Radiation Exposure Perturbs IL-17RA-Mediated Immunity Leading to Changes in Neutrophil Responses That Increase Susceptibility to Oropharyngeal Candidiasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Radiation Induced OM
2.3. Candida albicans Culturing and Handling
2.4. Murine Model of Oropharyngeal Candidiasis
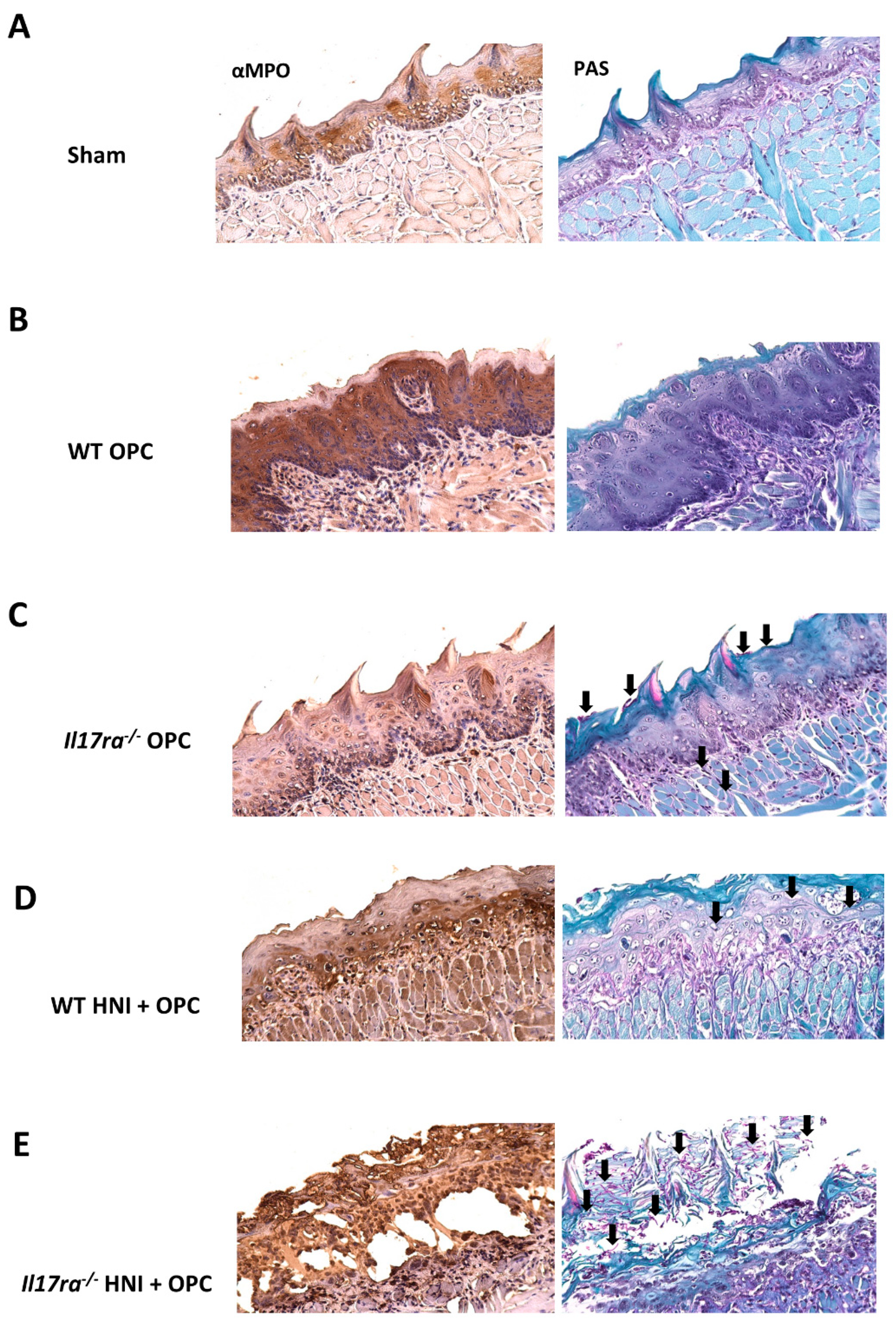
2.5. Macroscopic and Histopathologic Examination
2.6. Immunohistochemistry
2.7. Complete Blood Count
2.8. Realtime PCR
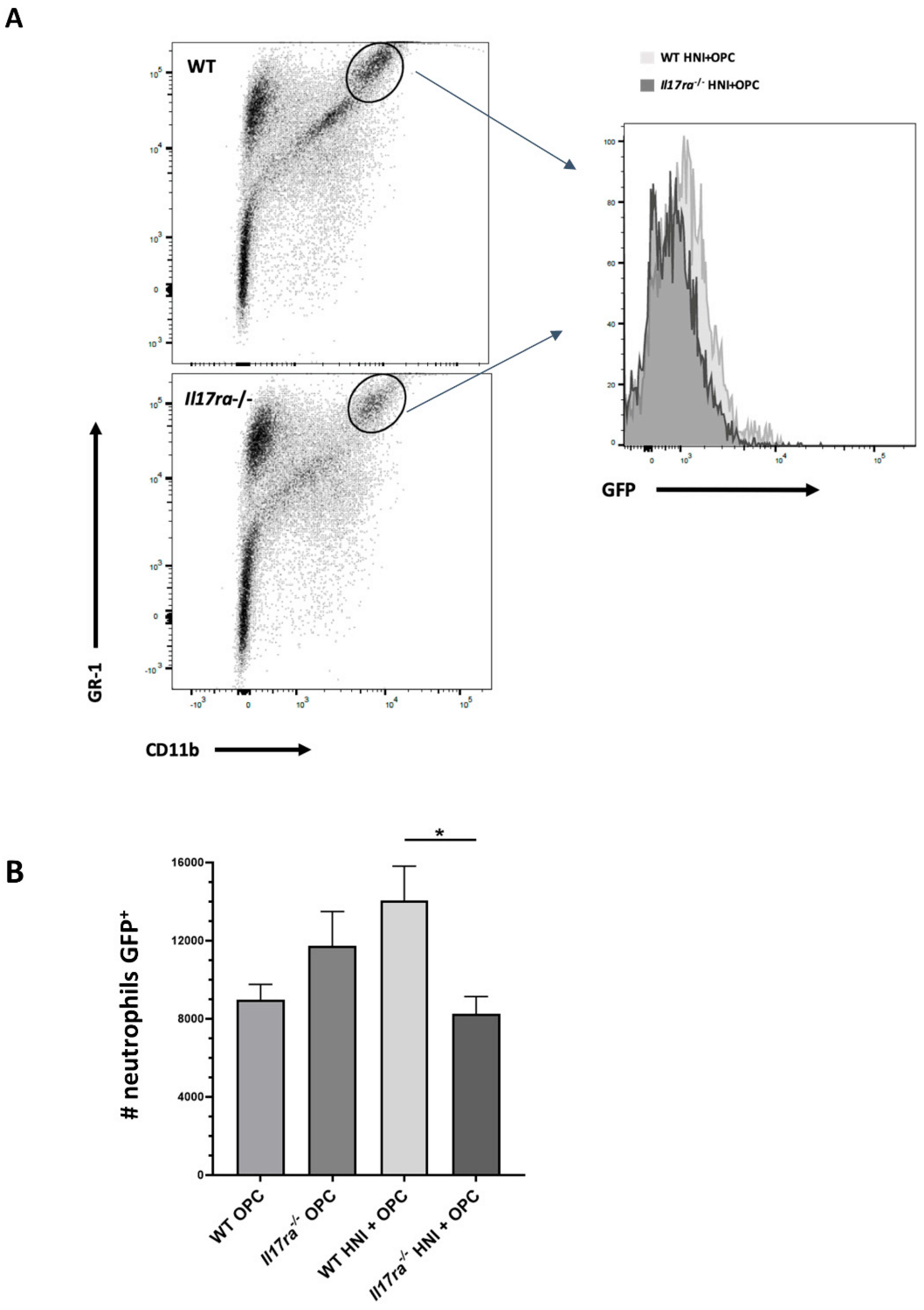
2.9. Flow Cytometry
2.10. Statistics
3. Results
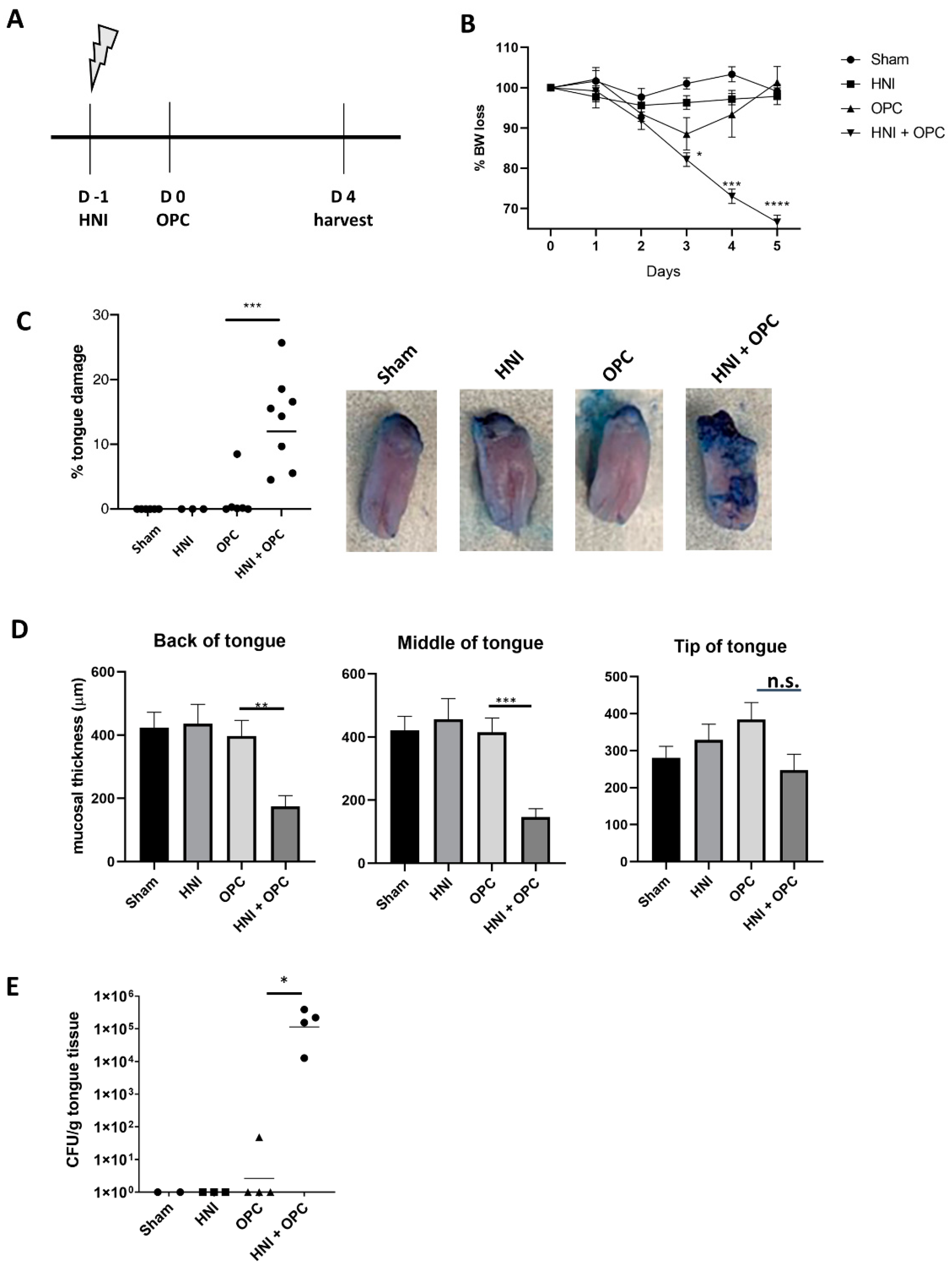
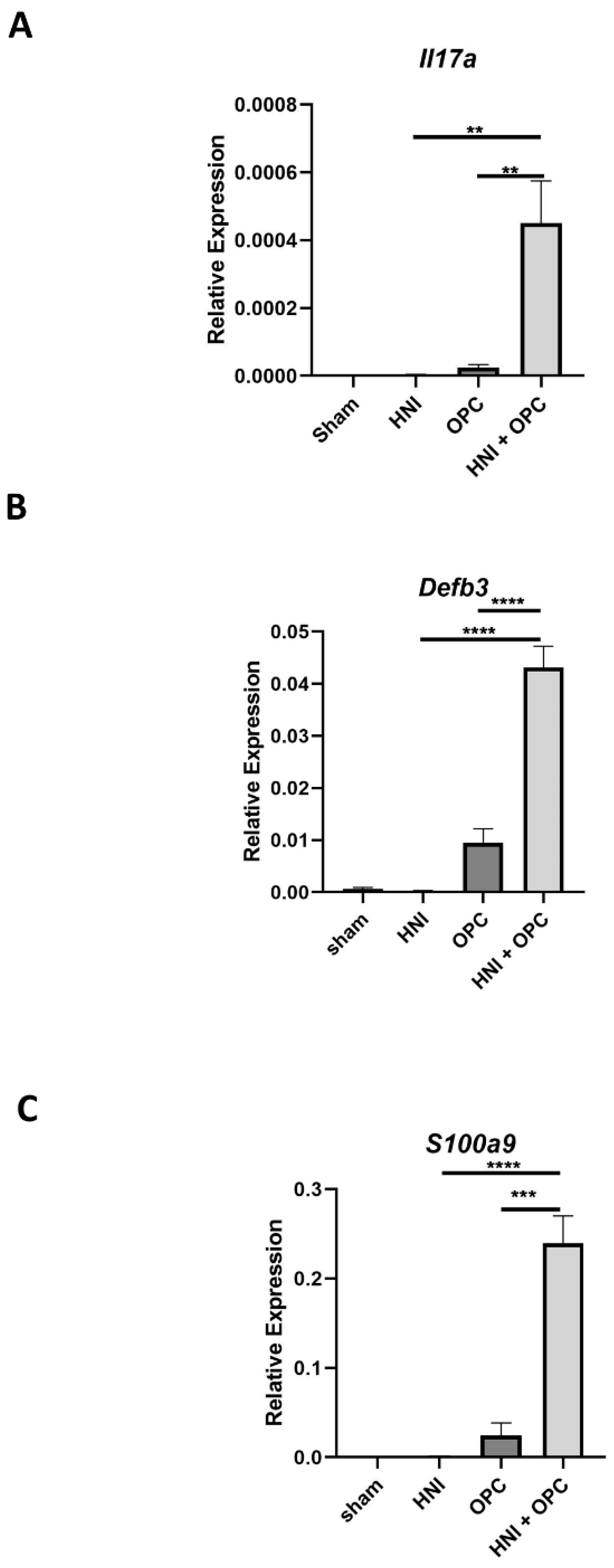
3.1. Exposure to Candida albicans after HNI Leads to Development of Severe OPC
3.2. IL-17RA Signaling Protects against OPC following HNI
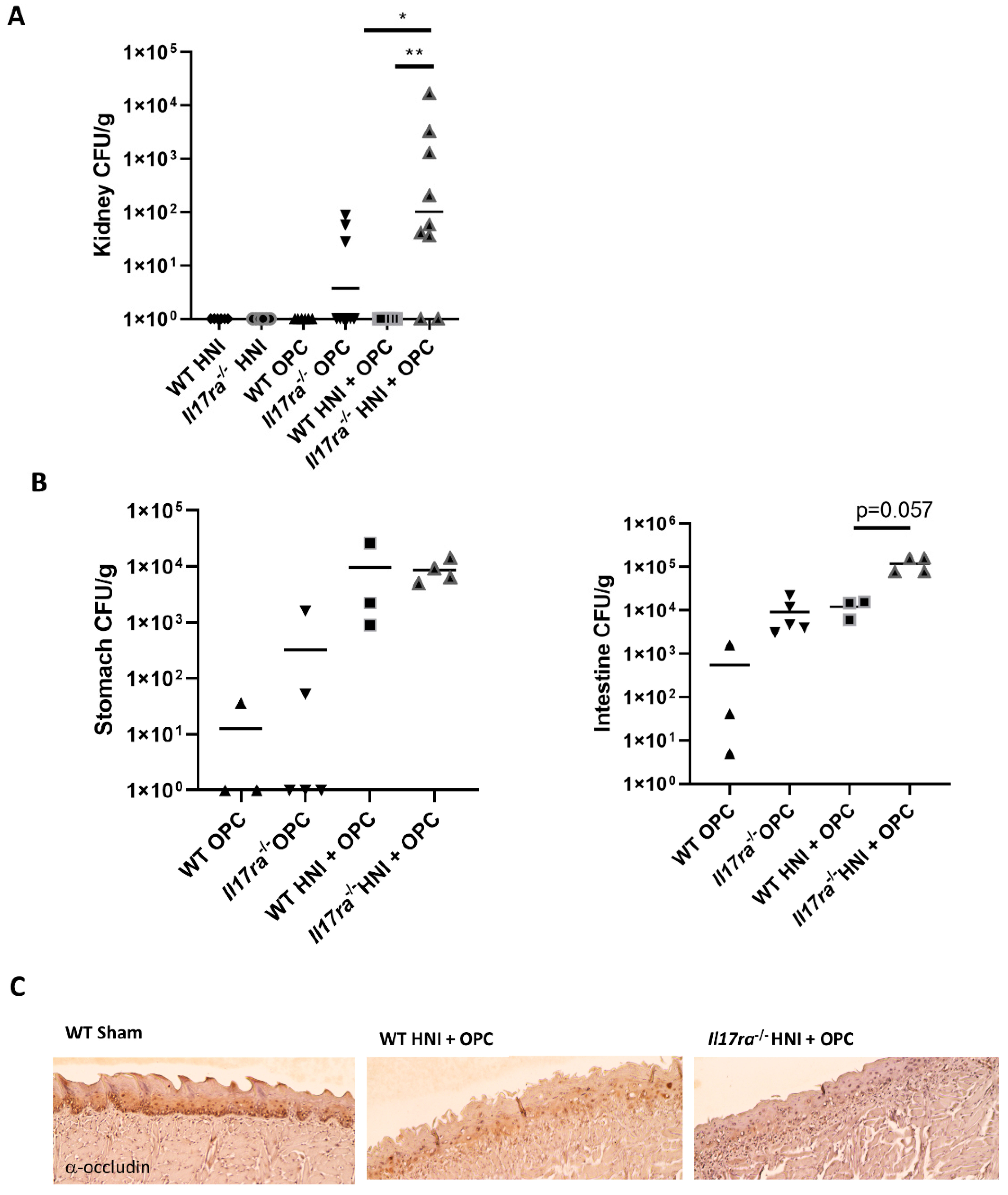
3.3. Lack of IL-17RA Leads to Dissemination of C. albicans after HNI
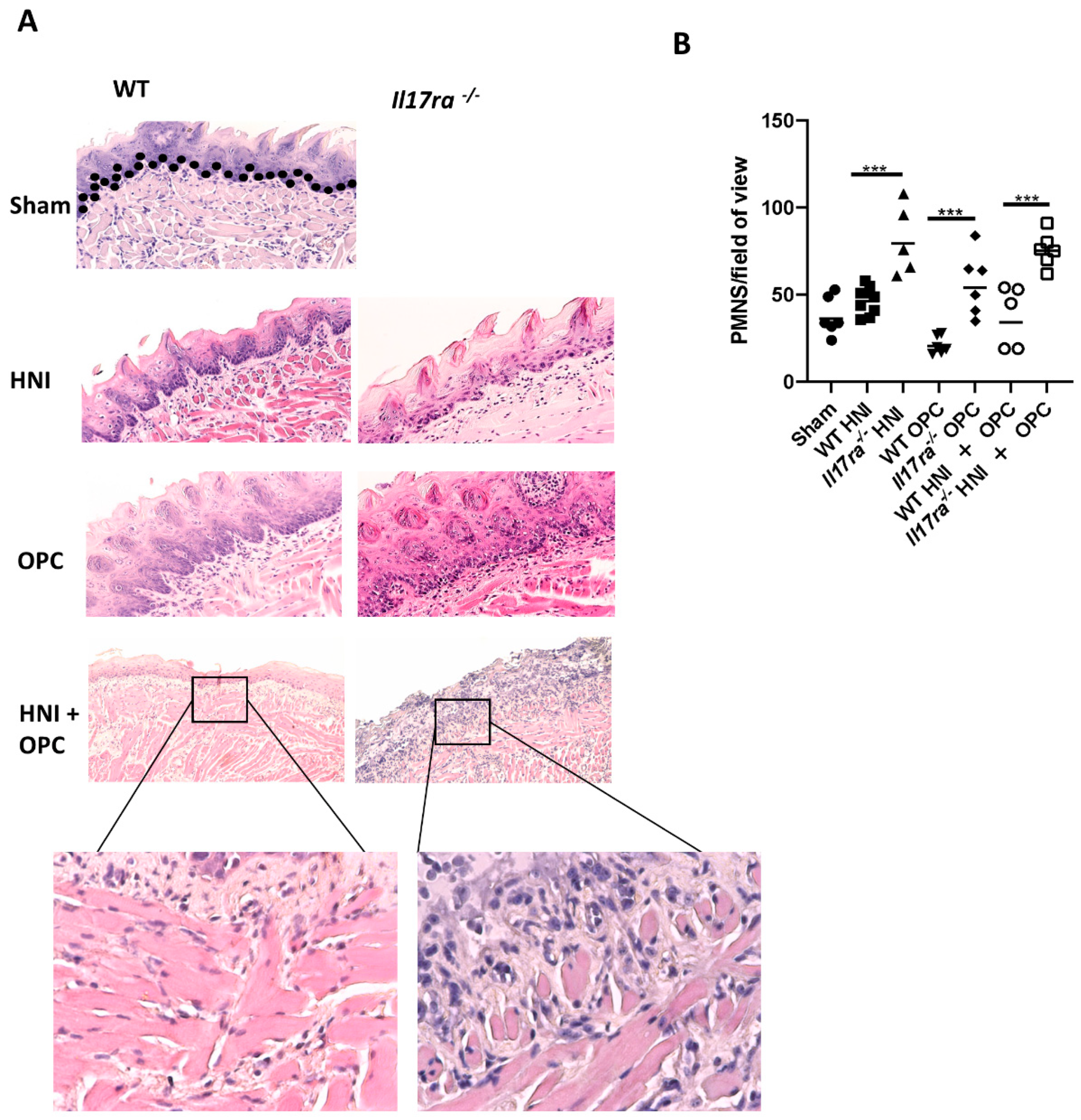
3.4. Heightened Neutrophil Recruitment in IL-17RA-Deficient Mice in Response to OPC after HNI
3.5. Inadequate Anti-Candida Neutrophil Response in IL-17RA-Deficient Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dongari-Bagtzoglou, A.; Fidel, P.L., Jr. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J. Dent. Res. 2005, 84, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Gaffen, S.L. Host responses to Candida albicans: Th17 cells and mucosal candidiasis. Microbes Infect. 2010, 12, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scully, C.; Epstein, J.; Sonis, S. Oral mucositis: A challenging complication of radiotherapy, chemotherapy, and radiochemotherapy. Part 2: Diagnosis and management of mucositis. Head Neck 2004, 26, 77–84. [Google Scholar] [CrossRef]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef]
- Lalla, R.V.; Latortue, M.C.; Hong, C.H.; Ariyawardana, A.; D’Amato-Palumbo, S.; Fischer, D.J.; Martof, A.; Nicolatou-Galitis, O.; Patton, L.L.; Elting, L.S.; et al. A systematic review of oral fungal infections in patients receiving cancer therapy. Support. Care Cancer 2010, 18, 985–992. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, A.P.; Miller Watelet, L.F.; Linder, T.; Eberly, S.; Raubertas, R.F.; Lipp, J.; Duerst, R.; Abboud, C.N.; Constine, L.; Andrews, J.; et al. Analysis of factors that correlate with mucositis in recipients of autologous and allogeneic stem-cell transplants. J. Clin. Oncol. 1999, 17, 2446–2453. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [Green Version]
- Conti, H.R.; Bruno, V.M.; Childs, E.E.; Daugherty, S.; Hunter, J.P.; Mengesha, B.G.; Saevig, D.L.; Hendricks, M.R.; Coleman, B.M.; Brane, L.; et al. IL-17 Receptor Signaling in Oral Epithelial Cells Is Critical for Protection against Oropharyngeal Candidiasis. Cell Host Microbe 2016, 20, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Conti, H.R.; Gaffen, S.L. IL-17-Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. J. Immunol. 2015, 195, 780–788. [Google Scholar] [CrossRef] [Green Version]
- Gaffen, S.L.; Moutsopoulos, N.M. Regulation of host-microbe interactions at oral mucosal barriers by type 17 immunity. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Puel, A. Human inborn errors of immunity underlying superficial or invasive candidiasis. Hum. Genet. 2020, 139, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Peterson, A.C.; Brane, L.; Huppler, A.R.; Hernandez-Santos, N.; Whibley, N.; Garg, A.V.; Simpson-Abelson, M.R.; Gibson, G.A.; Mamo, A.J.; et al. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J. Exp. Med. 2014, 211, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Shen, F.; Nayyar, N.; Stocum, E.; Sun, J.N.; Lindemann, M.J.; Ho, A.W.; Hai, J.H.; Yu, J.J.; Jung, J.W.; et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009, 206, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.W.; Shen, F.; Conti, H.R.; Patel, N.; Childs, E.E.; Peterson, A.C.; Hernandez-Santos, N.; Kolls, J.K.; Kane, L.P.; Ouyang, W.; et al. IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J. Immunol. 2010, 185, 1063–1070. [Google Scholar] [CrossRef]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Brembilla, N.C.; Senra, L.; Boehncke, W.H. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front. Immunol. 2018, 9, 1682. [Google Scholar] [CrossRef] [Green Version]
- Gaffen, S.L. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr. Rheumatol. Rep. 2009, 11, 365–370. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
- Lee, J.S.; Tato, C.M.; Joyce-Shaikh, B.; Gulen, M.F.; Cayatte, C.; Chen, Y.; Blumenschein, W.M.; Judo, M.; Ayanoglu, G.; McClanahan, T.K.; et al. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity 2015, 43, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, J.R.; Zhang, Y.; Brown, W.A.; Smith, C.L.; Byrne, F.R.; Fiorino, M.; Stevens, E.; Bigler, J.; Davis, J.A.; Rottman, J.B.; et al. Differential Roles for Interleukin-23 and Interleukin-17 in Intestinal Immunoregulation. Immunity 2015, 43, 739–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffen, S.L.; Hernandez-Santos, N.; Peterson, A.C. IL-17 signaling in host defense against Candida albicans. Immunol. Res. 2011, 50, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, B.M.; Coleman, B.M.; Willems, H.M.E.; Barker, K.S.; Aggor, F.E.Y.; Cipolla, E.; Verma, A.H.; Bishu, S.; Huppler, A.H.; Bruno, V.M.; et al. The Interleukin (IL) 17R/IL-22R Signaling Axis Is Dispensable for Vulvovaginal Candidiasis Regardless of Estrogen Status. J. Infect. Dis. 2020, 221, 1554–1563. [Google Scholar] [CrossRef]
- Davidson, L.; van den Reek, J.; Bruno, M.; van Hunsel, F.; Herings, R.M.C.; Matzaraki, V.; Boahen, C.K.; Kumar, V.; Groenewoud, H.M.M.; van de Veerdonk, F.L.; et al. Risk of candidiasis associated with interleukin-17 inhibitors: A real-world observational study of multiple independent sources. Lancet Reg. Health Eur. 2022, 13, 100266. [Google Scholar] [CrossRef] [PubMed]
- Pietrella, D.; Rachini, A.; Pines, M.; Pandey, N.; Mosci, P.; Bistoni, F.; d’Enfert, C.; Vecchiarelli, A. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS ONE 2011, 6, e22770. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Sobel, J.D.; Nyirjesy, P.; Sobel, R.; Williams, V.L.; Yu, Q.; Noverr, M.C.; Fidel, P.L., Jr. Current patient perspectives of vulvovaginal candidiasis: Incidence, symptoms, management and post-treatment outcomes. BMC Womens Health 2019, 19, 48. [Google Scholar] [CrossRef] [Green Version]
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Saul-McBeth, J.; Dillon, J.; Lee, A.; Launder, D.; Kratch, J.M.; Abutaha, E.; Williamson, A.A.; Schroering, A.G.; Michalski, G.; Biswas, P.; et al. Tissue Damage in Radiation-Induced Oral Mucositis Is Mitigated by IL-17 Receptor Signaling. Front. Immunol. 2021, 12, 687627. [Google Scholar] [CrossRef]
- Basile, D.; Di Nardo, P.; Corvaja, C.; Garattini, S.K.; Pelizzari, G.; Lisanti, C.; Bortot, L.; Da Ros, L.; Bartoletti, M.; Borghi, M.; et al. Mucosal Injury during Anti-Cancer Treatment: From Pathobiology to Bedside. Cancers 2019, 11, 857. [Google Scholar] [CrossRef] [Green Version]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Sonis, S.T. Oral mucositis in cancer therapy. J. Support. Oncol. 2004, 2, 3–8. [Google Scholar] [PubMed]
- Ye, P.; Garvey, P.B.; Zhang, P.; Nelson, S.; Bagby, G.; Summer, W.R.; Schwarzenberger, P.; Shellito, J.E.; Kolls, J.K. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am. J. Respir. Cell Mol. Biol. 2001, 25, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Huppler, A.R.; Whibley, N.; Gaffen, S.L. Animal models for candidiasis. Curr. Protoc. Immunol. 2014, 105, 19.6.1–19.6.17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamai, Y.; Kubota, M.; Kamai, Y.; Hosokawa, T.; Fukuoka, T.; Filler, S.G. New model of oropharyngeal candidiasis in mice. Antimicrob. Agents Chemother. 2001, 45, 3195–3197. [Google Scholar] [CrossRef] [Green Version]
- Huppler, A.R.; Conti, H.R.; Hernandez-Santos, N.; Darville, T.; Biswas, P.S.; Gaffen, S.L. Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J. Immunol. 2014, 192, 1745–1752. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef] [Green Version]
- Sroussi, H.Y.; Lu, Y.; Zhang, Q.L.; Villines, D.; Marucha, P.T. S100A8 and S100A9 inhibit neutrophil oxidative metabolism in-vitro: Involvement of adenosine metabolites. Free Radic. Res. 2010, 44, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Zee, J.M.; Patel, K.D. Regulation of matrix metalloproteinase-9 (MMP-9) in TNF-stimulated neutrophils: Novel pathways for tertiary granule release. J. Leukoc. Biol. 2006, 79, 214–222. [Google Scholar] [CrossRef]
- Sonis, S.T.; Oster, G.; Fuchs, H.; Bellm, L.; Bradford, W.Z.; Edelsberg, J.; Hayden, V.; Eilers, J.; Epstein, J.B.; LeVeque, F.G.; et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J. Clin. Oncol. 2001, 19, 2201–2205. [Google Scholar] [CrossRef]
- Lionel, D.; Christophe, L.; Marc, A.; Jean-Luc, C. Oral mucositis induced by anticancer treatments: Physiopathology and treatments. Ther. Clin. Risk Manag. 2006, 2, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, A.; Sonis, S.T. An update on pharmacotherapies in active development for the management of cancer regimen-associated oral mucositis. Expert Opin. Pharmacother. 2020, 21, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Naidu, M.U.; Ramana, G.V.; Rani, P.U.; Mohan, I.K.; Suman, A.; Roy, P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis--complicating the treatment of cancer. Neoplasia 2004, 6, 423–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maria, O.M.; Eliopoulos, N.; Muanza, T. Radiation-Induced Oral Mucositis. Front. Oncol. 2017, 7, 89. [Google Scholar] [CrossRef]
- Farah, C.S.; Hong, S.; Wanasaengsakul, S.; Elahi, S.; Pang, G.; Gotjamanos, T.; Seymour, G.J.; Clancy, R.L.; Ashman, R.B. Irradiation-induced oral candidiasis in an experimental murine model. Oral Microbiol. Immunol. 2001, 16, 358–363. [Google Scholar] [CrossRef]
- Barlow, M.L.; Cummings, R.J.; Pentland, A.P.; Love, T.M.; Haidaris, C.G.; Ryan, J.L.; Lord, E.M.; Gerber, S.A. Total-Body Irradiation Exacerbates Dissemination of Cutaneous Candida Albicans Infection. Radiat. Res. 2016, 186, 436–446. [Google Scholar] [CrossRef]
- da Silva, E.M.; Mansano, E.S.B.; Miazima, E.S.; Rodrigues, F.A.V.; Hernandes, L.; Svidzinski, T.I.E. Radiation used for head and neck cancer increases virulence in Candida tropicalis isolated from a cancer patient. BMC Infect. Dis. 2017, 17, 783. [Google Scholar] [CrossRef] [Green Version]
- Altmeier, S.; Toska, A.; Sparber, F.; Teijeira, A.; Halin, C.; LeibundGut-Landmann, S. IL-1 Coordinates the Neutrophil Response to C. albicans in the Oral Mucosa. PLoS Pathog. 2016, 12, e1005882. [Google Scholar] [CrossRef] [Green Version]
- Peiseler, M.; Kubes, P. More friend than foe: The emerging role of neutrophils in tissue repair. J. Clin. Investig. 2019, 129, 2629–2639. [Google Scholar] [CrossRef] [Green Version]
- Aggor, F.E.Y.; Break, T.J.; Trevejo-Nunez, G.; Whibley, N.; Coleman, B.M.; Bailey, R.D.; Kaplan, D.H.; Naglik, J.R.; Shan, W.; Shetty, A.C.; et al. Oral epithelial IL-22/STAT3 signaling licenses IL-17-mediated immunity to oral mucosal candidiasis. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Taylor, P.R.; Roy, S.; Leal, S.M., Jr.; Sun, Y.; Howell, S.J.; Cobb, B.A.; Li, X.; Pearlman, E. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat. Immunol. 2014, 15, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralph, B.A.; Lehoux, M.; Ostapska, H.; Snarr, B.D.; Caffrey-Carr, A.K.; Fraser, R.; Saleh, M.; Obar, J.J.; Qureshi, S.T.; Sheppard, D.C. The IL-1 Receptor Is Required to Maintain Neutrophil Viability and Function during Aspergillus fumigatus Airway Infection. Front. Immunol. 2021, 12, 675294. [Google Scholar] [CrossRef] [PubMed]
- Vonk, A.G.; Netea, M.G.; van Krieken, J.H.; Iwakura, Y.; van der Meer, J.W.; Kullberg, B.J. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J. Infect. Dis. 2006, 193, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Borghi, M.; De Luca, A.; Puccetti, M.; Jaeger, M.; Mencacci, A.; Oikonomou, V.; Pariano, M.; Garlanda, C.; Moretti, S.; Bartoli, A.; et al. Pathogenic NLRP3 Inflammasome Activity during Candida Infection Is Negatively Regulated by IL-22 via Activation of NLRC4 and IL-1Ra. Cell Host Microbe 2015, 18, 198–209. [Google Scholar] [CrossRef] [Green Version]
- Roselletti, E.; Perito, S.; Gabrielli, E.; Mencacci, A.; Pericolini, E.; Sabbatini, S.; Cassone, A.; Vecchiarelli, A. NLRP3 inflammasome is a key player in human vulvovaginal disease caused by Candida albicans. Sci. Rep. 2017, 7, 17877. [Google Scholar] [CrossRef] [Green Version]
- Yano, J.; Noverr, M.C.; Fidel, P.L., Jr. Cytokines in the host response to Candida vaginitis: Identifying a role for non-classical immune mediators, S100 alarmins. Cytokine 2012, 58, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Gladiator, A.; Wangler, N.; Trautwein-Weidner, K.; LeibundGut-Landmann, S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J. Immunol. 2013, 190, 521–525. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.R.; Leal, S.M., Jr.; Sun, Y.; Pearlman, E. Aspergillus and Fusarium corneal infections are regulated by Th17 cells and IL-17-producing neutrophils. J. Immunol. 2014, 192, 3319–3327. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saul-McBeth, J.; Dillon, J.; Launder, D.; Hickey, M.; Yi, E.M.-C.; Daboul, Y.; Biswas, P.; Salari, E.; Parsai, E.I.; Conti, H.R. Radiation Exposure Perturbs IL-17RA-Mediated Immunity Leading to Changes in Neutrophil Responses That Increase Susceptibility to Oropharyngeal Candidiasis. J. Fungi 2022, 8, 495. https://doi.org/10.3390/jof8050495
Saul-McBeth J, Dillon J, Launder D, Hickey M, Yi EM-C, Daboul Y, Biswas P, Salari E, Parsai EI, Conti HR. Radiation Exposure Perturbs IL-17RA-Mediated Immunity Leading to Changes in Neutrophil Responses That Increase Susceptibility to Oropharyngeal Candidiasis. Journal of Fungi. 2022; 8(5):495. https://doi.org/10.3390/jof8050495
Chicago/Turabian StyleSaul-McBeth, Jessica, John Dillon, Dylan Launder, Maura Hickey, Elise Mein-Chiain Yi, Yusuf Daboul, Priosmita Biswas, Elahheh Salari, E. Ishmael Parsai, and Heather R. Conti. 2022. "Radiation Exposure Perturbs IL-17RA-Mediated Immunity Leading to Changes in Neutrophil Responses That Increase Susceptibility to Oropharyngeal Candidiasis" Journal of Fungi 8, no. 5: 495. https://doi.org/10.3390/jof8050495





