Mycosynthesis, Characterization, and Mosquitocidal Activity of Silver Nanoparticles Fabricated by Aspergillus niger Strain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain
2.2. Mycosynthesis of Ag-NPs
2.3. Ag-NPs Characterization
2.4. Mosquito Culture
2.5. Laboratory Larvicidal/Pupicidal Toxicity of Ag-NPs
2.6. Field Larvicidal Bioassay
2.7. Smoke Toxicity Assay
2.8. Ovicidal Activity
2.9. Data Analysis
3. Results and Discussion
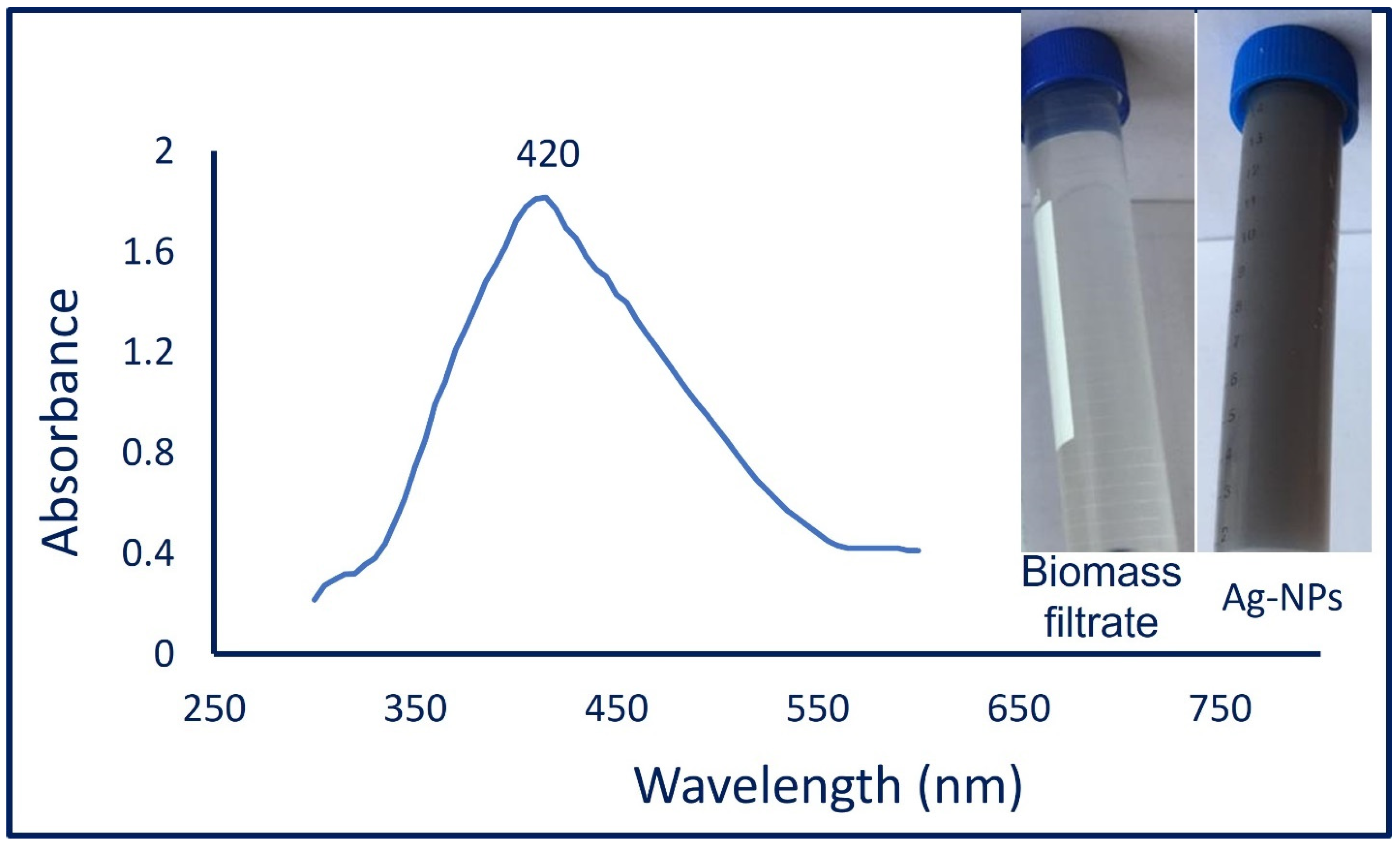
3.1. Mycosynthesis of Ag-NPs
3.2. Ag-NPs Characterizations
3.2.1. UV-Vis Spectroscopy
3.2.2. Fourier Transform Infrared (FT-IR) Analysis
3.2.3. Transmission Electron Microscopy (TEM)
3.2.4. X-ray Diffraction Pattern
3.3. Larvicidal/Pupicidal Toxicity of Ag-NPs under Laboratory and Field Conditions
3.4. Smoke Toxicity Assay
3.5. Ovicidal Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soliman, A.M.; Abdel-Latif, W.; Shehata, I.H.; Fouda, A.; Abdo, A.M.; Ahmed, Y.M. Green Approach to Overcome the Resistance Pattern of Candida spp. Using Biosynthesized Silver Nanoparticles Fabricated by Penicillium chrysogenum F9. Biol. Trace Elem. Res. 2021, 199, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Manimegalai, T.; Raguvaran, K.; Kalpana, M.; Maheswaran, R. Green synthesis of silver nanoparticle using Leonotis nepetifolia and their toxicity against vector mosquitoes of Aedes aegypti and Culex quinquefasciatus and agricultural pests of Spodoptera litura and Helicoverpa armigera. Environ. Sci. Pollut. Res. Int. 2020, 27, 43103–43116. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, T.I.; Fouda, A.; Salem, S.S. Integration of Cotton Fabrics with Biosynthesized CuO Nanoparticles for Bactericidal Activity in the Terms of Their Cytotoxicity Assessment. Ind. Eng. Chem. Res. 2021, 60, 1553–1563. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Maksoud, G.; Abdel-Rahman, M.A.; Eid, A.M.; Barghoth, M.G.; El-Sadany, M.A.-H. Monitoring the effect of biosynthesized nanoparticles against biodeterioration of cellulose-based materials by Aspergillus niger. Cellulose 2019, 26, 6583–6597. [Google Scholar] [CrossRef]
- Hamza, M.F.; Hamad, N.A.; Hamad, D.M.; Khalafalla, M.S.; Abdel-Rahman, A.A.H.; Zeid, I.F.; Wei, Y.; Hessien, M.M.; Fouda, A.; Salem, W.M. Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd(II) and Pb(II) from Contaminated Effluents. Materials 2021, 14, 2189. [Google Scholar] [CrossRef]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi Chem. Soc. 2015, 19, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Alsharif, S.M.; Salem, S.S.; Abdel-Rahman, M.A.; Fouda, A.; Eid, A.M.; El-Din Hassan, S.; Awad, M.A.; Mohamed, A.A. Multifunctional properties of spherical silver nanoparticles fabricated by different microbial taxa. Heliyon 2020, 6, e03943. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis of Metal and Metal Oxide Nanoparticles. ChemBioEng Rev. 2016, 3, 55–67. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R.D. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [Green Version]
- Jinu, U.; Rajakumaran, S.; Senthil-Nathan, S.; Geetha, N.; Venkatachalam, P. Potential larvicidal activity of silver nanohybrids synthesized using leaf extracts of Cleistanthus collinus (Roxb.) Benth. ex Hook.f. and Strychnos nuxvomica L. nuxvomica against dengue, Chikungunya and Zika vectors. Physiol. Mol. Plant Pathol. 2017, 101, 163–171. [Google Scholar] [CrossRef]
- Abdo, A.M.; Fouda, A.; Eid, A.M.; Fahmy, N.M.; Elsayed, A.M.; Khalil, A.M.A.; Alzahrani, O.M.; Ahmed, A.F.; Soliman, A.M. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) by Pseudomonas aeruginosa and Their Activity against Pathogenic Microbes and Common House Mosquito, Culex Pipiens. Materials 2021, 14, 6983. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.A.; El Aty, A.A.A.; El Awdan, S.A. Extracellular myco-synthesis of nano-silver using the fermentable yeasts Pichia kudriavzevii HA-NY2 and Saccharomyces uvarum HA-NY3, and their effective biomedical applications. Bioprocess Biosyst. Eng. 2021, 44, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Abd-Elsalam, K.A.; AboDalam, H.M.; Ahmed, F.K.; Ravichandran, M.; Kalia, A.; Rai, M. Trichoderma: An Eco-Friendly Source of Nanomaterials for Sustainable Agroecosystems. J. Fungi 2022, 8, 367. [Google Scholar] [CrossRef]
- Salem, S.S.; Mohamed, A.; El-Gamal, M.; Talat, M.; Fouda, A. Biological Decolorization and Degradation of Azo Dyes from Textile Wastewater Effluent by Aspergillus niger. Egypt. J. Chem. 2019, 62, 1799–1813. [Google Scholar]
- Khalil, A.M.A.; Hassan, S.E.; Alsharif, S.M.; Eid, A.M.; Ewais, E.E.; Azab, E.; Gobouri, A.A.; Elkelish, A.; Fouda, A. Isolation and Characterization of Fungal Endophytes Isolated from Medicinal Plant Ephedra pachyclada as Plant Growth-Promoting. Biomolecules 2021, 11, 140. [Google Scholar] [CrossRef]
- Clarance, P.; Luvankar, B.; Sales, J.; Khusro, A.; Agastian, P.; Tack, J.C.; Al Khulaifi, M.M.; Al-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J. Biol. Sci. 2020, 27, 706–712. [Google Scholar] [CrossRef]
- Zaki, S.A.; Ouf, S.A.; Albarakaty, F.M.; Habeb, M.M.; Aly, A.A.; Abd-Elsalam, K.A. Trichoderma harzianum-Mediated ZnO Nanoparticles: A Green Tool for Controlling Soil-Borne Pathogens in Cotton. J. Fungi 2021, 7, 952. [Google Scholar] [CrossRef]
- Benelli, G.; Romano, D. Mosquito vectors of Zika virus. Entomol. Gen. 2017, 36, 309–318. [Google Scholar] [CrossRef]
- Lee, H.; Halverson, S.; Ezinwa, N. Mosquito-Borne Diseases. Prim. Care 2018, 45, 393–407. [Google Scholar] [CrossRef]
- World Health Organization. Dengue and Severe Dengue. 2016. Available online: http://www.who.int/mediacentre/factsheets/fs117/en/ (accessed on 12 January 2017).
- Benelli, G.; Pavela, R.; Maggi, F.; Petrelli, R.; Nicoletti, M. Commentary: Making green pesticides greener? The potential of plant products for nanosynthesis and pest control. J. Clust. Sci. 2017, 28, 3–10. [Google Scholar] [CrossRef]
- Frierson, J.G. The yellow fever vaccine: A history. Yale J. Biol. Med. 2010, 83, 77–85. [Google Scholar] [PubMed]
- Saied, E.; Fouda, A.; Alemam, A.M.; Sultan, M.H.; Barghoth, M.G.; Radwan, A.A.; Desouky, S.G.; Azab, I.H.E.; Nahhas, N.E.; Hassan, S.E. Evaluate the Toxicity of Pyrethroid Insecticide Cypermethrin before and after Biodegradation by Lysinibacillus cresolivuorans Strain HIS7. Plants 2021, 10, 1903. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Hamad, D.M.; Hamad, N.A.; Abdel-Rahman, A.A.H.; Fouda, A.; Wei, Y.; Guibal, E.; El-Etrawy, A.-A.S. Functionalization of magnetic chitosan microparticles for high-performance removal of chromate from aqueous solutions and tannery effluent. Chem. Eng. J. 2022, 428, 131775. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Benelli, G.; Losic, D.; Usha Rani, P.; Desneux, N. Nanoparticles for pest control: Current status and future perspectives. J. Pest Sci. 2018, 91, 1–15. [Google Scholar] [CrossRef]
- Morad, M.Y.; El-Sayed, H.; Elhenawy, A.A.; Korany, S.M.; Aloufi, A.S.; Ibrahim, A.M. Myco-Synthesized Molluscicidal and Larvicidal Selenium Nanoparticles: A New Strategy to Control Biomphalaria alexandrina Snails and Larvae of Schistosoma mansoni with an In Silico Study on Induced Oxidative Stress. J. Fungi 2022, 8, 262. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Abdel-Rahman, M.A.; EL-Belely, E.F.; Awad, M.A.; Hassan, S.E.-D.; AL-Faifi, Z.E.; Hamza, M.F. Enhanced Antimicrobial, Cytotoxicity, Larvicidal, and Repellence Activities of Brown Algae, Cystoseira crinita-Mediated Green Synthesis of Magnesium Oxide Nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 849921. [Google Scholar] [CrossRef]
- Thelma, J.; Balasubramanian, C. Ovicidal, larvicidal and pupicidal efficacy of silver nanoparticles synthesized by Bacillus marisflavi against the chosen mosquito species. PLoS ONE 2021, 16, e0260253. [Google Scholar]
- Fouda, A.; Abdel-Maksoud, G.; Abdel-Rahman, M.A.; Salem, S.S.; Hassan, S.E.-D.; El-Sadany, M.A.-H. Eco-friendly approach utilizing green synthesized nanoparticles for paper conservation against microbes involved in biodeterioration of archaeological manuscript. Int. Biodeterior. Biodegrad. 2019, 142, 160–169. [Google Scholar] [CrossRef]
- Ismail, M.A.; Amin, M.A.; Eid, A.M.; Hassan, S.E.; Mahgoub, H.A.M.; Lashin, I.; Abdelwahab, A.T.; Azab, E.; Gobouri, A.A.; Elkelish, A.; et al. Comparative Study between Exogenously Applied Plant Growth Hormones versus Metabolites of Microbial Endophytes as Plant Growth-Promoting for Phaseolus vulgaris L. Cells 2021, 10, 1059. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Fouda, A.; Niedbała, G.; Hassan, S.E.; Salem, S.S.; Abdo, A.M.; Hetta, H.F.; Shaheen, T.I. Endophytic Streptomyces laurentii Mediated Green Synthesis of Ag-NPs with Antibacterial and Anticancer Properties for Developing Functional Textile Fabric Properties. Antibiotics 2020, 9, 641. [Google Scholar] [CrossRef]
- Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. Fungus-mediated green synthesis of nano-silver using Aspergillus sydowii and its antifungal/antiproliferative activities. Sci. Rep. 2021, 11, 10356. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Elgammal, W.E.; Eid, A.M.; Dawaba, A.M.; Ibrahim, A.G.; Fouda, A.; Hassan, S.M. Synthesis and characterization of new functionalized chitosan and its antimicrobial and in-vitro release behavior from topical gel. Int. J. Biol. Macromol. 2022, 207, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Hamza, M.F. Photocatalytic degradation of real textile and tannery effluent using biosynthesized magnesium oxide nanoparticles (MgO-NPs), heavy metal adsorption, phytotoxicity, and antimicrobial activity. J. Environ. Chem. Eng. 2021, 9, 105346. [Google Scholar] [CrossRef]
- Borchert, H.; Shevchenko, E.V.; Robert, A.; Mekis, I.; Kornowski, A.; Grübel, G.; Weller, H. Determination of nanocrystal sizes: A comparison of TEM, SAXS, and XRD studies of highly monodisperse CoPt3 particles. Langmuir 2005, 21, 1931–1936. [Google Scholar] [CrossRef]
- Sujitha, V.; Murugan, K.; Paulpandi, M.; Panneerselvam, C.; Suresh, U.; Roni, M.; Nicoletti, M.; Higuchi, A.; Madhiyazhagan, P.; Subramaniam, J.; et al. Green-synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes Aegypti. Parasitol. Res. 2015, 114, 3315–3325. [Google Scholar] [CrossRef]
- Kaleka, A.; Kaur, N.; Bali, G. Larval Development and Molting. In Edible Insects; 2020 Edition; Mikkola, H., Ed.; Intech Open: London, UK, 2019. [Google Scholar]
- Murugan, K.; Vahitha, R.; Baruah, I.; Das, S. Integration of botanical and microbial pesticides for the control of filarial vector, Culex quinquefasciatus Say (Diptera: Culicidae). Ann. Med. Entomol. 2003, 12, 12–23. [Google Scholar]
- Dinesh, D.; Murugan, K.; Madhiyazhagan, P.; Panneerselvam, C.; Mahesh Kumar, P.; Nicoletti, M.; Jiang, W.; Benelli, G.; Chandramohan, B.; Suresh, U. Mosquitocidal and antibacterial activity of green-synthesized silver nanoparticles from Aloe vera extracts: Towards an effective tool against the malaria vector Anopheles stephensi? Parasitol. Res. 2015, 114, 1519–1529. [Google Scholar] [CrossRef]
- Suresh, U.; Murugan, K.; Benelli, G.; Nicoletti, M.; Barnard, D.R.; Panneerselvam, C.; Kumar, P.M.; Subramaniam, J.; Dinesh, D.; Chandramohan, B. Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2015, 114, 1551–1562. [Google Scholar] [CrossRef]
- Su, T.; Mulla, M.S. Ovicidal activity of neem products (azadirachtin) against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae). J. Am. Mosq. Control Assoc. 1998, 14, 204–209. [Google Scholar] [PubMed]
- Finney, D.L. Probit Analysis, 2nd ed.; Cambridge University Press: New York, NY, USA, 1952; Volume 41, p. 627. [Google Scholar]
- Elsayed, M.A.; Othman, A.M.; Hassan, M.M.; Elshafei, A.M. Optimization of silver nanoparticles biosynthesis mediated by Aspergillus niger NRC1731 through application of statistical methods: Enhancement and characterization. 3 Biotech 2018, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Parial, D.; Patra, H.K.; Roychoudhury, P.; Dasgupta, A.K.; Pal, R. Gold nanorod production by cyanobacteria—A green chemistry approach. J. Appl. Phycol. 2012, 24, 55–60. [Google Scholar] [CrossRef]
- Namvar, F.; Azizi, S.; Ahmad, M.B.; Shameli, K.; Mohamad, R.; Mahdavi, M.; Tahir, P.M. Green synthesis and characterization of gold nanoparticles using the marine macroalgae Sargassum muticum. Res. Chem. Intermed. 2015, 41, 5723–5730. [Google Scholar] [CrossRef]
- Sastry, M.; Mayya, K.S.; Bandyopadhyay, K. pH Dependent changes in the optical properties of carboxylic acid derivatized silver colloidal particles. Colloids Surf. A Physicochem. Eng. Asp. 1997, 127, 221–228. [Google Scholar] [CrossRef]
- Zomorodian, K.; Pourshahid, S.; Sadatsharifi, A.; Mehryar, P.; Pakshir, K.; Rahimi, M.J.; Arabi Monfared, A. Biosynthesis and Characterization of Silver Nanoparticles by Aspergillus Species. BioMed Res. Int. 2016, 2016, 5435397. [Google Scholar] [CrossRef] [Green Version]
- Saada, N.S.; Abdel-Maksoud, G.; Abd El-Aziz, M.S.; Youssef, A.M. Green synthesis of silver nanoparticles, characterization, and use for sustainable preservation of historical parchment against microbial biodegradation. Biocatal. Agric. Biotechnol. 2021, 32, 101948. [Google Scholar] [CrossRef]
- Cruz-Luna, A.R.; Cruz-Martínez, H.; Vásquez-López, A.; Medina, D.I. Metal Nanoparticles as Novel Antifungal Agents for Sustainable Agriculture: Current Advances and Future Directions. J. Fungi 2021, 7, 1033. [Google Scholar] [CrossRef]
- Salem, S.S.; Ali, O.M.; Reyad, A.M.; Abd-Elsalam, K.A.; Hashem, A.H. Pseudomonas indica-Mediated Silver Nanoparticles: Antifungal and Antioxidant Biogenic Tool for Suppressing Mucormycosis Fungi. J. Fungi 2022, 8, 126. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Salem, S.S.; El-Belely, E.F.; Niedbała, G.; Alnoman, M.M.; Hassan, S.E.; Eid, A.M.; Shaheen, T.I.; Elkelish, A.; Fouda, A. Bactericidal and In-Vitro Cytotoxic Efficacy of Silver Nanoparticles (Ag-NPs) Fabricated by Endophytic Actinomycetes and Their Use as Coating for the Textile Fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef] [PubMed]
- Iqtedar, M.; Aslam, M.; Akhyar, M.; Shehzaad, A.; Abdullah, R.; Kaleem, A. Extracellular biosynthesis, characterization, optimization of silver nanoparticles (AgNPs) using Bacillus mojavensis BTCB15 and its antimicrobial activity against multidrug resistant pathogens. Prep. Biochem. Biotechnol. 2019, 49, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Taha, Z.K.; Hawar, S.N.; Sulaiman, G.M. Extracellular biosynthesis of silver nanoparticles from Penicillium italicum and its antioxidant, antimicrobial and cytotoxicity activities. Biotechnol. Lett. 2019, 41, 899–914. [Google Scholar] [CrossRef]
- Li, G.; He, D.; Qian, Y.; Guan, B.; Gao, S.; Cui, Y.; Yokoyama, K.; Wang, L. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 2012, 13, 466–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamza, M.F.; Abdel-Rahman, A.A.; Negm, A.S.; Hamad, D.M.; Khalafalla, M.S.; Fouda, A.; Wei, Y.; Amer, H.H.; Alotaibi, S.H.; Goda, A.E. Grafting of Thiazole Derivative on Chitosan Magnetite Nanoparticles for Cadmium Removal-Application for Groundwater Treatment. Polymers 2022, 14, 1240. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation. Infrared Spectrosc; Meyers, R.A., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2006. [Google Scholar]
- Saied, E.; Eid, A.M.; Hassan, S.E.; Salem, S.S.; Radwan, A.A.; Halawa, M.; Saleh, F.M.; Saad, H.A.; Saied, E.M.; Fouda, A. The Catalytic Activity of Biosynthesized Magnesium Oxide Nanoparticles (MgO-NPs) for Inhibiting the Growth of Pathogenic Microbes, Tanning Effluent Treatment, and Chromium Ion Removal. Catalysts 2021, 11, 821. [Google Scholar] [CrossRef]
- Hamza, M.F.; Lu, S.; Salih, K.A.M.; Mira, H.; Dhmees, A.S.; Fujita, T.; Wei, Y.; Vincent, T.; Guibal, E. As(V) sorption from aqueous solutions using quaternized algal/polyethyleneimine composite beads. Sci. Total Environ. 2020, 719, 137396. [Google Scholar] [CrossRef]
- Mohammadi, N.; Ganesan, A.; Chantler, C.T.; Wang, F. Differentiation of ferrocene D5d and D5h conformers using IR spectroscopy. J. Organomet. Chem. 2012, 713, 51–59. [Google Scholar] [CrossRef]
- Hamza, M.F.; Fouda, A.; Wei, Y.; El Aassy, I.E.; Alotaibi, S.H.; Guibal, E.; Mashaal, N.M. Functionalized biobased composite for metal decontamination—Insight on uranium and application to water samples collected from wells in mining areas (Sinai, Egypt). Chem. Eng. J. 2022, 431, 133967. [Google Scholar] [CrossRef]
- Wei, Y.; Rakhatkyzy, M.; Salih, K.A.M.; Wang, K.; Hamza, M.F.; Guibal, E. Controlled bi-functionalization of silica microbeads through grafting of amidoxime/methacrylic acid for Sr(II) enhanced sorption. Chem. Eng. J. 2020, 402, 125220. [Google Scholar] [CrossRef]
- Sutthanont, N.; Attrapadung, S.; Nuchprayoon, S. Larvicidal Activity of Synthesized Silver Nanoparticles from Curcuma zedoaria Essential Oil against Culex quinquefasciatus. Insects 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouda, A.; Hassan, S.E.-D.; Abdel-Rahman, M.A.; Farag, M.M.S.; Shehal-deen, A.; Mohamed, A.A.; Alsharif, S.M.; Saied, E.; Moghanim, S.A.; Azab, M.S. Catalytic degradation of wastewater from the textile and tannery industries by green synthesized hematite (α-Fe2O3) and magnesium oxide (MgO) nanoparticles. Curr. Res. Biotechnol. 2021, 3, 29–41. [Google Scholar] [CrossRef]
- Bansal, V.; Li, V.; O’Mullane, A.P.; Bhargava, S.K. Shape dependent electrocatalytic behaviour of silver nanoparticles. CrystEngComm 2010, 12, 4280–4286. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.A.; Ismail, M.A.; Badawy, A.A.; Awad, M.A.; Hamza, M.F.; Awad, M.F.; Fouda, A. The Potency of Fungal-Fabricated Selenium Nanoparticles to Improve the Growth Performance of Helianthus annuus L. and Control of Cutworm Agrotis ipsilon. Catalysts 2021, 11, 1551. [Google Scholar] [CrossRef]
- Sribenjarat, P.; Jirakanjanakit, N.; Jirasripongpun, K. Selenium nanoparticles biosynthesized by garlic extract as antimicrobial agent. Sci. Eng. Health Stud. 2020, 14, 22–31. [Google Scholar]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef] [Green Version]
- Lashin, I.; Fouda, A.; Gobouri, A.A.; Azab, E.; Mohammedsaleh, Z.M.; Makharita, R.R. Antimicrobial and In Vitro Cytotoxic Efficacy of Biogenic Silver Nanoparticles (Ag-NPs) Fabricated by Callus Extract of Solanum incanum L. Biomolecules 2021, 11, 341. [Google Scholar] [CrossRef]
- Thomas, B.; Vithiya, B.S.M.; Prasad, T.A.A.; Mohamed, S.B.; Magdalane, C.M.; Kaviyarasu, K.; Maaza, M. Antioxidant and Photocatalytic Activity of Aqueous Leaf Extract Mediated Green Synthesis of Silver Nanoparticles Using Passiflora edulis f. flavicarpa. J. Nanosci. Nanotechnol. 2019, 19, 2640–2648. [Google Scholar] [CrossRef]
- Suresh, U.; Murugan, K.; Panneerselvam, C.; Rajaganesh, R.; Roni, M.; Aziz, A.T.; Naji Al-Aoh, H.A.; Trivedi, S.; Rehman, H.; Kumar, S.; et al. Suaeda maritima-based herbal coils and green nanoparticles as potential biopesticides against the dengue vector Aedes aegypti and the tobacco cutworm Spodoptera litura. Physiol. Mol. Plant Pathol. 2018, 101, 225–235. [Google Scholar] [CrossRef]
- Hassan, S.E.; Fouda, A.; Saied, E.; Farag, M.M.S.; Eid, A.M.; Barghoth, M.G.; Awad, M.A.; Hamza, M.F.; Awad, M.F. Rhizopus oryzae-Mediated Green Synthesis of Magnesium Oxide Nanoparticles (MgO-NPs): A Promising Tool for Antimicrobial, Mosquitocidal Action, and Tanning Effluent Treatment. J. Fungi 2021, 7, 372. [Google Scholar] [CrossRef]
- Deepak, P.; Sowmiya, R.; Ramkumar, R.; Balasubramani, G.; Aiswarya, D.; Perumal, P. Structural characterization and evaluation of mosquito-larvicidal property of silver nanoparticles synthesized from the seaweed, Turbinaria ornata (Turner) J. Agardh 1848. Artif. Cells Nanomed. Biotechnol. 2017, 45, 990–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badawy, A.A.; Abdelfattah, N.A.H.; Salem, S.S.; Awad, M.F.; Fouda, A. Efficacy Assessment of Biosynthesized Copper Oxide Nanoparticles (CuO-NPs) on Stored Grain Insects and Their Impacts on Morphological and Physiological Traits of Wheat (Triticum aestivum L.) Plant. Biology 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Baskar, K.; Muthu, C.; Ignacimuthu, S. Effect of pectolinaringenin, a flavonoid from Clerodendrum phlomidis, on total protein, glutathione S-transferase and esterase activities of Earias vittella and Helicoverpa armigera. Phytoparasitica 2014, 42, 323–331. [Google Scholar] [CrossRef]
- Haldar, K.M.; Ghosh, P.; Chandra, G. Larvicidal, adulticidal, repellency and smoke toxic efficacy of Ficus krishnae against Anopheles stephensi Liston and Culex vishnui group mosquitoes. Asian Pac. J. Trop. Dis. 2014, 4, S214–S220. [Google Scholar] [CrossRef]
- Pauluhn, J. Mosquito coil smoke inhalation toxicity. Part I: Validation of test approach and acute inhalation toxicity. J. Appl. Toxicol. Int. J. 2006, 26, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Dion, E.; Monteiro, A. Haze smoke impacts survival and development of butterflies. Sci. Rep. 2018, 8, 15667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajaganesh, R.; Murugan, K.; Panneerselvam, C.; Jayashanthini, S.; Aziz, A.T.; Roni, M.; Suresh, U.; Trivedi, S.; Rehman, H.; Higuchi, A.; et al. Fern-synthesized silver nanocrystals: Towards a new class of mosquito oviposition deterrents? Res. Vet. Sci. 2016, 109, 40–51. [Google Scholar] [CrossRef]




| Target | LC50 (LC90) (ppm) | 95 % Confidence Limit LC50 (LC90) | |
|---|---|---|---|
| LCL | UCL | ||
| I instar | 12.4 (22.4) | 10.01 (20.3) | 16.03 (25.5) |
| II instar | 13.6 (24.6) | 11.9 (25.2) | 14.8 (27.1) |
| III instar | 15.04 (27.1) | 12.3 (21.1) | 16.03 (29.8) |
| IV instar | 20.9 (37.6) | 18.4 (30.6) | 24.4 (39.1) |
| Pupa | 22.9 (41.4) | 19.1 (37.7) | 25.3 (46.3) |
| Treatment | Larval Density with Reduction Percentages (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| before Treatment | Reduction % | after Treatment | ||||||
| 24 h | Reduction % | 48 h | Reduction % | 72 h | Reduction % | |||
| Ag-NPs (10× LC50) | 127.1 ± 4.3 a | 0.0 | 51.4 ± 6.3 b | 59.6 | 32.2 ± 4.1 c | 74.7 | 0.0 ± 0.0 d | 100 |
| Treatment | Fed Mosquitoes (%) | Unfed Mosquitoes (%) | Total (%) | |
|---|---|---|---|---|
| Alive | Dead | |||
| Ag-NPs based coil | 14.4 ± 1.7 b | 22.6 ± 1.9 a | 59.0 ± 2.1 a | 81.6 ± 0.5 b |
| Negative control | 73.9 ± 0.9 a | 23.9 ± 1.6 a | 0.0 ± 0.0 c | 23.9 ± 1.2 c |
| Positive control | 9.03 ± 1.7 c | 37.9 ± 1.7 b | 48.2 ± 1.5 b | 86.1 ± 1.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, M.A.; Eid, A.M.; Elsheikh, T.M.Y.; Al-Faifi, Z.E.; Saad, N.; Sultan, M.H.; Selim, S.; Al-Khalaf, A.A.; Fouda, A. Mycosynthesis, Characterization, and Mosquitocidal Activity of Silver Nanoparticles Fabricated by Aspergillus niger Strain. J. Fungi 2022, 8, 396. https://doi.org/10.3390/jof8040396
Awad MA, Eid AM, Elsheikh TMY, Al-Faifi ZE, Saad N, Sultan MH, Selim S, Al-Khalaf AA, Fouda A. Mycosynthesis, Characterization, and Mosquitocidal Activity of Silver Nanoparticles Fabricated by Aspergillus niger Strain. Journal of Fungi. 2022; 8(4):396. https://doi.org/10.3390/jof8040396
Chicago/Turabian StyleAwad, Mohamed A., Ahmed M. Eid, Tarek M. Y. Elsheikh, Zarraq E. Al-Faifi, Nadia Saad, Mahmoud H. Sultan, Samy Selim, Areej A. Al-Khalaf, and Amr Fouda. 2022. "Mycosynthesis, Characterization, and Mosquitocidal Activity of Silver Nanoparticles Fabricated by Aspergillus niger Strain" Journal of Fungi 8, no. 4: 396. https://doi.org/10.3390/jof8040396
APA StyleAwad, M. A., Eid, A. M., Elsheikh, T. M. Y., Al-Faifi, Z. E., Saad, N., Sultan, M. H., Selim, S., Al-Khalaf, A. A., & Fouda, A. (2022). Mycosynthesis, Characterization, and Mosquitocidal Activity of Silver Nanoparticles Fabricated by Aspergillus niger Strain. Journal of Fungi, 8(4), 396. https://doi.org/10.3390/jof8040396









