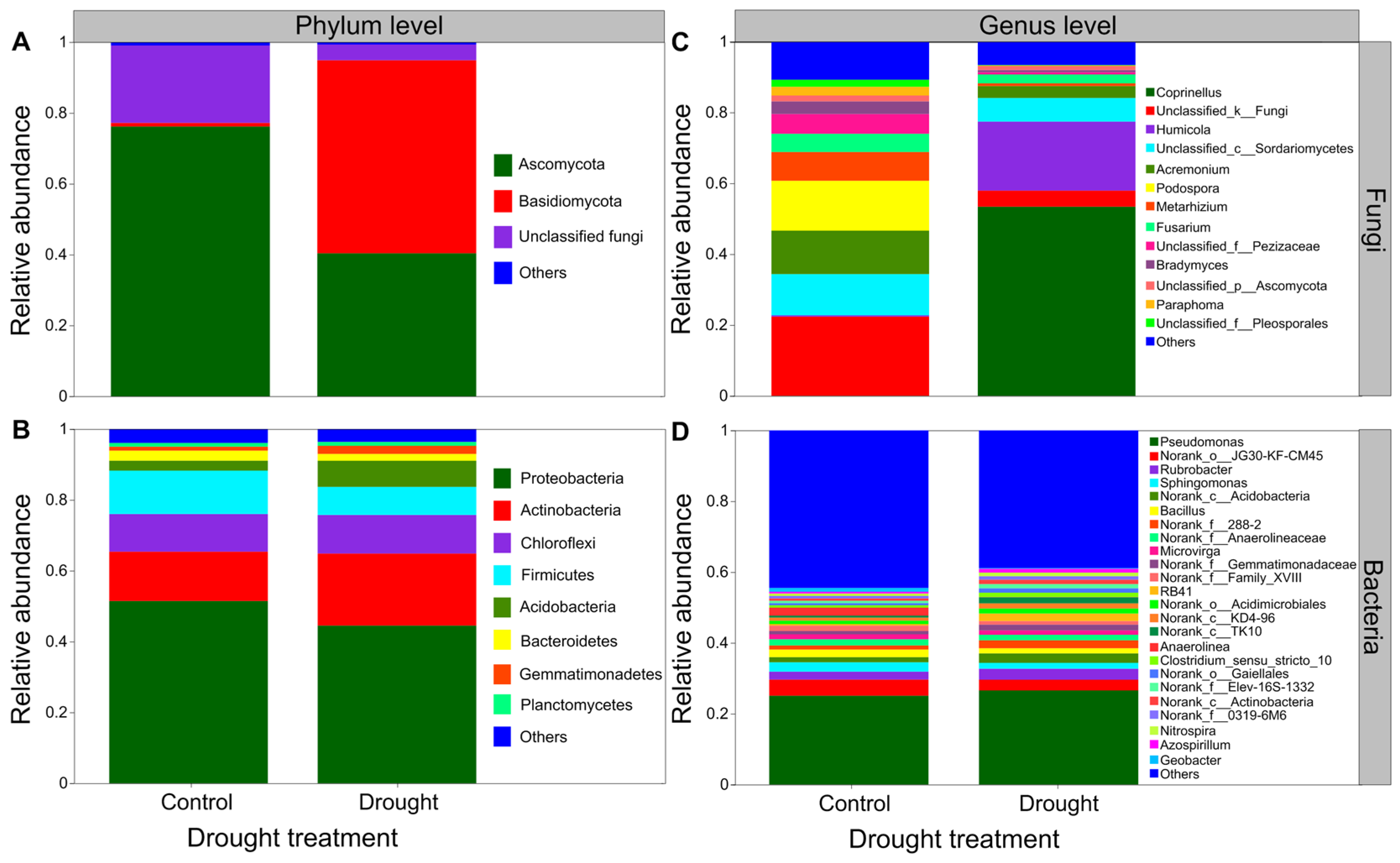
3.5. The Composition of Soil Microbial Communities
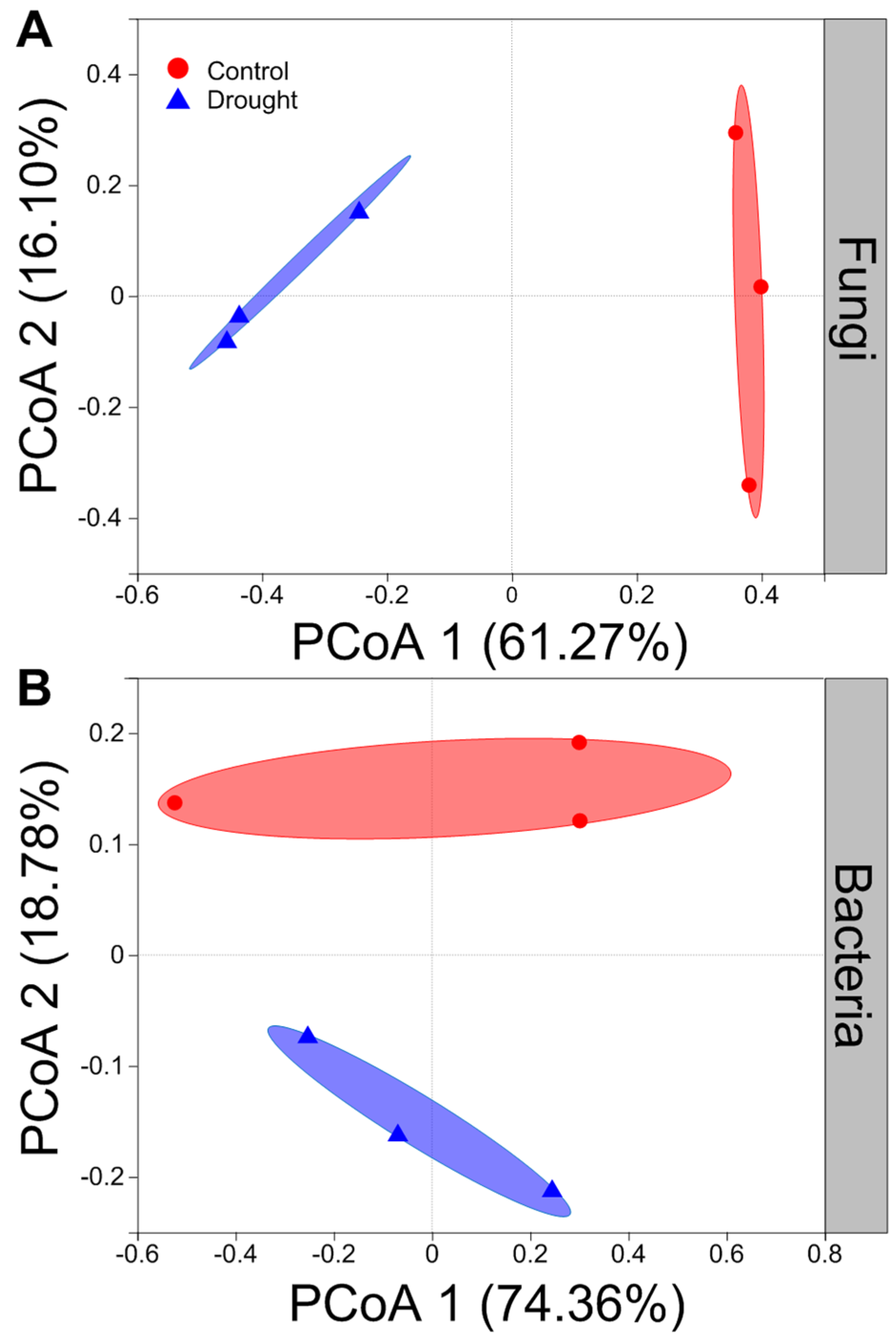
Drought strongly altered the composition of soil fungal communities at both the phylum and/or genus levels (Figure 6A,C). Drought clearly decreased the relative abundance of Ascomycota but increased that of the Basidiomycota (Figure 6A). Interestingly, drought strongly increased the relative abundances of Coprinellus and Humicola but decreased those of Podospora and Acremonium (Figure 6C). In contrast, drought did not clearly alter the composition of soil bacterial communities at both the phylum and/or genus levels (Figure 6B,D). Proteobacteria was the most abundant phylum between the drought and control conditions (Figure 6B). Drought slightly decreased the relative abundances of Proteobacteria and Firmicutes but increased those of Actinobacteria and Acidobacteria to some extent (Figure 6B). Moreover, drought increased the relative abundances of Acidobacteria, Pseudomonas and Rubrobacter to some degree but mildly decreased those of Sphingomonas and Bacillus (Figure 6D). The results of rarefaction curves indicated that the number of reads was enough to detect most of the types of soil bacterial and fungal sequences from the soil samples since the curves arrived at balanced plateaus (Figure S2). In addition, the results of PCoA showed that the composition of soil fungal and bacterial communities was clearly separated between the drought and control (Figure 7).
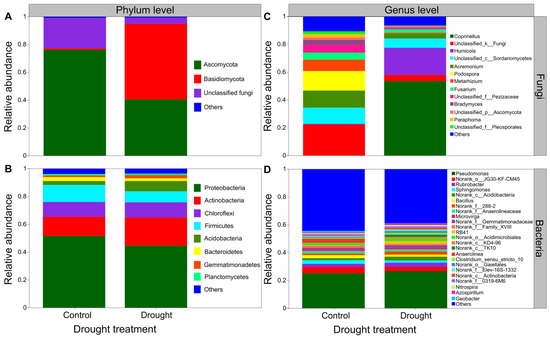
Figure 6.
Effects of drought on the composition of fungal and bacterial community. The relative abundance of (A) soil fungal and (B) bacterial community at the phylum level, (C) soil fungal and (D) bacterial community at the genus level (n = 3).
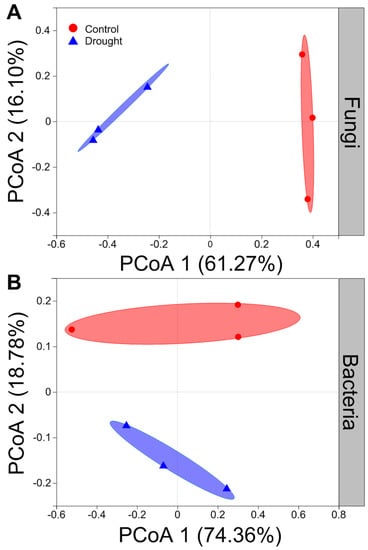
Figure 7.
Effects of drought on the structure of fungal and bacterial community. Principal coordinates analysis (PCoA) of (A) fungal and (B) bacterial community at the operational taxonomic units (OTUs) level based on the Bray–Curtis dissimilarities (n = 3).
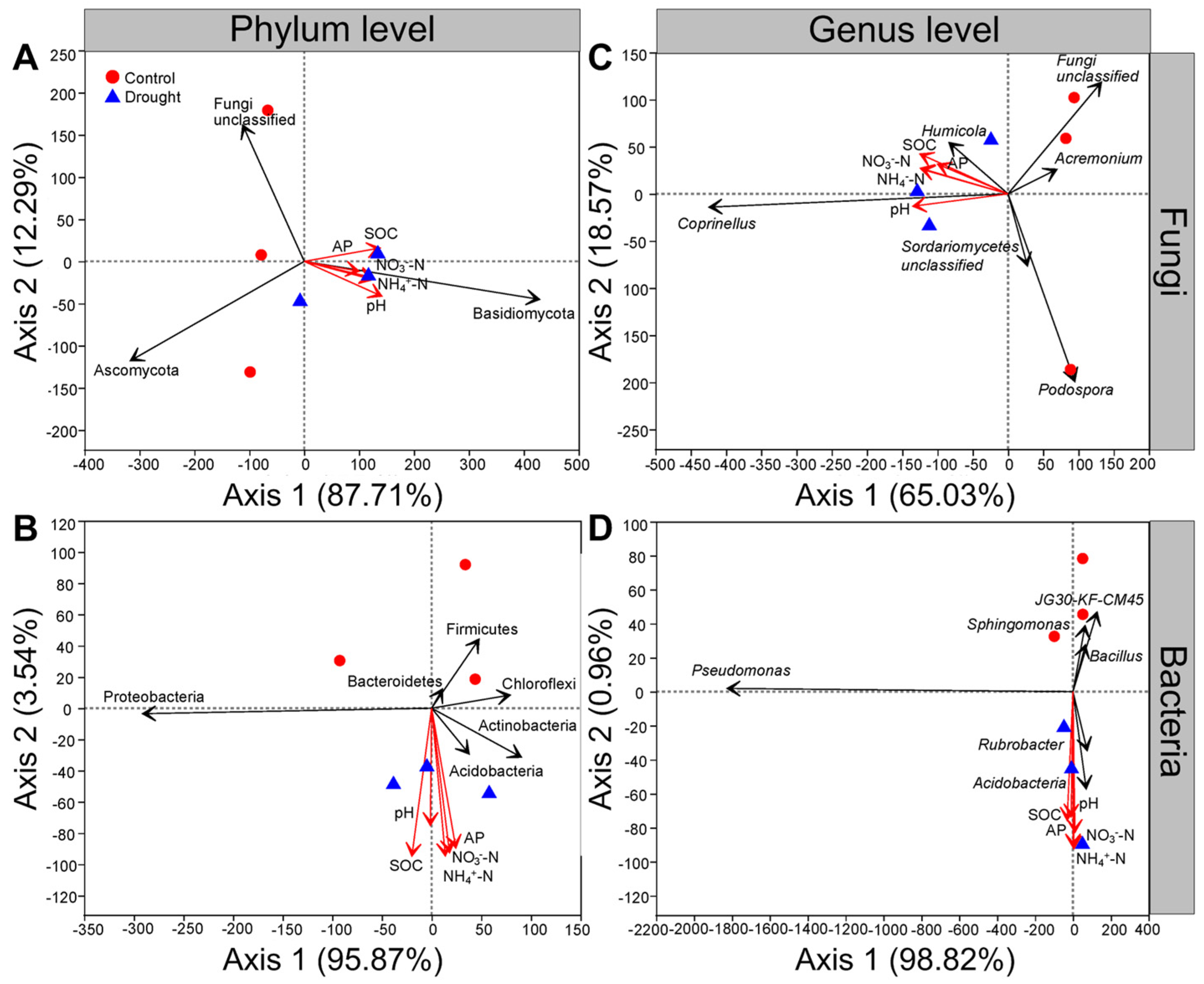
3.6. Relationships between the Soil Microbial Communities and Soil Properties
At the phylum level, the first and second axes of RDA explained 87.71% and 12.29% of the variance for fungal communities, respectively; soil organic carbon, soil pH, available P, NH4+-N and NO3−-N were positively related to the abundance of Basidiomycota but negatively related to that of Ascomycota under drought conditions (Figure 8A). The first and second axes of the RDA explained 95.87% and 3.54% of the variance in bacterial communities, respectively, and the abundance of Proteobacteria was positively related to the soil organic carbon and soil pH but negatively related to the soil available P, NH4+-N and NO3−-N under drought conditions (Figure 8B). Moreover, the soil organic carbon, soil pH, available P, NH4+-N and NO3−-N were positively related to the abundances of Acidobacteria and Actinobacteria but negatively related to those of Firmicutes, Chloroflexi and Bacteroidetes under drought conditions (Figure 8B).
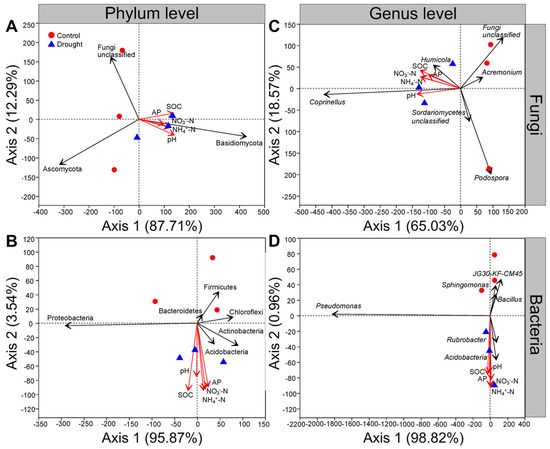
Figure 8.
Effects of drought on the relationships among soil microbial communities and soil properties. Redundancy analysis (RDA) of relative abundance of (A) soil fungal or (B) bacterial community and soil properties at the phylum level, (C) soil fungal or (D) bacterial community and soil properties at the genus level after L. davurica grown in the glasshouse under drought treatment. The black solid line indicates fungal and bacterial phyla or genera, and the red solid line indicates soil properties. Soil properties indicated include soil organic carbon (SOC), available phosphorus (AP), ammonium nitrogen (NH4+-N), nitrate nitrogen (NO3−-N) and pH.
At the genus level, the first and second axes of the RDA explained 65.03% and 18.57% of the variance for fungal communities, respectively; the abundances of Coprinellus and Humicola were positively related to the soil organic carbon, soil pH, available P, NH4+-N and NO3−-N under drought conditions (Figure 8C). In contrast, the abundances of Podospora and Acremonium were negatively related to the soil organic carbon, soil pH, available P, NH4+-N and NO3−-N (Figure 8C). The first and second axes of the RDA explained 98.82% and 0.96% of the variance in bacterial communities, respectively, and the soil organic carbon, soil pH, available P, NH4+-N and NO3−-N were positively related to the abundances of Acidobacteria and Rubrobacter but were negatively related to those of Pseudomonas, Sphingomonas and Bacillus under drought conditions (Figure 8D).
3.7. The Contribution of Drought and Soil Properties to the Variation in Soil Fungal Community
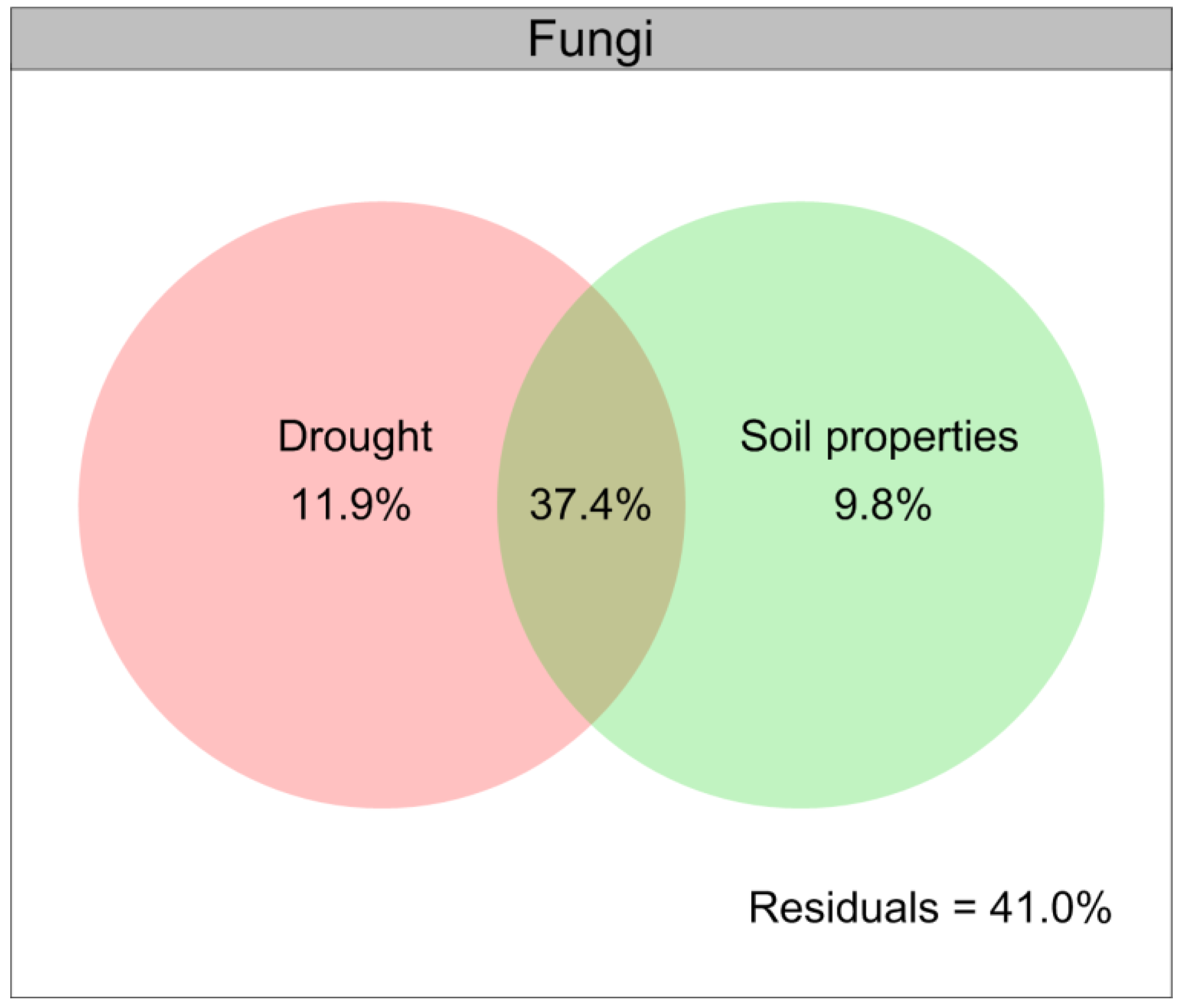
Drought contributed a larger role of variation relative to the composition of the soil fungal community than that for the soil bacterial community (Figure 6), and the soil properties were closely related to the soil microbial communities under drought conditions (Figure 8). We further illustrated the contribution of drought and soil properties to the soil fungal community variation with a modified VPA (Figure 9). The results of VPA indicated that the complete set of all variables together explained 59.1% of the variation in the soil fungal community, and drought (11.9%) clearly contributed a larger proportion of variation of the soil fungal community compared with soil properties (9.8%) (Figure 9).
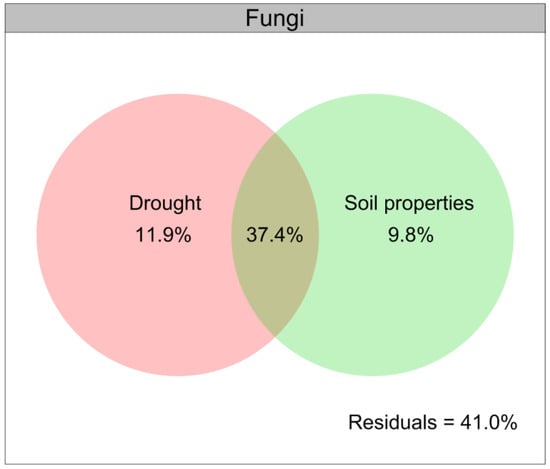
Figure 9.
Variation partitioning analysis (VPA) of the relative contributions of drought and soil properties to variation of fungal community. Soil properties indicated include soil organic carbon, total nitrogen, total phosphorus, available phosphorus, ammonium nitrogen, nitrate nitrogen and pH.
4. Discussion
Water-limited ecosystems are likely to be highly responsive to drought [78]. Water is considered to be one of the key environmental factors that limits the growth of plants in the Loess Plateau of northwest China [8]. Soil microbes play an important role in plant drought stress. Lespedeza davurica is one of the predominant species in natural semiarid grasslands in northwest China [52]. Therefore, we examined how drought alters the abiotic properties and microbial communities of soil and mediates the growth of L. davurica.
In this study, drought indeed strongly altered the growth and development of L. davurica, including its biomass and the morphological characteristics of shoot and root. We found that drought significantly reduced the RGR, shoot biomass, root biomass and total biomass of L. davurica, which is consistent with previous findings that drought was the primary limiting factor for the production of biomass by L. davurica [53]. Under drought conditions, roots act as sensors and are responsible for resource uptake and storage, and they grow faster than leaves [79,80]. Consistent with previous research, we found that drought decreased the plant height but increased the root:shoot ratio and length of primary roots of L. davurica, supporting our first hypothesis that drought would increase the root biomass allocation of L. davurica. Root morphological traits are important for plants to manage in an environment that has limited water and/or nutrients, such as those in arid and semiarid ecosystems [53,81]. Numerous studies have shown that drought increased the root mass ratio, proportion of thin roots, root length and surface area and the numbers of root hairs [81,82,83,84,85], which help the plant to acquire nutrients from severe environments. A previous study showed that the root average diameter values of L. davurica are significantly affected by water levels, efficiently compete for the limited water resources and increase the acquisition of nutrients under drought conditions [53]. In this study, our findings indicated that L. davurica may absorb more water and soil nutrients under drought conditions by shifting its biomass allocation and morphological characteristics, which is consistent with the findings of previous studies [8,53]. However, the root vertical development could be largely constrained by the pot size as well.
Since drought limits the uptake of nutrients from the soil nutrients [86], and long-term drought is expected to alter plant physiology and metabolic pathways [56], plants are likely to alter the level of their hormones to adapt to resource-limited environments. Plant hormones play significant functions in the establishment of signaling networks to regulate stress-related responses and plant development in response to drought. Therefore, we tested whether hormones and chitinase modified the growth and development of L. davurica under drought conditions. Numerous reports have suggested that JA and SA have an important role in the response of plants to drought. For example, a prolonged water deficit can reduce the contents of JA and SA of common sage (Salvia officinalis) during leaf senescence [87]. Long-term drought decreased the contents of JA and SA in banana leaves (Musa spp.) [88]. Drought decreased the contents of JA and SA of two contrasting genotypes of Catalpa bungee [89]. Long-term drought decreased the contents of JA and SA in the roots of tea (Camellia sinensis) [90]. A recent study found that drought also decreased the contents of JA and SA in gray-leaved Cistus (Cistus albidus) seedlings [91]. In the study, we found that drought decreased the content of JA in the L. davurica roots, which was consistent with a previous study that the concentrations of endogenous JA first increased rapidly following drought stress and then decreased to normal levels if the stress periods were prolonged [92,93,94]. Another study also reported that severe drought slightly decreased the JA content of Arabidopsis thaliana [95]. In addition, a previous study also reported that SA, auxin, such as IAA, and other plant hormones interacted with JA, thus regulating the adaptation of plant to its surroundings [57]. Thus, we expected that the SA content in the L. davurica roots could decrease under drought as well, which was indeed confirmed by our results. Recently, a study found that prolonged drought decreased the SA content in Brassica napus leaves [96]. In contrast, we found that drought increased the contents of endogenous IAA in L. davurica roots, indicating that IAA possibly enhanced the drought tolerance of L. davurica. Our results were also consistent with previous findings that the application of exogenous IAA could weaken the negative effects caused by drought, and thus improved the growth of barley (Hordeum vulgare) [97]. In addition, we found that drought decreased the activity of chitinase in L. davurica roots, which was consistent with previous findings that drought decreased the activities of enzymes because of its negative effects on soil properties [98]. Similar results were reported that indicated the involvement of chitinase in the drought tolerance of tomato (Solanum lycopersicum) when subjected to drought [58].
Drought can typically induce a reduction in the mobility of soil nutrients, leading to some substantially considerable effects on plant performance [99,100,101]. Consistent with previous findings that drought has negative effects on soil properties and increased the concentrations of soil nutrients [33,98], we found that the concentrations of soil organic carbon, total N, available P, NH4+-N and NO3−-N were higher in drought conditions compared with the control, supporting our second hypothesis that drought would reduce the mobility of soil nutrients. In contrast, a recent study reported that drought decreased the contents of soil organic carbon, total N, NH4+-N and NO3−-N in topsoil (0–10 cm) compared with ambient water in alpine grassland [102]. The difference in this study could by owing to use of a pot experiment in a greenhouse that utilized poor quality soil from a semiarid grassland, which could have resulted in the depletion of soil nutrients.
Furthermore, drought can alter the activity, abundance, and community structure and composition of soil microbes by altering the availability of soil water and nutrients [15,24,25,27,30,103,104], which, in turn, may influence plant performance [29,32,33]. Indeed, our results indicated that drought altered the structure and composition of soil fungal and bacterial communities. Soil microbes are more likely to aggregate to avoid death or dehydration under drought conditions [105]. Consistent with previous findings that drought reduced the diffusion of substrates, microbial activities and biomass [106], we found that drought significantly decreased the soil microbial biomass carbon, because the microbial population of soil would decline considerably if the soil water content was reduced to less than a particular level [14,107]. There is no consensus about the response of soil microbial diversity to drought, although many studies have focused on the responses of soil microbes to drought [103,108,109]. In this study, we found that drought altered the structure and composition of soil microbial communities, partially supporting our third hypothesis that drought would have negative effects on the soil microbial communities. Drought can reduce the mobility of soil nutrients and limit the reproduction of soil microbes by reducing the supply of substrates and the availability of soil water to them [110,111] and decreasing soil microbial diversity [112,113,114,115]. In addition, several meta-analyses indicated that drought had a negative effect on soil microbial diversity [105,116,117,118,119]. In this study, we found that drought tended to decrease the Chao1 and Shannon diversity indices of soil bacteria but increase them in soil fungi to some extent, suggesting that drought could have a negative effect on the diversity of soil fungal communities [119]. Drought increases the fungal richness but does not alter the bacterial richness, for the reason that fungi are thought to have a greater ability to cope with drought than bacteria, owing to their capability to cumulate osmoregulatory solutes to protect their filamentous structure and metabolism [113,120,121]. This was partly consistent with our results that drought strongly altered the composition of soil fungal communities but did not significantly affect the composition of soil bacterial communities. Recently, a study also found that drought was related with an increase in the Gram-positive:Gram-negative bacteria ratio [101]. Soil fungi may remain active at a very low content of soil water compared with soil bacteria [107,116]. Since fungi can produce a large mass of hyphae that improves the transfer of moisture across long distances [114], they are likely to be more tolerant to drought compared with bacteria. Moreover, the fungi:bacteria ratio has a positive relationship with soil water content, and fungi may easily have been water-restricted compared to bacteria [45,122,123]. Besides, specialized microbes show some different responses to global change factors effects compared with fungi and bacteria [118]. For example, specialized microbes may play some key soil functions (such as ammonia oxidizer, methanotrophic, diazotrophic, phosphorus mineralizer, etc.), which are vulnerable to diversity loss owing to their lower richness [124]. The root system may improve diversity of microbes, most of which (e.g., Pseudomonas, Bacillus) can synthesize phytohormones, hydrolase enzymes or siderophores, helping plants to cope with abiotic stress (such as drought). In contrast, our results indicated that drought mildly decreased the relative abundances of Bacillus. Plant resistance was improved by the root-associated bacterial microbiome to drought by water stress-induced promotion capability as well [125]. Mycorrhizal fungi also can help plants for water and nutrients uptake through extraradical hyphae [126]. However, in this study, we did not find changes in these specialized microbes, possibly because the soil samples used for high-throughput sequencing were from non-rhizosphere. Furthermore, fungi tend to have slow turnover rates and utilize nitrogen-poor substrates, while bacteria are characterized by high nutrient requirements and usually dominate in soil habitats that contain high-quality substrates, such as those with lower C:P and C:N ratios [36,127,128]. In this study, we found that drought increased the C:P and C:N ratios in soils, which would be likely to favor fungal growth compared with that of bacteria. In addition, one study indicated that soil fungi are more sensitive to the wetting–drying cycle than soil bacteria under drier conditions [129]. However, another study concluded that there was no difference [130].
Although drought did not significantly affect the soil microbial diversity in this study, we found that drought clearly altered the composition of soil microbes. We found that the composition of soil fungal communities was more influenced by drought than bacteria, regardless of the phylum or genus level. A previous study suggested that Bacteroidetes and Proteobacteria are sensitive to drought, while Actinobacteria and Firmicutes are resistant to drought [131], which was not entirely consistent with our results. We found that drought decreased the relative abundances of Ascomycota, Proteobacteria and Firmicutes but increased those of Basidiomycota, Actinobacteria and Acidobacteria at the phylum level, which is consistent with previous findings that drought increased the relative abundance of Actinobacteria [132]. This is because soil microbes may show quite different responses to drought based on their adaptation to specific environmental conditions [42,133]. In addition, we found that drought strongly increased the relative abundances of Coprinellus and Humicola but decreased those of Podospora and Acremonium at the genus level. Moreover, we found that drought increased the relative abundances of Acidobacteria, Pseudomonas and Rubrobacter to some degree but mildly decreased those of Sphingomonas and Bacillus. Taken together, these results imply that the adaptation of key soil fungi and/or bacteria are important for plants to manage drought [132].
Moreover, we found that drought indirectly affected the diversity and composition of soil microbial communities by altering the soil physical and chemical factors, particularly the soil organic carbon, soil pH, available P, NH4+-N and NO3−-N, which were consistent with previous findings that the availability of water affected the soil microbial communities by altering the availability of soil pH and nutrients [118,134,135,136,137]. In particular, we found that the abundances of Basidiomycota, Proteobacteria, Acidobacteria and Actinobacteria were closely positively related to the soil organic carbon and soil pH under drought conditions, while the soil available P, NH4+-N and NO3−-N were closely positively related to those of Ascomycota, Chloroflexi, Firmicutes and Bacteroidetes. Given that the soil fungal community could potentially play an important role in mediating the growth of L. davurica in response to drought, the results of VPA further indicated that drought contributed a larger proportion of variation to the soil fungal β-diversity compared with the soil properties, thus indicating a stronger effect of drought in driving the soil fungal community.
5. Conclusions
This study first revealed the effects of drought on L. davurica owing to changes in the soil microbial communities and the availability of nutrients. Our findings demonstrated that drought considerably altered the performance and endogenous hormones of L. davurica, soil physicochemical properties, soil microbial biomass, and the composition of soil fungal and bacterial communities. The abundances of Coprinellus had the strongest positive relationship with the soil organic carbon, soil pH, available P, NH4+-N and NO3−-N under drought conditions, and possibly mediating the response of L. davurica growth to drought. Drought clearly contributes a larger proportion of variation relative to soil properties to soil fungal communities. An additional benefit of this study is its analysis of forage production and natural grassland vegetation recovery in semiarid regions of the Loess Plateau of northwest China. However, future experiments under natural conditions are necessary to fully understand how drought alters the abiotic and biotic properties of soil to regulate the growth of L. davurica.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8040384/s1, Figure S1: Effects of drought on (A) plant height and (B) length of primary root of Lespedeza davurica; Figure S2. Rarefaction curves of OTUs for (A) fungal and (B) bacterial communities. Table S1: Basic concentrations of soil organic carbon (SOC), total nitrogen (TN), total phosphorus (TP), available phosphorus (AP), inorganic nitrogen (NH4+-N and NO3−-N) and pH before the sowing in glasshouse.
Author Contributions
D.D., T.C. and Z.N. designed the experiments. D.D., F.J., W.L., Z.T., N.W. and X.F. carried out the experiments. D.D., F.J. and T.C. analyzed the experimental results. D.D. and T.C. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Nature Science Foundation of China (31901378), the Fundamental Research Funds for the Central Universities (lzujbky-2020-cd01), the Start-up Funds of Introduced Talent in Lanzhou University (561119207), the 111 Program (B12002) and the Science and Technology Program of Gansu Province (19ZD2NA002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The fungal and bacterial raw DNA sequences used in this study have been deposited in the Sequence Read Achieve (SRA) of the NCBI database under the accession number PRJNA755514 for open access.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Orlowsky, B.; Seneviratne, S.I. Global changes in extreme events: Regional and seasonal dimension. Clim. Chang. 2012, 110, 669–696. [Google Scholar] [CrossRef]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- IPCC. Summary for policy makers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group 1 to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 2–21. [Google Scholar]
- Mullet, J.E.; Whitsitt, M.S. Plant cellular responses to water deficit. Plant Growth Regul. 1996, 20, 119–124. [Google Scholar] [CrossRef]
- Rowell, D.P.; Jones, R.G. Causes and uncertainty of future summer drying over Europe. Clim. Dyn. 2006, 27, 281–299. [Google Scholar] [CrossRef]
- Vergni, L.; Todisco, F. Spatio-temporal variability of precipitation, temperature and agricultural drought indices in Central Italy. Agric. For. Meteorol. 2011, 151, 301–313. [Google Scholar] [CrossRef]
- Bray, E.A. Plant responses to water deficit. Trends Plant Sci. 1997, 2, 48–54. [Google Scholar] [CrossRef]
- Christensen, L.; Coughenour, M.B.; Ellis, J.E.; Chen, Z.Z. Vulnerability of the Asian typical steppe to grazing and climate change. Clim. Chang. 2004, 63, 351–368. [Google Scholar] [CrossRef]
- Schwalm, C.R.; Williams, C.A.; Schaefer, K.; Arneth, A.; Bonal, D.; Buchmann, N.; Chen, J.; Law, B.E.; Lindroth, A.; Luyssaert, S.; et al. Assimilation exceeds respiration sensitivity to drought: A FLUXNET synthesis. Glob. Chang. Biol. 2010, 16, 657–670. [Google Scholar] [CrossRef]
- Kardol, P.; Cregger, M.A.; Campany, C.E.; Classen, A.T. Soil ecosystem functioning under climate change: Plant species and community effects. Ecology 2010, 91, 767–781. [Google Scholar] [CrossRef]
- Cleland, E.E.; Collins, S.L.; Dickson, T.L.; Farrer, E.C.; Gross, K.L.; Gherardi, L.A.; Hallett, L.M.; Hobbs, R.J.; Hsu, J.S.; Turnbull, L.; et al. Sensitivity of grassland plant community composition to spatial vs. temporal variation in precipitation. Ecology 2013, 94, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; O’Brien, M.J.; Vogel, A.; Scherer-Lorenzen, M.; Eisenhauer, N.; Schmid, B.; Weigelt, A. Plant diversity maintains long-term ecosystem productivity under frequent drought by increasing short-term variation. Ecology 2017, 98, 2952–2961. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-R.; Delgado-Baquerizo, M.; Wang, J.-T.; Hu, H.-W.; Yang, Z.; He, J.-Z. New insights into the role of microbial community composition in driving soil respiration rates. Soil Biol. Biochem. 2018, 118, 35–41. [Google Scholar] [CrossRef]
- Shan, L.; Chen, G.L.; Xu, T. Theory and Practice of Dryland Farming on the Loess Plateau; Chinese Science Press: Beijing, China, 1993. [Google Scholar]
- Campbell, C.A.; de Jong, R. Root-to-straw ratios—Influence of moisture and rate of N fertilizer. Can. J. Soil Sci. 2001, 81, 39–43. [Google Scholar] [CrossRef]
- Bat-Oyun, T.; Shinoda, M.; Cheng, Y.; Purevdorj, Y. Effects of grazing and precipitation variability on vegetation dynamics in a Mongolian dry steppe. J. Plant Ecol. 2016, 9, 508–519. [Google Scholar] [CrossRef]
- Ruppert, J.C.; Harmoney, K.; Henkin, Z.; Snyman, H.A.; Sternberg, M.; Willms, W.; Linstaedter, A. Quantifying drylands’ drought resistance and recovery: The importance of drought intensity, dominant life history and grazing regime. Glob. Chang. Biol. 2015, 21, 1258–1270. [Google Scholar] [CrossRef]
- Lucci, G.M. Pastures and drought: A review of processes and implications for nitrogen and phosphorus cycling in grassland systems. Soil Res. 2019, 57, 101–112. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Langan, L.; Linstaedter, A.; Martens, C.; Gaillard, C.; Ruppert, J.C.; Higgins, S.I.; Mudongo, E.I.; Scheiter, S. Grazing and aridity reduce perennial grass abundance in semi-arid rangelands—Insights from a trait-based dynamic vegetation model. Ecol. Model. 2019, 395, 11–22. [Google Scholar] [CrossRef]
- Swemmer, A.M.; Knapp, A.K.; Snyman, H.A. Intra-seasonal precipitation patterns and above-ground productivity in three perennial grasslands. J. Ecol. 2007, 95, 780–788. [Google Scholar] [CrossRef]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; Penuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Chang. Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Knapp, A.K.; Carroll, C.J.W.; Denton, E.M.; La Pierre, K.J.; Collins, S.L.; Smith, M.D. Differential sensitivity to regional-scale drought in six central US grasslands. Oecologia 2015, 177, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Knorr, K.-H.; Oosterwoud, M.R.; Blodau, C. Experimental drought alters rates of soil respiration and methanogenesis but not carbon exchange in soil of a temperate fen. Soil Biol. Biochem. 2008, 40, 1781–1791. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, S.-H.; Freeman, C.; Fenner, N.; Kang, H. Comparative analysis of soil microbial communities and their responses to the short-term drought in bog, fen, and riparian wetlands. Soil Biol. Biochem. 2008, 40, 2874–2880. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Kivlin, S.N.; Rocca, J.D.; Huguet, V.; Thomsen, M.A.; Suttle, K.B. Fungal community responses to precipitation. Glob. Chang. Biol. 2011, 17, 1637–1645. [Google Scholar] [CrossRef]
- Ma, K.; Conrad, R.; Lu, Y. Dry/Wet Cycles Change the Activity and Population Dynamics of Methanotrophs in Rice Field Soil. Appl. Environ. Microbiol. 2013, 79, 4932–4939. [Google Scholar] [CrossRef]
- de Vries, F.T.; Shade, A. Controls on soil microbial community stability under climate change. Front. Microbiol. 2013, 4, 265. [Google Scholar] [CrossRef]
- Meisner, A.; De Deyn, G.B.; de Boer, W.; van der Putten, W.H. Soil biotic legacy effects of extreme weather events influence plant invasiveness. Proc. Natl. Acad. Sci. USA 2013, 110, 9835–9838. [Google Scholar] [CrossRef]
- Potter, C.; Freeman, C.; Golyshin, P.N.; Ackermann, G.; Fenner, N.; Mcdonald, J.E.; Ehbair, A.; Jones, T.G.; Murphy, L.M.; Creer, S. Subtle shifts in microbial communities occur alongside the release of carbon induced by drought and rewetting in contrasting peatland ecosystems. Sci. Rep. 2017, 7, 11314. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Kaisermann, A.; de Vries, F.T.; Griffiths, R.I.; Bardgett, R.D. Legacy effects of drought on plant-soil feedbacks and plant-plant interactions. New Phytol. 2017, 215, 1413–1424. [Google Scholar] [CrossRef]
- Fry, E.L.; Johnson, G.N.; Hall, A.L.; Pritchard, W.J.; Bullock, J.M.; Bardgett, R.D. Drought neutralises plant-soil feedback of two mesic grassland forbs. Oecologia 2018, 186, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Locey, K.J.; Lennon, J.T. Scaling laws predict global microbial diversity. Proc. Natl. Acad. Sci. USA 2016, 113, 5970–5975. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Lett. 2009, 12, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Putten, W.D. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Franz, B.S.; Widmer, F.; Van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Schimel, J. Microbial ecology: Linking omics to biogeochemistry. Nat. Microbiol. 2016, 1, 15028. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Henry, H.A. Reprint of “Soil extracellular enzyme dynamics in a changing climate”. Soil Biol. Biochem. 2013, 56, 53–59. [Google Scholar] [CrossRef]
- Schimel, J.P.; Gulledge, J.M.; Clein-Curley, J.S.; Lindstrom, J.E.; Braddock, J.F. Moisture effects on microbial activity and community structure in decomposing birch litter in the Alaskan taiga. Soil Biol. Biochem. 1999, 31, 831–838. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Influence of drying–rewetting frequency on soil bacterial community structure. Microb. Ecol. 2003, 45, 63–71. [Google Scholar] [CrossRef]
- Evans, S.E.; Wallenstein, M.D. Climate change alters ecological strategies of soil bacteria. Ecol. Lett. 2014, 17, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Bérard, A.; Sassi, M.B.; Kaisermann, A.; Renault, P. Soil microbial community responses to heat wave components: Drought and high temperature. Clim. Res. 2015, 66, 243–264. [Google Scholar] [CrossRef]
- Cregger, M.A.; Schadt, C.W.; McDowell, N.G.; Pockman, W.T.; Classen, A.T. Response of the soil microbial community to changes in precipitation in a semiarid ecosystem. Appl. Environ. Microbiol. 2012, 78, 8587–8594. [Google Scholar] [CrossRef] [PubMed]
- Van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A. Plant–soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Lau, J.A.; Lennon, J.T. Evolutionary ecology of plant-microbe interactions: Soil microbial structure alters selection on plant traits. New Phytol. 2011, 192, 215–224. [Google Scholar] [CrossRef]
- Lau, J.A.; Lennon, J.T. Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc. Natl. Acad. Sci. USA 2012, 109, 14058–14062. [Google Scholar] [CrossRef]
- He, Q.; Bertness, M.D. Extreme stresses, niches, and positive species interactions along stress gradients. Ecology 2014, 95, 1437–1443. [Google Scholar] [CrossRef]
- Soliveres, S.; Smit, C.; Maestre, F.T. Moving forward on facilitation research: Response to changing environments and effects on the diversity, functioning and evolution of plant communities. Biol. Rev. 2015, 90, 297–313. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Ding, S. Characteristic on the steppe of Bothriochloa ischaemum in Loess Plateau and its geographical significance. Acta Bot. Boreali-Occident. Sin. 1997, 17, 88–93. [Google Scholar]
- Chen, T.; Christensen, M.; Nan, Z.; Hou, F. The effects of different intensities of long-term grazing on the direction and strength of plant-soil feedback in a semiarid grassland of Northwest China. Plant Soil 2017, 413, 303–317. [Google Scholar] [CrossRef]
- Xu, B.-C.; Niu, F.-R.; Duan, D.-P.; Xu, W.-Z.; Huang, J. Root morphological characteristics of Lespedeza davurica (L.) Intercropped with Bothriochloa ischaemum (L.) Keng under water stress and P application conditions. Pak. J. Bot. 2012, 44, 1857–1864. [Google Scholar]
- Ren, J. Grassland Research Methods; China Agriculture Press: Beijing, China, 1998. [Google Scholar]
- Zhang, J.T. A study on relations of vegetation, climate and soils in Shanxi province, China. Plant Ecol. 2002, 162, 23–31. [Google Scholar]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Zhang, K. Jasmonic Acid Signaling Pathway in Plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Yu, L.X.; Djebrouni, M.; Chamberland, H.; Lafontaine, J.G.; Tabaeizadeh, Z. Chitinase: Differential induction of gene expression and enzyme activity by drought stress in the wild (Lycopersicon chilense Dun.) and cultivated (L. esculentum Mill.) tomatoes. J. Plant Physiol. 1998, 153, 745–753. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, Z.B.; Dong, K.H.; Yang, W.D.; Zhu, H.S.; Liang, P.F. Effects of Drought Stress and Rewatering on Enzymatic Defensive System in Lespedeza davurica (Laxm.) Schindl. Acta Agrestia Sin. 2010, 18, 199–211. [Google Scholar]
- Xu, B.; Xu, W.; Wang, Z.; Chen, Z.; Palta, J.A.; Chen, Y. Accumulation of N and P in the Legume Lespedeza davurica in Controlled Mixtures with the Grass Bothriochloa ischaemum under Varying Water and Fertilization Conditions. Front. Plant Sci. 2018, 9, 165. [Google Scholar] [CrossRef]
- Chinese Soil Taxonomy Research Group. Chinese Soil Taxonomy (Revised Proposal); Chinese Agricultural Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Verheijen, F.G.A.; Zhuravel, A.; Silva, F.C.; Amaro, A.; Ben-Hur, M.; Keizer, J.J. The influence of biochar particle size and concentration on bulk density and maximum water holding capacity of sandy vs. sandy loam soil in a column experiment. Geoderma 2019, 347, 194–202. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, D.; Jiang, F.; Tian, Z.; Feng, X.; Wu, N.; Hou, F.; Kardol, P.; Nan, Z.; Chen, T. Long-term heavy grazing increases community-level foliar fungal diseases by shifting plant composition. J. Appl. Ecol. 2021, 59, 791–800. [Google Scholar] [CrossRef]
- Chen, H.; Shao, M.; Li, Y. The characteristics of soil water cycle and water balance on steep grassland under natural and simulated rainfall conditions in the Loess Plateau of China. J. Hydrol. 2008, 360, 242–251. [Google Scholar] [CrossRef]
- Jiao, F.; Wen, Z.-M.; An, S.-S. Soil water storage capacity under chronosequence of revegetation in Yanhe watershed on the Loess Plateau, China. SpringerPlus 2013, 2, S15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Miscellaneous Paper Institute for Agricultural Research: Samaru, Nigeria, 1954. [Google Scholar]
- Brookes, P.C.; Kragt, J.F.; Powlson, D.S.; Jenkinson, D.S. Chloroform Fumigation and the Release of Soil-Nitrogen—The Effects of Fumigation Time and Temperature. Soil Biol. Biochem. 1985, 17, 831–835. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Zhu, Q.S.; Wang, W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol. 2001, 127, 315–323. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmed, S.H.; Namich, A.A.M.; Abdel-Sattar, R.R. Effect of Salicylic Acid and Potassium Citrate on Cotton Plant under Salt Stress. Fresenius Environ. Bull. 2017, 26, 1091–1100. [Google Scholar]
- Wang, X. Principles and Techniques of Plant Physiology and Biochemistry Experiment, 2nd ed.; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Yang, H.X.; Deng, J.J.; Zhang, J.; Zhao, G.H. Review on purification, enzyme assay and application of plant chitinases. Sci. Technol. Food Ind. 2011. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Hoiland, K.; Kjoller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi—Recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Wagner, H. Package ‘vegan’—Community Ecology Package. R Package Version 2 2015, 1997, 15–17. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.r-project.org/ (accessed on 9 October 2021).
- Schwinning, S.; Ehleringer, J.R. The Prediction of Plant Functional Diversity in Water-Limited Ecosystems. AGU Fall Meet. Abstr. 2001, 2001, H31F-01. [Google Scholar]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.; Foulkes, M.J. Roots and Uptake of Water and Nutrients. Crop Sci. 2019, 107–130. [Google Scholar]
- Ho, M.D.; Rosas, J.C.; Brown, K.M.; Lynch, J.P. Root architectural tradeoffs for water and phosphorus acquisition. Funct. Plant Biol. 2005, 32, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef]
- Pang, J.; Ryan, M.H.; Tibbett, M.; Cawthray, G.R.; Siddique, K.H.M.; Bolland, M.D.A.; Denton, M.D.; Lambers, H. Variation in morphological and physiological parameters in herbaceous perennial legumes in response to phosphorus supply. Plant Soil 2010, 331, 241–255. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.B.; Ryan, M.H.; Renton, M.; Lambers, H. Multiple adaptive responses of Australian native perennial legumes with pasture potential to grow in phosphorus- and moisture-limited environments. Ann. Bot. 2010, 105, 755–767. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.B.; Ryan, M.H.; Renton, M.; Lambers, H. Plant Responses to Limited Moisture and Phosphorus Availability: A Meta-Analysis. Adv. Agron. 2014, 124, 143–200. [Google Scholar]
- Bechtold, U.; Field, B. Molecular mechanisms controlling plant growth during abiotic stress. J. Exp. Bot. 2018, 69, 2753–2758. [Google Scholar] [CrossRef]
- Elizabeth Abreu, M.; Munne-Bosch, S. Salicylic acid may be involved in the regulation of drought-induced leaf senescence in perennials: A case study in field-grown Salvia officinalis L. plants. Environ. Exp. Bot. 2008, 64, 105–112. [Google Scholar] [CrossRef]
- Mahouachi, J.; Lopez-Climent, M.F.; Gomez-Cadenas, A. Hormonal and Hydroxycinnamic Acids Profiles in Banana Leaves in Response to Various Periods of Water Stress. Sci. World J. 2014, 2014, 540962. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, X.; Ma, W.; Song, J.; Rahman, S.U.; Wang, J.; Zhang, Y. Morphological and physiological responses to cyclic drought in two contrasting genotypes of Catalpa bungei. Environ. Exp. Bot. 2017, 138, 77–87. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.; Chen, J.; Gao, X.; Shen, C. Survival strategies based on the hydraulic vulnerability segmentation hypothesis, for the tea plant [Camellia sinensis(L.) O. Kuntze] in long-term drought stress condition. Plant Physiol. Biochem. 2020, 156, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Perez-Llorca, M.; Caselles, V.; Muller, M.; Munne-Bosch, S. The threshold between life and death in Cistus albidus L. seedlings: Mechanisms underlying drought tolerance and resilience. Tree Physiol. 2021, 41, 1861–1876. [Google Scholar] [CrossRef]
- Balbi, V.; Devoto, A. Jasmonate signalling network in Arabidopsis thaliana: Crucial regulatory nodes and new physiological scenarios. New Phytol. 2008, 177, 301–318. [Google Scholar] [CrossRef]
- de Ollas, C.; Hernando, B.; Arbona, V.; Gomez-Cadenas, A. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol. Plant. 2013, 147, 296–306. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Xing, Q.; Zhang, X.; Li, Y.; Shao, Q.; Cao, S.; Wang, F.; Qi, H. The lipoxygenase CmLOX13 from oriental melon enhanced severe drought tolerance via regulating ABA accumulation and stomatal closure in Arabidopsis. Environ. Exp. Bot. 2019, 167, 103815. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, B.R.; Al Mamun, M.; Bae, D.W.; Kim, T.H. Characterization of salicylic acid- and abscisic acid-mediated photosynthesis, Ca2+ and H2O2 accumulation in two distinct phases of drought stress intensity in Brassica napus. Environ. Exp. Bot. 2021, 186, 104434. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Azhar, N.; Hussain, M. Indole acetic acid (IAA) induced changes in growth, relative water contents and gas exchange attributes of barley (Hordeum vulgare L.) grown under water stress conditions. Plant Growth Regul. 2006, 50, 85–90. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Knapp, D.G.; Kovacs, G.M.; Zajta, E.; Groenewald, J.Z.; Crous, P.W. Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia 2015, 35, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Mariotte, P.; Canarini, A.; Dijkstra, F.A. Stoichiometric N:P flexibility and mycorrhizal symbiosis favour plant resistance against drought. J. Ecol. 2017, 105, 958–967. [Google Scholar] [CrossRef]
- Xi, N.; Chu, C.; Bloor, J.M.G. Plant drought resistance is mediated by soil microbial community structure and soil-plant feedbacks in a savanna tree species. Environ. Exp. Bot. 2018, 155, 695–701. [Google Scholar] [CrossRef]
- Zhu, E.; Cao, Z.; Jia, J.; Liu, C.; Zhang, Z.; Wang, H.; Dai, G.; He, J.S.; Feng, X. Inactive and inefficient: Warming and drought effect on microbial carbon processing in alpine grassland at depth. Glob. Chang. Biol. 2021, 27, 2241–2253. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Jiang, L.; Niu, S.; Zhou, X. Nonlinear responses of land ecosystems to variation in precipitation. New Phytol. 2017, 214, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Chen, B.; Rillig, M.C.; Jansa, J.; Ma, W.; Xu, C.; Luo, W.; Wu, H.; Hao, Z.; Wu, H.; et al. Community response of arbuscular mycorrhizal fungi to extreme drought in a cold-temperate grassland. New Phytol. 2021. [Google Scholar] [CrossRef]
- Ren, G.; Wang, C.; Dong, K.; Zhu, H.; Wang, Y.; Zhao, X. Effects of grazing exclusion on soil-vegetation relationships in a semiarid grassland on the Loess Plateau, China. Land Degrad. Dev. 2018, 29, 4071–4079. [Google Scholar] [CrossRef]
- Hueso, S.; García, C.; Hernández, T. Severe drought conditions modify the microbial community structure, size and activity in amended and unamended soils. Soil Biol. Biochem. 2012, 50, 167–173. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Collins, S.L.; Delgado-Baquerizo, M.; Hamonts, K.; Pockman, W.T.; Sinsabaugh, R.L.; Smith, M.D.; Knapp, A.K.; Power, S.A. Drought consistently alters the composition of soil fungal and bacterial communities in grasslands from two continents. Glob. Chang. Biol. 2018, 24, 2818–2827. [Google Scholar] [CrossRef]
- Knapp, A.K.; Ciais, P.; Smith, M.D. Reconciling inconsistencies in precipitation-productivity relationships: Implications for climate change. New Phytol. 2017, 214, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Luo, Y. Response of soil microbial communities to altered precipitation: A global synthesis. Glob. Ecol. Biogeogr. 2018, 27, 1121–1136. [Google Scholar] [CrossRef]
- Crowther, T.W.; Thomas, S.M.; Maynard, D.S.; Baldrian, P.; Covey, K.; Frey, S.D.; van Diepen, L.T.A.; Bradford, M.A. Biotic interactions mediate soil microbial feedbacks to climate change. Proc. Natl. Acad. Sci. USA 2015, 112, 7033–7038. [Google Scholar] [CrossRef] [PubMed]
- Field, K.J.; Pressel, S. Unity in diversity: Structural and functional insights into the ancient partnerships between plants and fungi. New Phytol. 2018, 220, 996–1011. [Google Scholar] [CrossRef]
- Kerfeld, C.A.; Heinhorst, S.; Cannon, G.C. Bacterial Microcompartments. Annu. Rev. Microbiol. 2010, 64, 391–408. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Caballero, E.; Belnap, J.; Buedel, B.; Crutzen, P.J.; Andreae, M.O.; Poeschl, U.; Weber, B. Dryland photoautotrophic soil surface communities endangered by global change. Nat. Geosci. 2018, 11, 185–189. [Google Scholar] [CrossRef]
- Meyer, K.M.; Memiaghe, H.; Korte, L.; Kenfack, D.; Alonso, A.; Bohannan, B.J.M. Why do microbes exhibit weak biogeographic patterns? ISME J. 2018, 12, 1404–1413. [Google Scholar] [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Luis Quero, J.; Garcia-Gomez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Y.-P.; Su, F.; Jiang, J.; Wang, C.; Yu, M.; Yan, J. The response of soil respiration to precipitation change is asymmetric and differs between grasslands and forests. Glob. Chang. Biol. 2020, 26, 6015–6024. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, T.; Wang, Y.; Cheng, H.; Chang, S.X.; Liang, C.; An, S. Negative effects of multiple global change factors on soil microbial diversity. Soil Biol. Biochem. 2021, 156, 108229. [Google Scholar] [CrossRef]
- Manzoni, S.; Schaeffer, S.; Katul, G.; Porporato, A.; Schimel, J. A theoretical analysis of microbial eco-physiological and diffusion limitations to carbon cycling in drying soils. Soil Biol. Biochem. 2014, 73, 69–83. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Ball, B.A. Impacts of altered precipitation regimes on soil communities and biogeochemistry in arid and semi-arid ecosystems. Glob. Chang. Biol. 2015, 21, 1407–1421. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, N.; Liang, Y.; Yang, H.; Ma, K. Interactive effects of water and nitrogen addition on soil microbial communities in a semiarid steppe. J. Plant Ecol. 2012, 5, 320–329. [Google Scholar] [CrossRef]
- Bell, C.W.; Tissue, D.T.; Loik, M.E.; Wallenstein, M.D.; Acosta-Martinez, V.; Erickson, R.A.; Zak, J.C. Soil microbial and nutrient responses to 7 years of seasonally altered precipitation in a Chihuahuan Desert grassland. Glob. Chang. Biol. 2014, 20, 1657–1673. [Google Scholar] [CrossRef]
- Trivedi, C.; Delgado-Baquerizo, M.; Hamonts, K.; Lai, K.; Reich, P.B.; Singh, B.K. Losses in microbial functional diversity reduce the rate of key soil processes. Soil Biol. Biochem. 2019, 135, 267–274. [Google Scholar] [CrossRef]
- Rolli, E.; Marasco, R.; Vigani, G.; Ettoumi, B.; Mapelli, F.; Deangelis, M.L.; Gandolfi, C.; Casati, E.; Previtali, F.; Gerbino, R. Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ. Microbiol. 2015, 17, 316–331. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Arbuscular mycorrhizal fungi and tolerance of drought stress in plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Singapore, 2017; Volume 2, pp. 25–41. [Google Scholar]
- Chen, J.; Sun, X.; Li, L.; Liu, X.; Zhang, B.; Zheng, J.; Pan, G. Change in active microbial community structure, abundance and carbon cycling in an acid rice paddy soil with the addition of biochar. Eur. J. Soil Sci. 2016, 67, 857–867. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Liang, C.; Xu, Q.; Li, Y.; Hua, Q.; Fuhrmann, J.J. Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: Effect of particle size and addition rate. Sci. Total Environ. 2017, 574, 24–33. [Google Scholar] [CrossRef]
- Engelhardt, I.C.; Welty, A.; Blazewicz, S.J.; Bru, D.; Rouard, N.; Breuil, M.-C.; Gessler, A.; Galiano, L.; Carlos Miranda, J.; Spor, A.; et al. Depth matters: Effects of precipitation regime on soil microbial activity upon rewetting of a plant-soil system. ISME J. 2018, 12, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Scheu, S.; Parkinson, D. Changes in bacterial and fungal biomass C, bacterial and fungal biovolume and ergosterol content after drying, remoistening and incubation of different layers of cool temperate forest soils. Soil Biol. Biochem. 1994, 26, 1515–1525. [Google Scholar] [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought Stress and Root-Associated Bacterial Communities. Front. Plant Sci. 2018, 8, 2223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Myrold, D.D.; Shi, L.; Kuzyakov, Y.; Dai, H.; Thu Hoang, D.T.; Dippold, M.A.; Meng, X.; Song, X.; Li, Z.; et al. Resistance of microbial community and its functional sensitivity in the rhizosphere hotspots to drought. Soil Biol. Biochem. 2021, 161, 108360. [Google Scholar] [CrossRef]
- Evans, S.E.; Wallenstein, M.D. Soil microbial community response to drying and rewetting stress: Does historical precipitation regime matter? Biogeochemistry 2012, 109, 101–116. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H.; Chen, Q.; Han, X. The counteractive effects of nitrogen addition and watering on soil bacterial communities in a steppe ecosystem. Soil Biol. Biochem. 2014, 72, 26–34. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loque, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Yang, S.; Zheng, Q.; Yang, Y.; Yuan, M.; Ma, X.; Chiariello, N.R.; Docherty, K.M.; Field, C.B.; Gutknecht, J.L.M.; Hungate, B.A.; et al. Fire affects the taxonomic and functional composition of soil microbial communities, with cascading effects on grassland ecosystem functioning. Glob. Chang. Biol. 2020, 26, 431–442. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).