Current Landscape of Coccidioidomycosis
Abstract
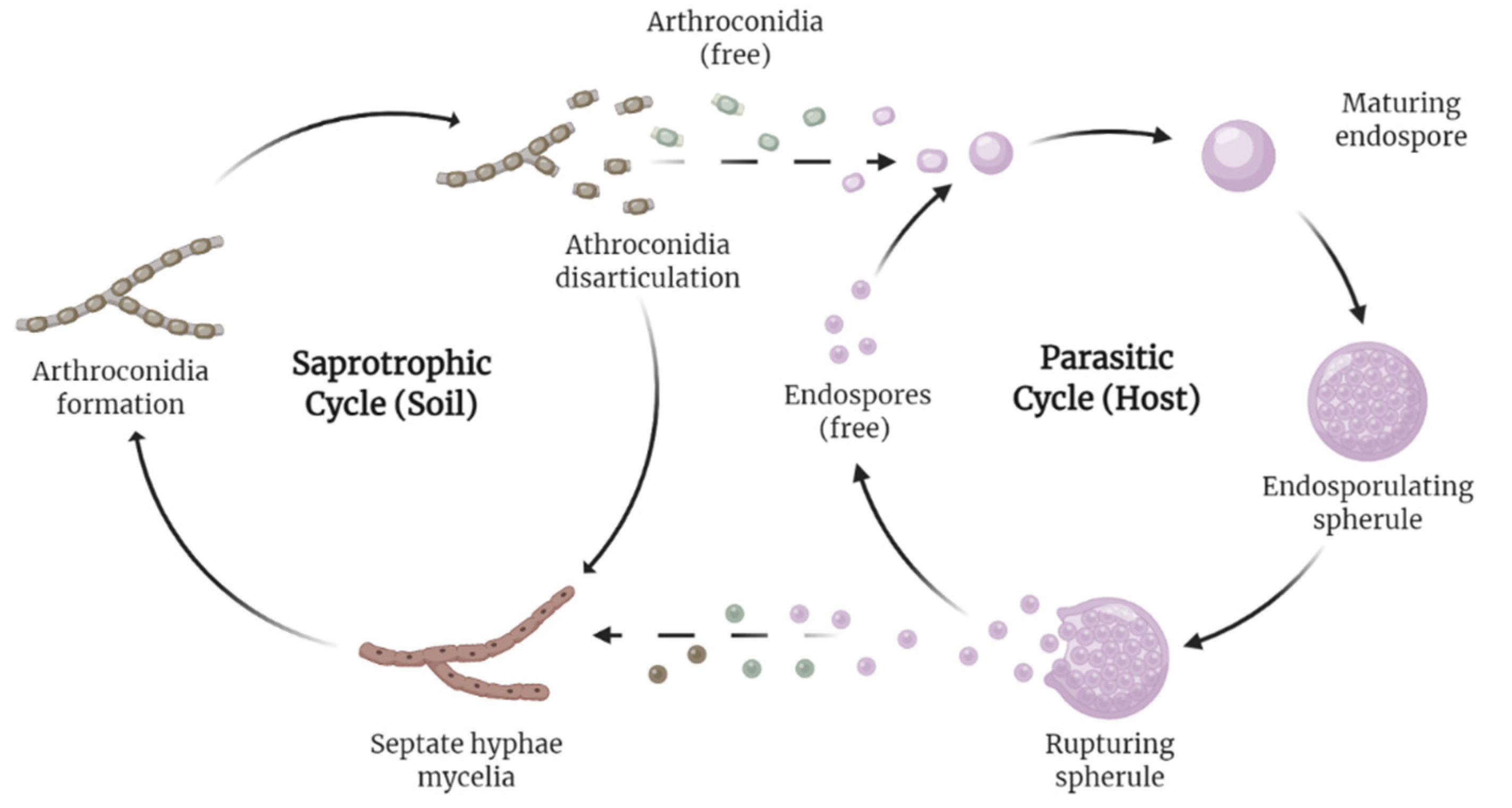
1. Introduction
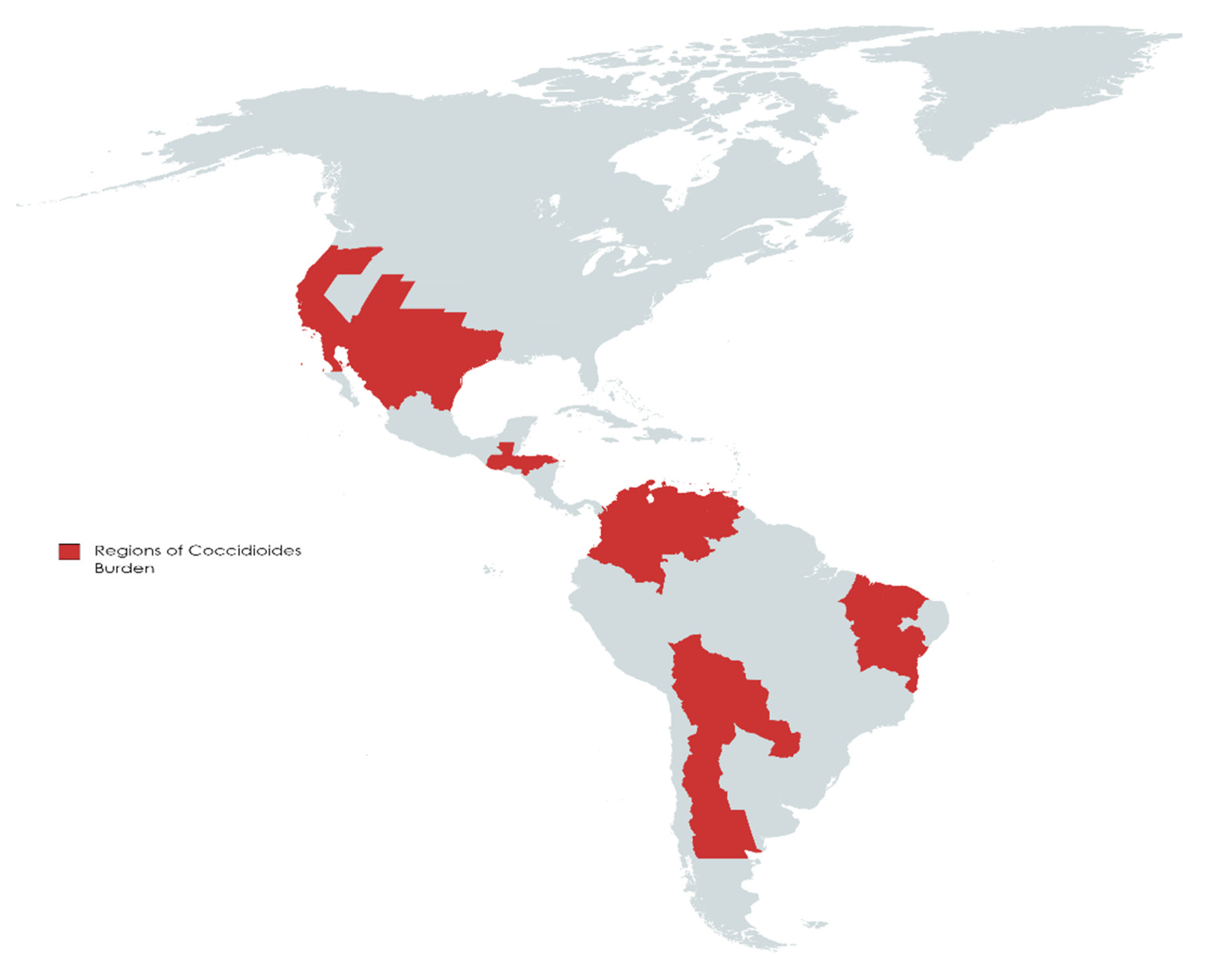
2. Burden and Projections
3. Diagnosis
4. Currently Approved Drugs
5. New Drugs in the Pipeline
6. Immunological Therapies
7. Vaccines
8. CM and COVID-19
9. Special Populations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, J.; Benedict, K.; Park, B.J.; Thompson, G.R., 3rd. Coccidioidomycosis: Epidemiology. Clin. Epidemiol. 2013, 5, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Chaffee, A.W.; Harrigan, R.J.; Schoenberg, F.P. A non-parametric Hawkes model of the spread of Ebola in west Africa. J. Appl. Stat. 2020, 49, 621–637. [Google Scholar] [CrossRef]
- Posadas, A. Un nuevo caso de micosis fungoidea con posrospemias. Ann. Cir. Med. Argent 1892, 15, 585–597. [Google Scholar]
- Fisher, M.C.; Koenig, G.L.; White, T.J.; Taylor, J.W. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 2002, 94, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, A.K.; Rongkavilit, C. Update on Coccidioidomycosis in the United States and Beyond. Glob. Pediatr. Health 2020, 7, 2333794x20969282. [Google Scholar] [CrossRef] [PubMed]
- Laniado-Laborín, R.; Arathoon, E.G.; Canteros, C.; Muñiz-Salazar, R.; Rendon, A. Coccidioidomycosis in Latin America. Med. Mycol. 2019, 57, S46–S55. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.; Borges, M.C.M.; Cavalcante, L.; Motoyama, P.V.P.; Libório, M.P.; Távora, L.G.F. Coccidioidomycosis in a reference center in Northeast Brazil: Clinical/epidemiological profile and most common radiological findings. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200249. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Toda, M.; Benedict, K.; Caceres, D.H.; Litvintseva, A.P. Endemic and Other Dimorphic Mycoses in The Americas. J. Fungi 2021, 7, 151. [Google Scholar] [CrossRef]
- Huppert, M.; Sun, S.H.; Harrison, J.L. Morphogenesis throughout saprobic and parasitic cycles of Coccidioides immitis. Mycopathologia 1982, 78, 107–122. [Google Scholar] [CrossRef]
- Nguyen, C.; Barker, B.M.; Hoover, S.; Nix, D.E.; Ampel, N.M.; Frelinger, J.A.; Orbach, M.J.; Galgiani, J.N. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin. Microbiol. Rev. 2013, 26, 505–525. [Google Scholar] [CrossRef]
- Sharpton, T.J.; Stajich, J.E.; Rounsley, S.D.; Gardner, M.J.; Wortman, J.R.; Jordar, V.S.; Maiti, R.; Kodira, C.D.; Neafsey, D.E.; Zeng, Q.; et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009, 19, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.W.; Barker, B.M. The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Med. Mycol. 2019, 57, S16–S20. [Google Scholar] [CrossRef] [PubMed]
- Shubitz, L.E.; Butkiewicz, C.D.; Dial, S.M.; Lindan, C.P. Incidence of coccidioides infection among dogs residing in a region in which the organism is endemic. J. Am. Vet. Med. Assoc. 2005, 226, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Shubitz, L.F. Comparative aspects of coccidioidomycosis in animals and humans. Ann. N. Y. Acad. Sci. 2007, 1111, 395–403. [Google Scholar] [CrossRef]
- Wilson, L.; Ting, J.; Lin, H.; Shah, R.; MacLean, M.; Peterson, M.W.; Stockamp, N.; Libke, R.; Brown, P. The Rise of Valley Fever: Prevalence and Cost Burden of Coccidioidomycosis Infection in California. Int. J. Environ. Res. Public Health 2019, 16, 1113. [Google Scholar] [CrossRef]
- Increase in reported coccidioidomycosis—United States, 1998–2011. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 217–221.
- Oltean, H.N.; Springer, M.; Bowers, J.R.; Barnes, R.; Reid, G.; Valentine, M.; Engelthaler, D.M.; Toda, M.; McCotter, O.Z. Suspected Locally Acquired Coccidioidomycosis in Human, Spokane, Washington, USA. Emerg. Infect. Dis. 2020, 26, 606–609. [Google Scholar] [CrossRef]
- Johnson, S.M.; Carlson, E.L.; Fisher, F.S.; Pappagianis, D. Demonstration of Coccidioides immitis and Coccidioides posadasii DNA in soil samples collected from Dinosaur National Monument, Utah. Med. Mycol. 2014, 52, 610–617. [Google Scholar] [CrossRef]
- Yoo, S.D.; Lusiba, J.K.; Lukande, R.; Shin, K. Disseminated Coccidioidomycosis in Africa. Eur. J. Case Rep. Intern. Med. 2020, 7, 001659. [Google Scholar] [CrossRef]
- Hernandez, H.; Erives, V.H.; Martinez, L.R. Coccidioidomycosis: Epidemiology, Fungal Pathogenesis, and Therapeutic Development. Curr. Trop. Med. Rep. 2019, 6, 132–144. [Google Scholar] [CrossRef]
- Huang, J.Y.; Bristow, B.; Shafir, S.; Sorvillo, F. Coccidioidomycosis-associated Deaths, United States, 1990–2008. Emerg. Infect. Dis. 2012, 18, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, L.; Nix, D.; Wright, M.; Lindberg, E.; Fagan, T.; Lieberman, D.; Stoffer, T.; Ampel, N.M.; Galgiani, J.N. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg. Infect. Dis. 2006, 12, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.H.; Sharma, R.; Kuran, R.; Fong, I.; Heidari, A. Coccidioidomycosis: A review. J. Investig. Med. 2021, 69, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N.; Ampel, N.M.; Blair, J.E.; Catanzaro, A.; Johnson, R.H.; Stevens, D.A.; Williams, P.L. Coccidioidomycosis. Clin. Infect. Dis. 2005, 41, 1217–1223. [Google Scholar] [CrossRef]
- Johnson, R.; Caldwell, J.; Welch, G.; Einstein, H. The great coccidioidomycosis epidemic: Clinical features. In Proceedings of the Coccidioidomycosis: Fifth International Conference; National Foundation for Infectious Diseases: Washington, DC, USA, 1996. [Google Scholar]
- Odio, C.D.; Marciano, B.E.; Galgiani, J.N.; Holland, S.M. Risk Factors for Disseminated Coccidioidomycosis, United States. Emerg. Infect. Dis. 2017, 23, 308–311. [Google Scholar] [CrossRef]
- Goldstein, E.J.C.; Johnson, R.H.; Einstein, H.E. Coccidioidal Meningitis. Clin. Infect. Dis. 2006, 42, 103–107. [Google Scholar] [CrossRef]
- McCotter, O.Z.; Benedict, K.; Engelthaler, D.M.; Komatsu, K.; Lucas, K.D.; Mohle-Boetani, J.C.; Oltean, H.; Vugia, D.; Chiller, T.M.; Sondermeyer Cooksey, G.L.; et al. Update on the Epidemiology of coccidioidomycosis in the United States. Med. Mycol. 2019, 57, S30–S40. [Google Scholar] [CrossRef]
- Gorris, M.E.; Neumann, J.E.; Kinney, P.L.; Sheahan, M.; Sarofim, M.C. Economic Valuation of Coccidioidomycosis (Valley Fever) Projections in the United States in Response to Climate Change. Weather Clim. Soc. 2021, 13, 107–123. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M. A global view on fungal infections in humans and animals: Infections caused by dimorphic fungi and dermatophytoses. J. Appl. Microbiol. 2021, 131, 2688–2704. [Google Scholar] [CrossRef]
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds. mBio 2019, 10, e01397-19. [Google Scholar] [CrossRef]
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-drawing the Maps for Endemic Mycoses. Mycopathologia 2020, 185, 843–865. [Google Scholar] [CrossRef] [PubMed]
- Pearson, D.; Ebisu, K.; Wu, X.; Basu, R. A Review of Coccidioidomycosis in California: Exploring the Intersection of Land Use, Population Movement, and Climate Change. Epidemiol. Rev. 2019, 41, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Matlock, M.; Hopfer, S.; Ogunseitan, O.A. Communicating Risk for a Climate-Sensitive Disease: A Case Study of Valley Fever in Central California. Int. J. Environ. Res. Public Health 2019, 16, 3254. [Google Scholar] [CrossRef] [PubMed]
- Shiu, J.; Thai, M.; Elsensohn, A.N.; Nguyen, N.Q.; Lin, K.Y.; Cassarino, D.S. A case series of primary cutaneous coccidioidomycosis after a record-breaking rainy season. JAAD Case Rep. 2018, 4, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Zender, C.S.; Talamantes, J. Climate controls on valley fever incidence in Kern County, California. Int. J. Biometeorol. 2006, 50, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Coates, S.J.; Fox, L.P. Disseminated coccidioidomycosis as a harbinger of climate change. JAAD Case Rep. 2018, 4, 424–425. [Google Scholar] [CrossRef]
- Gorris, M.E.; Treseder, K.K.; Zender, C.S.; Randerson, J.T. Expansion of Coccidioidomycosis Endemic Regions in the United States in Response to Climate Change. Geohealth 2019, 3, 308–327. [Google Scholar] [CrossRef]
- Wang, J.; Harrigan, R.J.; Schoenberg, F.P. Point Process Models for the Spread of Coccidioidomycosis in California. Infect. Dis. Rep. 2021, 13, 52. [Google Scholar] [CrossRef]
- Mitchell, W.; Bhatia, R.; Zebardast, N. Retrospective cross-sectional analysis of the changes in marijuana use in the USA, 2005–2018. BMJ Open 2020, 10, e037905. [Google Scholar] [CrossRef]
- McHardy, I.; Romanelli, A.; Harris, L.J.; Opp, G.; Gaudino, R.; Torres, A.; Polage, C.R.; Tuscano, J.M.; Thompson, G.R., 3rd. Infectious risks associated with medicinal Cannabis: Potential implications for immunocompromised patients? J. Infect. 2018, 76, 500–501. [Google Scholar] [CrossRef]
- Shapiro Bb Md, M.P.H.; Hedrick, R.; Vanle, B.C.; Becker, C.A.; Nguyen, C.; Underhill, D.M.; Morgan, M.A.; Kopple, J.D.; Danovitch, I.; IsHak, W.W. Cryptococcal meningitis in a daily cannabis smoker without evidence of immunodeficiency. BMJ Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Thompson, G.R., 3rd; Jackson, B.R. Cannabis Use and Fungal Infections in a Commercially Insured Population, United States, 2016. Emerg. Infect. Dis. 2020, 26, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Akram, S.M.; Koirala, J. Coccidioidomycosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Balderas-Sosa, E.Y.; De la Torre, J.L.; Soualhi, A.; Leyva-Moraga, F.A.; Leyva-Moraga, F.; Leyva-Moraga, E. Coccidioidomycosis mimicking testicular cancer: A case report. Andrologia 2021, 53, e14151. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cano, E.J.; Shweta, F.; Shah, A.S.; Schuetz, A.N.; Bois, M.; Gurram, P.R. Infected Aneurysm of the Native Aorta due to Coccidioides posadasii. Open Forum Infect. Dis. 2021, 8, ofab266. [Google Scholar] [CrossRef]
- Nasrawi, F.; Heidari, A.; Aljashamy, T.; Mangat, N.; Bhaika, J.; Kaur, S.; Kuran, R.; Johnson, R. Disseminated Coccidioidomycosis Presenting as Polyarticular Septic Arthritis: A Case Report. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620974894. [Google Scholar] [CrossRef]
- Converse, C.; Dey, A.; Decker, S.; Arabian, S.; Neeki, M. Coccidioidomycosis of the Vocal Cords Presenting in Sepsis: A Case Report and Literature Review. Case Rep. Crit. Care 2020, 2020, 8025391. [Google Scholar] [CrossRef]
- Aduroja, O.; Okudo, J.; Padilla, A. Disseminated Coccidioidomycosis Presenting as Septic Shock with Multiorgan Failure. Case Rep. Infect. Dis. 2021, 2021, 8837493. [Google Scholar] [CrossRef]
- Pu, J.; Donovan, F.M.; Ellingson, K.; Leroy, G.; Stone, J.; Bedrick, E.; Galgiani, J.N. Clinician Practice Patterns that Result in the Diagnosis of Coccidioidomycosis Before or During Hospitalization. Clin. Infect. Dis. 2020, 73, e1587–e1593. [Google Scholar] [CrossRef]
- Durkin, M.; Connolly, P.; Kuberski, T.; Myers, R.; Kubak, B.M.; Bruckner, D.; Pegues, D.; Wheat, L.J. Diagnosis of Coccidioidomycosis with Use of the Coccidioides Antigen Enzyme Immunoassay. Clin. Infect. Dis. 2008, 47, e69–e73. [Google Scholar] [CrossRef]
- Saubolle, M.A. Laboratory Aspects in the Diagnosis of Coccidioidomycosis. Ann. N. Y. Acad. Sci. 2007, 1111, 301–314. [Google Scholar] [CrossRef]
- Kassis, C.; Durkin, M.; Holbrook, E.; Myers, R.; Wheat, L. Advances in Diagnosis of Progressive Pulmonary and Disseminated Coccidioidomycosis. Clin. Infect. Dis. 2020, 72, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N.; Ampel, N.M.; Blair, J.E.; Catanzaro, A.; Geertsma, F.; Hoover, S.E.; Johnson, R.H.; Kusne, S.; Lisse, J.; MacDonald, J.D.; et al. 2016 Infectious Diseases Society of America (IDSA) Clinical Practice Guideline for the Treatment of Coccidioidomycosis. Clin. Infect. Dis. 2016, 63, e112–e146. [Google Scholar] [CrossRef] [PubMed]
- Greiner, B.; Essex, R.; Wheeler, D. An analysis of research quality underlying IDSA clinical practice guidelines: A cross-sectional study. J. Osteopath. Med. 2021, 121, 319–323. [Google Scholar] [CrossRef] [PubMed]
- DIFLUCAN (Fluconazole). New York, New York: Pfizer. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019949s051lbl.pdf (accessed on 24 February 2022).
- Amphotericin, B. Big Flats, NY: X-Gen Pharmaceuticals, Inc. Available online: http://xgenpharmadjb.com/wp-content/uploads/sites/21/2021/12/ampho.pdf. (accessed on 24 February 2022).
- SPORANOX (Itraconazole). Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020083s063lbl.pdf (accessed on 24 February 2022).
- Thompson, G.R., III; Lewis, J.S., II; Nix, D.E.; Patterson, T.F. Current Concepts and Future Directions in the Pharmacology and Treatment of Coccidioidomycosis. Med. Mycol. 2019, 57, S76–S84. [Google Scholar] [CrossRef]
- Bercovitch, R.S.; Catanzaro, A.; Schwartz, B.S.; Pappagianis, D.; Watts, D.H.; Ampel, N.M. Coccidioidomycosis During Pregnancy: A Review and Recommendations for Management. Clin. Infect. Dis. 2011, 53, 363–368. [Google Scholar] [CrossRef]
- Davis, M.R.; Nguyen, M.-V.H.; Donnelley, M.A.; Thompson, G.R., III. Tolerability of long-term fluconazole therapy. J. Antimicrob. Chemother. 2018, 74, 768–771. [Google Scholar] [CrossRef]
- Thompson, G.R.; Barker, B.M.; Wiederhold, N.P. Large-Scale Evaluation of In Vitro Amphotericin B, Triazole, and Echinocandin Activity against Coccidioides Species from U.S. Institutions. Antimicrob. Agents Chemother. 2017, 61, e02634-02616. [Google Scholar] [CrossRef]
- Decembrino, N.; Perruccio, K.; Zecca, M.; Colombini, A.; Calore, E.; Muggeo, P.; Soncini, E.; Comelli, A.; Molinaro, M.; Goffredo, B.M.; et al. A Case Series and Literature Review of Isavuconazole Use in Pediatric Patients with Hemato-oncologic Diseases and Hematopoietic Stem Cell Transplantation. Antimicrob. Agents Chemother. 2020, 64, e01783-01719. [Google Scholar] [CrossRef]
- Thompson, G.R., 3rd; Wiederhold, N.P. Isavuconazole: A comprehensive review of spectrum of activity of a new triazole. Mycopathologia 2010, 170, 291–313. [Google Scholar] [CrossRef]
- Heidari, A.; Quinlan, M.; Benjamin, D.J.; Laurence, B.; Mu, A.; Ngai, T.; Hoffman, W.J.; Cohen, S.H.; McHardy, I.; Johnson, R.; et al. Isavuconazole in the Treatment of Coccidioidal Meningitis. Antimicrob. Agents Chemother. 2019, 63, e02232-02218. [Google Scholar] [CrossRef]
- Kovanda, L.L.; Sass, G.; Martinez, M.; Clemons, K.V.; Nazik, H.; Kitt, T.M.; Wiederhold, N.; Hope, W.W.; Stevens, D.A. Efficacy and Associated Drug Exposures of Isavuconazole and Fluconazole in an Experimental Model of Coccidioidomycosis. Antimicrob. Agents Chemother. 2021, 65, e02344-02320. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.R.; Chang, S.; Gaynor, P.; McCreary, E.K.; Allyn, P. Isavuconazole for treatment of refractory coccidioidal meningitis with concomitant cerebrospinal fluid and plasma therapeutic drug monitoring. Med. Mycol. 2021, 59, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef] [PubMed]
- Deresinski, S.; Mirels, L.F. Coccidioidomycosis: What a long strange trip it’s been. Med. Mycol. 2019, 57, S3–S15. [Google Scholar] [CrossRef]
- Halde, C.; Newcomer, V.D.; Wright, E.T.; Sternberg, T.H. An Evaluation of Amphotericin B In Vitro and In Vivo in Mice Against Coccidioides Immitis and Candida Albicans, and Preliminary Observations Concerning the Administration of Amphotericin B to Man. J. Investig. Dermatol. 1957, 28, 217–232. [Google Scholar] [CrossRef]
- Hamill, R.J. Amphotericin B formulations: A comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef]
- Sidhu, R.; Lash, D.B.; Heidari, A.; Natarajan, P.; Johnson, R.H. Evaluation of Amphotericin B Lipid Formulations for Treatment of Severe Coccidioidomycosis. Antimicrob. Agents Chemother. 2018, 62, e02293-02217. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Shubitz, L.F.; Najvar, L.K.; Jaramillo, R.; Olivo, M.; Catano, G.; Trinh, H.T.; Yates, C.M.; Schotzinger, R.J.; Garvey, E.P.; et al. The Novel Fungal Cyp51 Inhibitor VT-1598 Is Efficacious in Experimental Models of Central Nervous System Coccidioidomycosis Caused by Coccidioides posadasii and Coccidioides immitis. Antimicrob. Agents Chemother. 2018, 62, e02258-02217. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Jaramillo, R.; Olivo, M.; Birch, M.; Law, D.; Rex, J.H.; Catano, G.; Patterson, T.F. The Orotomide Olorofim Is Efficacious in an Experimental Model of Central Nervous System Coccidioidomycosis. Antimicrob. Agents Chemother. 2018, 62, e00999-00918. [Google Scholar] [CrossRef]
- Rauseo, A.M.; Coler-Reilly, A.; Larson, L.; Spec, A. Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect. Dis. 2020, 7, ofaa016. [Google Scholar] [CrossRef]
- F2G. F2G Receives Second US FDA Breakthrough Therapy Designation for Olorofim. Available online: https://www.prnewswire.com/news-releases/f2g-receives-second-us-fda-breakthrough-therapy-designation-for-olorofim-301157698.html (accessed on 15 August 2021).
- Jallow, S.; Govender, N.P. Ibrexafungerp: A First-in-Class Oral Triterpenoid Glucan Synthase Inhibitor. J. Fungi 2021, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.J.; Ibrahim, A.S. Fosmanogepix: A Review of the First-in-Class Broad Spectrum Agent for the Treatment of Invasive Fungal Infections. J. Fungi 2020, 6, 239. [Google Scholar] [CrossRef] [PubMed]
- Larwood, D.J. Nikkomycin Z—Ready to Meet the Promise? J. Fungi 2020, 6, 261. [Google Scholar] [CrossRef] [PubMed]
- Nix, D.E.; Swezey, R.R.; Hector, R.; Galgiani, J.N. Pharmacokinetics of Nikkomycin Z after Single Rising Oral Doses. Antimicrob. Agents Chemother. 2009, 53, 2517–2521. [Google Scholar] [CrossRef]
- Shubitz, L.F.; Trinh, H.T.; Perrill, R.H.; Thompson, C.M.; Hanan, N.J.; Galgiani, J.N.; Nix, D.E. Modeling nikkomycin Z dosing and pharmacology in murine pulmonary coccidioidomycosis preparatory to phase 2 clinical trials. J. Infect. Dis. 2014, 209, 1949–1954. [Google Scholar] [CrossRef]
- Sass, G.; Larwood, D.J.; Martinez, M.; Shrestha, P.; Stevens, D.A. Efficacy of nikkomycin Z in murine CNS coccidioidomycosis: Modelling sustained-release dosing. J. Antimicrob. Chemother. 2021, 76, 2629–2635. [Google Scholar] [CrossRef]
- Ringel, S.M.; Greenough, R.C.; Roemer, S.; Connor, D.; Gutt, A.L.; Blair, B.; Kanter, G.; Von Strandtmann, M. Ambruticin (W7783), a new antifungal antibiotic. J. Antibiot. 1977, 30, 371–375. [Google Scholar] [CrossRef]
- Levine, H.B.; Ringel, S.M.; Cobb, J.M. Therapeutic properties of oral ambruticin (W7783) in experimental pulmonary coccidioidomycosis of mice. Chest 1978, 73, 202–206. [Google Scholar] [CrossRef]
- Knauth, P.; Reichenbach, H. On the mechanism of action of the myxobacterial fungicide ambruticin. J. Antibiot. 2000, 53, 1182–1190. [Google Scholar] [CrossRef]
- Chiang, L.Y.; Ejzykowicz, D.E.; Tian, Z.-Q.; Katz, L.; Filler, S.G. Efficacy of ambruticin analogs in a murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2006, 50, 3464–3466. [Google Scholar] [CrossRef][Green Version]
- Shubitz, L.F.; Galgiani, J.N.; Tian, Z.Q.; Zhong, Z.; Timmermans, P.; Katz, L. Efficacy of ambruticin analogs in a murine model of coccidioidomycosis. Antimicrob. Agents Chemother. 2006, 50, 3467–3469. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.Q.; Wang, Z.; Xu, Y.; Tran, C.Q.; Myles, D.C.; Zhong, Z.; Simmons, J.; Vetcher, L.; Katz, L.; Li, Y.; et al. Investigating amine derivatives of ambruticin VS-5 and VS-4. ChemMedChem 2008, 3, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Vetcher, L.; Menzella, H.G.; Kudo, T.; Motoyama, T.; Katz, L. The Antifungal Polyketide Ambruticin Targets the HOG Pathway. Antimicrob. Agents Chemother. 2007, 51, 3734–3736. [Google Scholar] [CrossRef] [PubMed]
- Shubitz, L.F.; Trinh, H.T.; Galgiani, J.N.; Lewis, M.L.; Fothergill, A.W.; Wiederhold, N.P.; Barker, B.M.; Lewis, E.R.G.; Doyle, A.L.; Hoekstra, W.J.; et al. Evaluation of VT-1161 for Treatment of Coccidioidomycosis in Murine Infection Models. Antimicrob. Agents Chemother. 2015, 59, 7249–7254. [Google Scholar] [CrossRef]
- Shubitz, L.F.; Roy, M.E.; Trinh, H.T.; Hoekstra, W.J.; Schotzinger, R.J.; Garvey, E.P. Efficacy of the Investigational Antifungal VT-1161 in Treating Naturally Occurring Coccidioidomycosis in Dogs. Antimicrob. Agents Chemother. 2017, 61, e00111-00117. [Google Scholar] [CrossRef]
- O’Shaughnessy, E.; Yasinskaya, Y.; Dixon, C.; Higgins, K.; Moore, J.; Reynolds, K.; Ampel, N.M.; Angulo, D.; Blair, J.E.; Catanzaro, A.; et al. FDA Public Workshop Summary-Coccidioidomycosis (Valley Fever): Considerations for Development of Antifungal Drugs. Clin. Infect. Dis. 2021, ciab904. [Google Scholar] [CrossRef]
- Tsai, M.; Thauland, T.J.; Huang, A.Y.; Bun, C.; Fitzwater, S.; Krogstad, P.; Douine, E.D.; Nelson, S.F.; Lee, H.; Garcia-Lloret, M.I.; et al. Disseminated Coccidioidomycosis Treated with Interferon-γ and Dupilumab. N. Engl. J. Med. 2020, 382, 2337–2343. [Google Scholar] [CrossRef]
- Paul, S.; Mortimer, R.B.; Mitchell, M. Sertraline demonstrates fungicidal activity in vitro for Coccidioides immitis. Mycology 2016, 7, 99–101. [Google Scholar] [CrossRef]
- Duplessis, C.A.; Tilley, D.; Bavaro, M.; Hale, B.; Holland, S.M. Two cases illustrating successful adjunctive interferon-γ immunotherapy in refractory disseminated coccidioidomycosis. J. Infect. 2011, 63, 223–228. [Google Scholar] [CrossRef]
- Kuberski, T.T.; Servi, R.J.; Rubin, P.J. Successful Treatment of a Critically Ill Patient with Disseminated Coccidioidomycosis, Using Adjunctive Interferon-γ. Clin. Infect. Dis. 2004, 38, 910–912. [Google Scholar] [CrossRef]
- De la Hoz, A.; Malek, A.; Hasbun, R. Interferon-γ and voriconazole combined therapy for refractory meningeal coccidioidomycosis in a patient with interferon-γ deficiency. IDCases 2020, 21, e00835. [Google Scholar] [CrossRef] [PubMed]
- Trainor, M.; Henkel, E.; Diaz, L.Z.; Carrasco, R. Disseminated coccidioidomycosis in a patient with juvenile idiopathic arthritis receiving infliximab. Pediatr. Rheumatol. 2021, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, T.N. The Quest for a Vaccine Against Coccidioidomycosis: A Neglected Disease of the Americas. J. Fungi 2016, 2, 34. [Google Scholar] [CrossRef]
- Barnato, A.E.; Sanders, G.D.; Owens, D.K. Cost-effectiveness of a potential vaccine for Coccidioides immitis. Emerg. Infect. Dis. 2001, 7, 797–806. [Google Scholar] [CrossRef] [PubMed]
- B R Da Silva, L.; P Taborda, C.; D Nosanchuk, J. Advances in Fungal Peptide Vaccines. J. Fungi 2020, 6, 119. [Google Scholar] [CrossRef]
- Tarcha, E.J.; Basrur, V.; Hung, C.Y.; Gardner, M.J.; Cole, G.T. A recombinant aspartyl protease of Coccidioides posadasii induces protection against pulmonary coccidioidomycosis in mice. Infect. Immun. 2006, 74, 516–527. [Google Scholar] [CrossRef]
- Tarcha, E.J.; Basrur, V.; Hung, C.Y.; Gardner, M.J.; Cole, G.T. Multivalent recombinant protein vaccine against coccidioidomycosis. Infect. Immun. 2006, 74, 5802–5813. [Google Scholar] [CrossRef]
- Hurtgen, B.J.; Hung, C.Y.; Ostroff, G.R.; Levitz, S.M.; Cole, G.T. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against Coccidioidomycosis. Infect. Immun. 2012, 80, 3960–3974. [Google Scholar] [CrossRef]
- Hung, C.Y.; Zhang, H.; Castro-Lopez, N.; Ostroff, G.R.; Khoshlenar, P.; Abraham, A.; Cole, G.T.; Negron, A.; Forsthuber, T.; Peng, T.; et al. Glucan-Chitin Particles Enhance Th17 Response and Improve Protective Efficacy of a Multivalent Antigen (rCpa1) against Pulmonary Coccidioides posadasii Infection. Infect. Immun. 2018, 86, 86. [Google Scholar] [CrossRef]
- Powell, D.A.; Hsu, A.P.; Butkiewicz, C.D.; Trinh, H.T.; Frelinger, J.A.; Holland, S.M.; Galgiani, J.N.; Shubitz, L.F. Vaccine Protection of Mice With Primary Immunodeficiencies Against Disseminated Coccidioidomycosis. Front. Cell. Infect. Microbiol. 2021, 11, 790488. [Google Scholar] [CrossRef]
- Shubitz, L.F.; Powell, D.A.; Trinh, H.T.; Lewis, M.L.; Orbach, M.J.; Frelinger, J.A.; Galgiani, J.N. Viable spores of Coccidioides posadasii Δcps1 are required for vaccination and provide long lasting immunity. Vaccine 2018, 36, 3375–3380. [Google Scholar] [CrossRef] [PubMed]
- Shubitz, L.F.; Robb, E.J.; Powell, D.A.; Bowen, R.A.; Bosco-Lauth, A.; Hartwig, A.; Porter, S.M.; Trinh, H.; Moale, H.; Bielefeldt-Ohmann, H.; et al. Δcps1 vaccine protects dogs against experimentally induced coccidioidomycosis. Vaccine 2021, 39, 6894–6901. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.K.; O’Neil, D.A. Innate Inspiration: Antifungal Peptides and Other Immunotherapeutics From the Host Immune Response. Front. Immunol. 2020, 11, 2177. [Google Scholar] [CrossRef] [PubMed]
- Frías-De-León, M.G.; Pinto-Almazán, R.; Hernández-Castro, R.; García-Salazar, E.; Meza-Meneses, P.; Rodríguez-Cerdeira, C.; Arenas, R.; Conde-Cuevas, E.; Acosta-Altamirano, G.; Martínez-Herrera, E. Epidemiology of Systemic Mycoses in the COVID-19 Pandemic. J. Fungi 2021, 7, 556. [Google Scholar] [CrossRef]
- Yousaf, Z.; Siddiqui, M.Y.A.; Mushtaq, K.; Feroz, S.E.; Aboukamar, M.; Mohamedali, M.G.H.; Chaudhary, H. Avoiding Anchoring Bias in the Times of the Pandemic! Case Rep. Neurol. 2020, 12, 359–364. [Google Scholar] [CrossRef]
- Benedict, K.; Williams, S.; Beekmann, S.E.; Polgreen, P.M.; Jackson, B.R.; Toda, M. Testing Practices for Fungal Respiratory Infections and SARS-CoV-2 among Infectious Disease Specialists, United States. J. Fungi 2021, 7, 605. [Google Scholar] [CrossRef]
- Pemán, J.; Ruiz-Gaitán, A.; García-Vidal, C.; Salavert, M.; Ramírez, P.; Puchades, F.; García-Hita, M.; Alastruey-Izquierdo, A.; Quindós, G. Fungal co-infection in COVID-19 patients: Should we be concerned? Rev. Iberoamer. Micol. 2020, 37, 41–46. [Google Scholar] [CrossRef]
- Heaney, A.K.; Head, J.R.; Broen, K.; Click, K.; Taylor, J.; Balmes, J.R.; Zelner, J.; Remais, J.V. Coccidioidomycosis and COVID-19 Co-Infection, United States, 2020. Emerg. Infect. Dis. 2021, 27, 1266–1273. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Reynoso, D.; Ren, P.; Burnham, C.-A.D. The Brief Case: A Fatal Case of SARS-CoV-2 Coinfection with Coccidioides in Texas—Another Challenge We Face. J. Clin. Microbiol. 2021, 59, e00163-00121. [Google Scholar] [CrossRef]
- Chang, C.C.; Senining, R.; Kim, J.; Goyal, R. An Acute Pulmonary Coccidioidomycosis Coinfection in a Patient Presenting With Multifocal Pneumonia With COVID-19. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620972244. [Google Scholar] [CrossRef]
- Chen, J.C.; Wong, D.; Rabi, S.; Worswick, S.; DeClerck, B.; Gibb, J. All That Coughs Is Not COVID-19: A Delayed Diagnosis of Disseminated Coccidioidomycosis Following Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Open Forum Infect. Dis. 2021, 8, ofab246. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.A.; James, S.; Uche, I.U.; Sharer, R.; Radhakrishnan, P. Cutaneous and Pulmonary Manifestations: COVID-19 Virus or Coccidioidomycosis? Cureus 2021, 13, e15060. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Heidari, A.; Civelli, V.F.; Sharma, R.; Clark, C.S.; Munoz, A.D.; Ragland, A.S.; Johnson, R.H. The Coincidence of 2 Epidemics, Coccidioidomycosis and SARS-CoV-2: A Case Report. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620930540. [Google Scholar] [CrossRef]
- Zavala, A.; Stark, C.M. Chest Pain and Fever in a Healthcare Provider During the Global Coronavirus Pandemic. Mil. Med. 2021, usab435. [Google Scholar] [CrossRef] [PubMed]
- Krauth, D.S.; Jamros, C.M.; Rivard, S.C.; Olson, N.H.; Maves, R.C. Accelerated Progression of Disseminated Coccidioidomycosis Following SARS-CoV-2 Infection: A Case Report. Mil. Med. 2021, 186, 1254–1256. [Google Scholar] [CrossRef] [PubMed]
- Nassif, E.F.; Maloney, N.; Conley, A.P.; Keung, E.Z. Disseminated Coccidioidomycosis Following COVID-19 Mimicking Metastatic Thoracic Relapse of Well-Differentiated Liposarcoma: A Case Report. Front. Med. 2021, 8, 715939. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov (accessed on 15 August 2021).
- Azadeh, N.; Chang, Y.H.; Kusne, S.; Vikram, H.R.; Seville, M.T.; Orenstein, R.; Blair, J.E. The impact of early and brief corticosteroids on the clinical course of primary pulmonary coccidioidomycosis. J. Infect. 2013, 67, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, A.; Falahi, S.; Kenarkoohi, A. COVID-19-associated opportunistic infections: A snapshot on the current reports. Clin. Experimentl. Med. 2021. [Google Scholar] [CrossRef]
- Group, T.W.R.E.A.f.C.-T.W. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Gupta, P.; Chow, V.; Wang, R.; Kaplan, I.; Chan, G.; Alvey, C.; Ni, G.; Ndongo, M.-N.; LaBadie, R.R.; Krishnaswami, S. Evaluation of the effect of fluconazole and ketoconazole on the pharmacokinetics of tofacitinib in healthy adult subjects. Clin. Pharmacol. Drug Dev. 2014, 3, 72–77. [Google Scholar] [CrossRef]
- Saling, C.F.; Gea-Banacloche, J.; Trickett, J.S.; Blair, J.E. Coccidioidomycosis in Allogeneic Stem Cell Transplant Recipients: Case Series and Review of the Literature. J. Fungi 2021, 7, 339. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, J.; Tablizo, M.A.; Zayed, Z.; Hepple, R.R.; McCarty, J.M.; Naeem, F. Neonatal Coccidioidomycosis: A Single-center Experience and Review of the Literature. Pediatr. Infect. Dis. J. 2021, 41, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Naeem, F.; Vijayan, V.; Kim, B.Y.; Rahmati, E.; McCarty, J. Congenital Coccidioidomycosis: A Case Report and Review of the Literature. J. Ped. Infect. Dis. Soc. 2021, 10, 789–792. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boro, R.; Iyer, P.C.; Walczak, M.A. Current Landscape of Coccidioidomycosis. J. Fungi 2022, 8, 413. https://doi.org/10.3390/jof8040413
Boro R, Iyer PC, Walczak MA. Current Landscape of Coccidioidomycosis. Journal of Fungi. 2022; 8(4):413. https://doi.org/10.3390/jof8040413
Chicago/Turabian StyleBoro, Ryan, Prema C. Iyer, and Maciej A. Walczak. 2022. "Current Landscape of Coccidioidomycosis" Journal of Fungi 8, no. 4: 413. https://doi.org/10.3390/jof8040413
APA StyleBoro, R., Iyer, P. C., & Walczak, M. A. (2022). Current Landscape of Coccidioidomycosis. Journal of Fungi, 8(4), 413. https://doi.org/10.3390/jof8040413





