Chitosan and Nano-Chitosan for Management of Harpophora maydis: Approaches for Investigating Antifungal Activity, Pathogenicity, Maize-Resistant Lines, and Molecular Diagnosis of Plant Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pathogen Isolation, Identification and Pathogenicity Test
2.2. Plant Materials
2.3. Synthesis of Chitosan Nanoparticles
2.4. Characterization of Nanomaterials
2.5. Chitosan Nanoparticles (CH NPs) Transmission Electron Microscopy (TEM) Analysis
2.6. Antifungal Activity of Chitosan Products
2.6.1. In Vitro Tests
2.6.2. In Vivo Tests
2.7. Disease Severity Assessments
2.8. Growth Parameters
2.9. Molecular Diagnosis of Late Wilt Pathogenesis
2.9.1. DNA Extraction
2.9.2. H. maydis Detection via qPCR Analysis
2.10. Statistical Analysis
3. Results
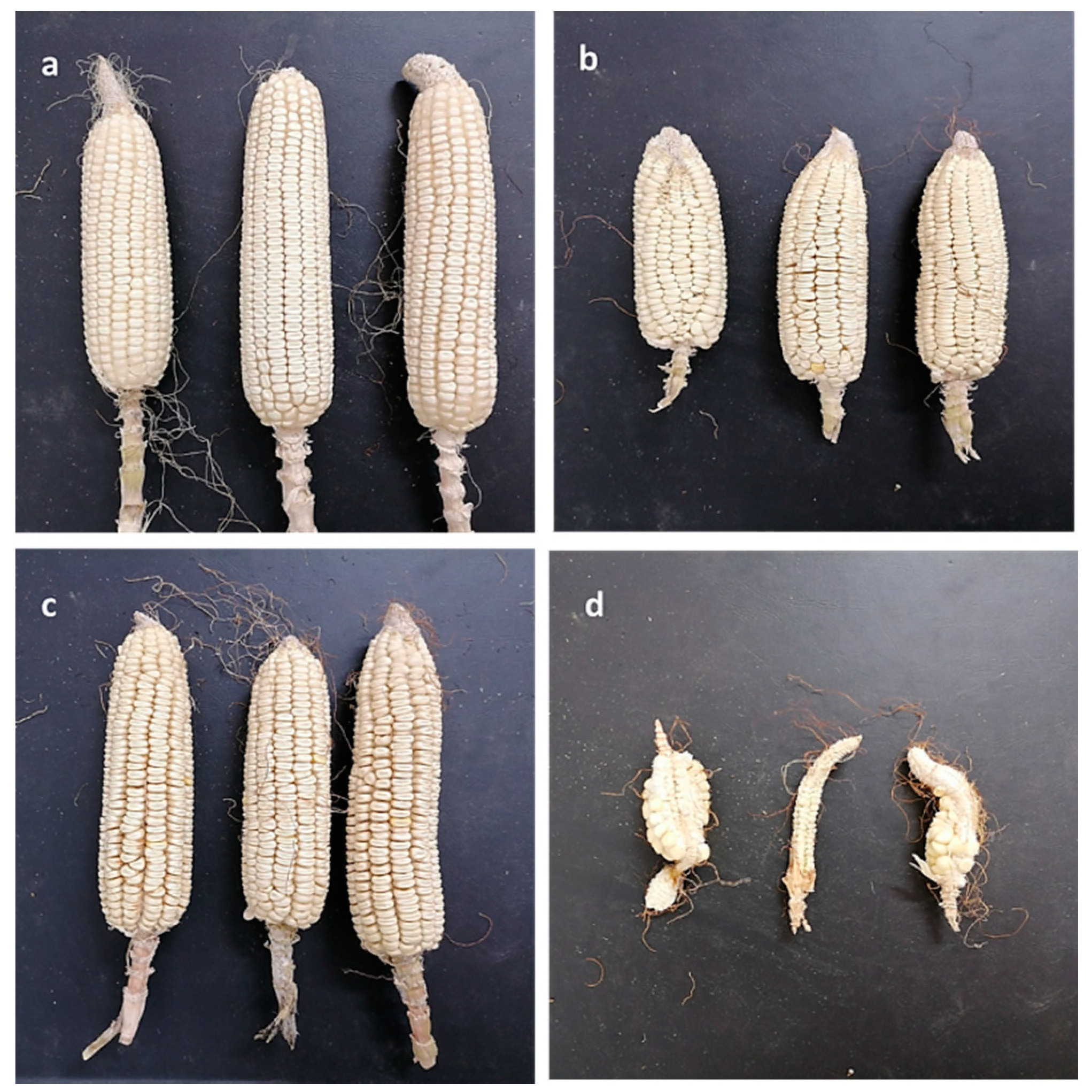
3.1. Isolation and Pathogenicity Test of Late Wilt Pathogen
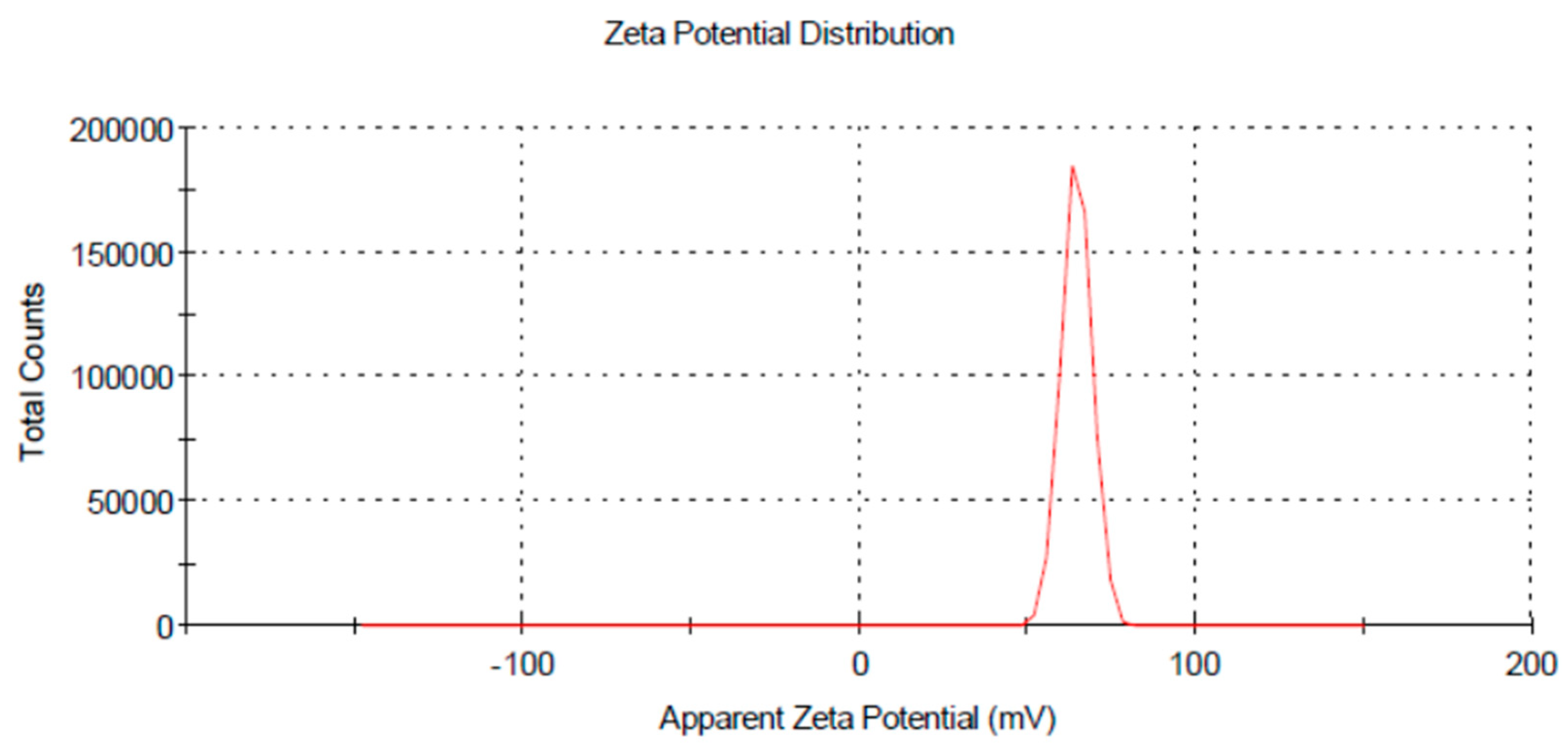
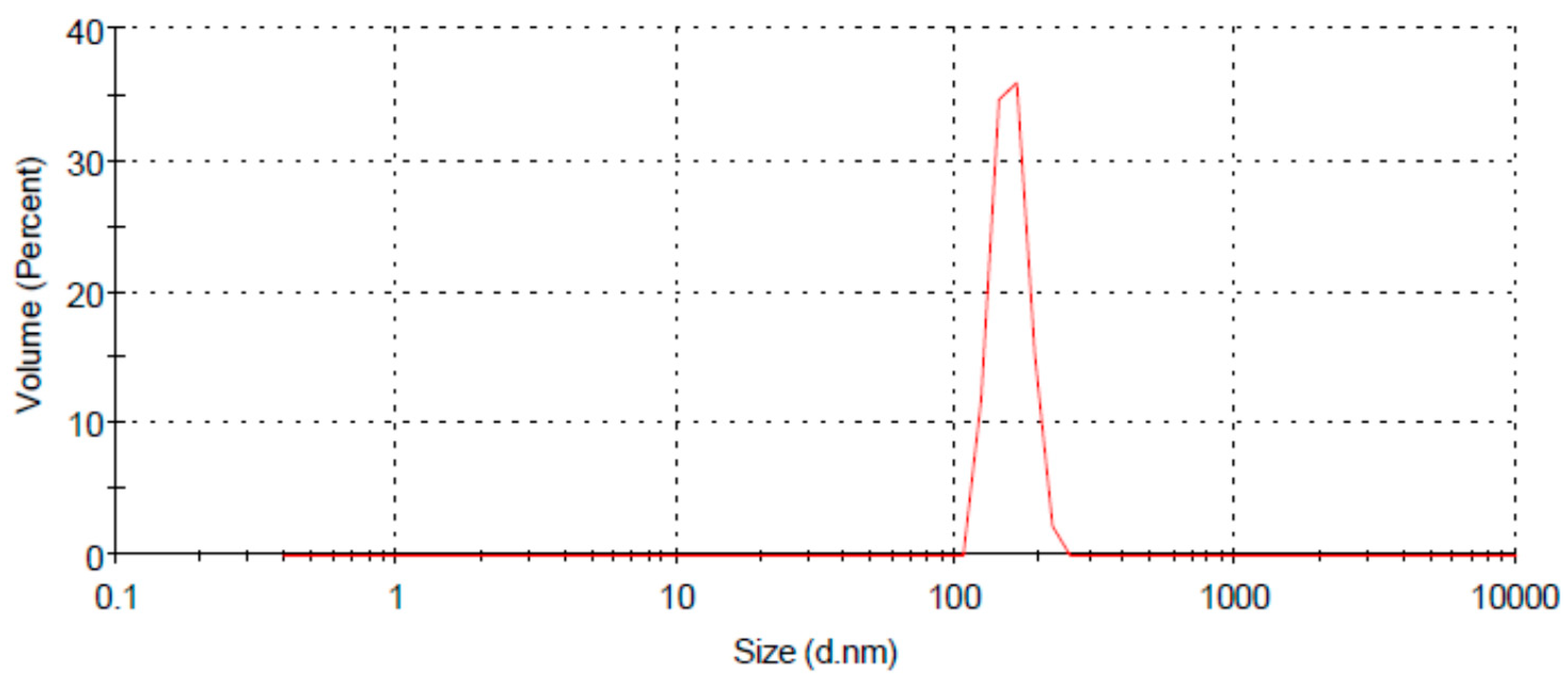
3.2. Characterization of Chitosan Nanoparticles
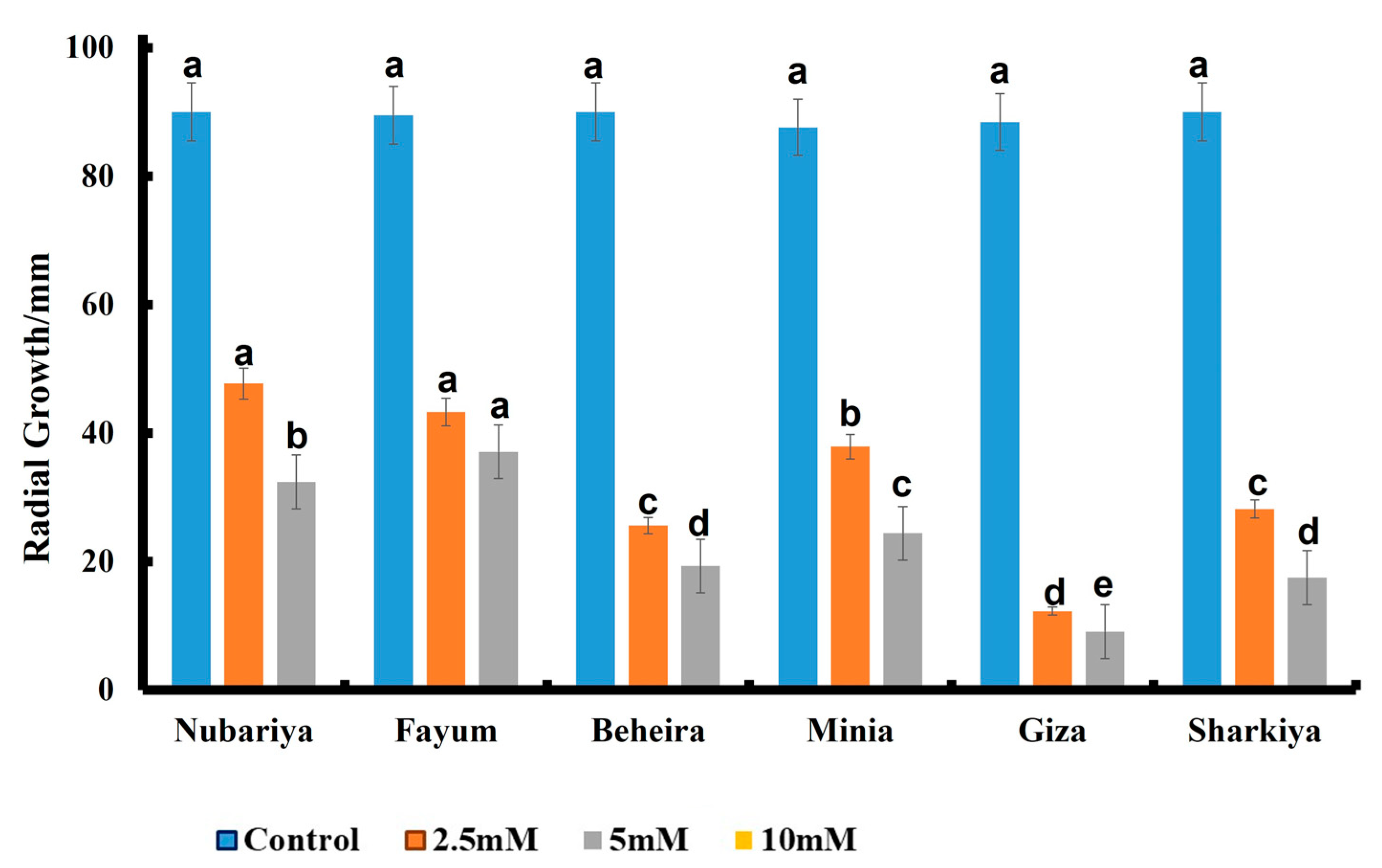
3.3. Effect of Fungicide Concentration on In Vitro Antifungal Activity
3.4. In Vivo Antifungal Activity of Chitosan Products
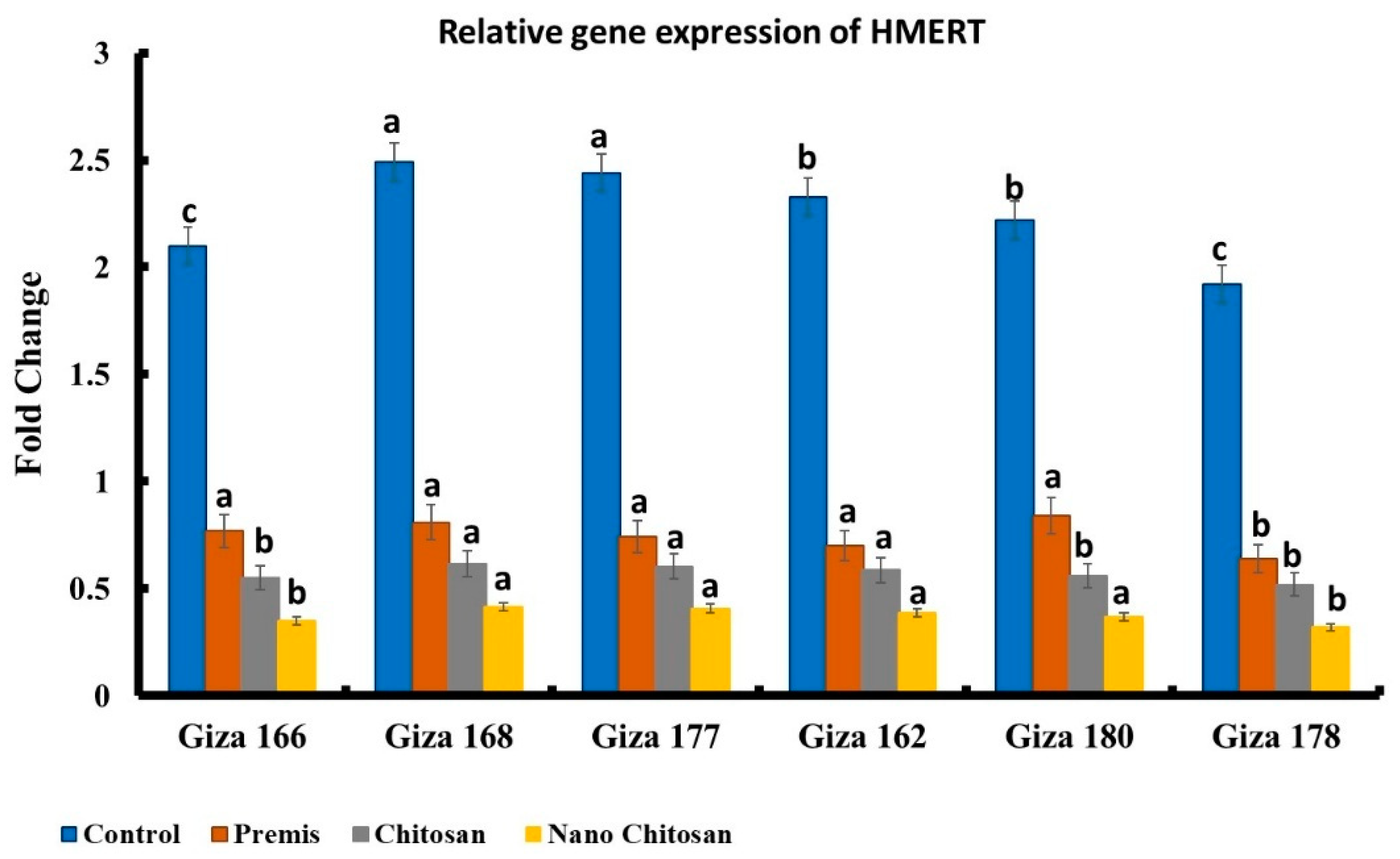
3.5. Molecular Diagnosis of Late Wilt Pathogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michail, S.H.; Abou-Elseoud, M.S.; Eldin, M.S.N. Seed health testing of corn for Cephalosporium maydis. Acta Phytopathol. Entomol. Hung. 1999, 34, 35–41. [Google Scholar]
- Charles, L.M.F.; McGillivray, L. The Crop Protection Compendium: Information for the Management of Crop Pests. Rice Black Bugs 2007, 707. Available online: https://www.cabdirect.org/cabdirect/abstract/20073035028 (accessed on 17 March 2022).
- Munkvold, G.P.; White, D.G. Compendium of Corn Diseases; Am Phytopath Society: Saint Paul, MN, USA, 2016; Volume 165. [Google Scholar]
- Johal, L.; Huber, D.M.; Martyn, R. Late wilt of corn (maize) pathway analysis: Intentional introduction of Cephalosporium maydis. In Pathways Analysis for the Introduction to the US of Plant Pathogens of Economic Importance; Animal and Plant Health Inspection Service, United States Department of Agriculture: Riverdale Park, MD, USA, 2004. [Google Scholar]
- Shalaby, M.; El-Moghazy, S.; Mehesen, A.A. Biological control of maize late wilt disease caused by Cephalosporium maydis. J. Agric. Res. Kafrelsheikh Univ. 2009, 35, 1–19. [Google Scholar]
- Sabet, K.A.; Zaher, A.M.; Samra, A.S.; Mansour, I.M. Pathogenic behaviour of Cephalosporium maydis and C. acremonium. Ann. Appl. Biol. 1970, 66, 257–263. [Google Scholar] [CrossRef]
- Singh, S.D.; Siradhana, B.S. Survival of Cephalosporium maydis, incitant of late wilt disease of maize. Indian J. Mycol. Plant Pathol. 1987, 17, 83–85. [Google Scholar]
- Bergstrom, G.; Leslie, J.; Huber, D.; Lipps, P.; Warren, H.; Esker, P.; Grau, C.; Botratynski, T.; Bulluck, R.; Floyd, J. Recovery Plan for Late Wilt of Corn Caused by Harpophora Maydis Syn. Cephalosporium Maydis; National Plant Disease Recovery System (NPDRS): Washington, DC, USA, 2008; Volume 24.
- Ortiz-Bustos, C.M.; López-Bernal, A.; Testi, L.; Molinero-Ruiz, L.J.P.P. Environmental and irrigation conditions can mask the effect of Magnaporthiopsis maydis on growth and productivity of maize. Plant Pathol. 2019, 68, 1555–1564. [Google Scholar] [CrossRef]
- El-Hosary, A.A.A.; El-Fiki, I.A.I. Diallel cross analysis for earliness, yield, its components and resistance to late wilt in maize. Int. J. Agric. Sci. Res. 2015, 5, 199–210. [Google Scholar]
- Soliman, F.H.S.; Sadek, S.E. Combining ability of new maize inbred lines and its utilisation in the Egyptian hybrid program. Bull. Agric. Univ. CAIRO 1999, 50, 1–20. [Google Scholar]
- Khokhar, M.K.; Hooda, K.S.; Sharma, S.S.; Singh, V. Post flowering stalk rot complex of maize-present status and future prospects. Maydica 2014, 59, 226–242. [Google Scholar]
- El-Assiuty, E.M.; Ismael, A.M.; Zeller, K.A.; Leslie, J.F. Relative colonization ability of greenhouse-grown maize by four lineages of Cephalosporium maydis from Egypt. Phytopathology 1999, 89, 23. [Google Scholar]
- Ortiz-Bustos, C.M.; Testi, L.; García-Carneros, A.B.; Molinero-Ruiz, L. Geographic distribution and aggressiveness of Harpophora maydis in the Iberian peninsula, and thermal detection of maize late wilt. Eur. J. Plant Pathol. 2016, 144, 383–397. [Google Scholar] [CrossRef]
- Degani, O.; Cernica, G. Diagnosis and control of Harpophora maydis, the cause of late wilt in maize. Adv. Microbiol. 2014, 2014, 42212. [Google Scholar]
- Sharma, K.K.; Singh, U.S.; Sharma, P.; Kumar, A.; Sharma, L. Seed treatments for sustainable agriculture-A review. J. Appl. Nat. Sci. 2015, 7, 521–539. [Google Scholar] [CrossRef]
- Begum, H.; Mohammad, S.; Rao, G.K.; Raj, R.B. Influence of seed dressing fungicides on the incidence of post flowering stalk rot (late wilt and charcoal rot), yield and profitability of maize. Crop. Res. Hisar. 1989, 2, 142–146. [Google Scholar]
- Satyanarayana, E.; Begum, H. Relative efficacy of fungicides (seed dressers) and irrigation schedule for the control of late wilt in maize. Curr. Res. Agric. Sci. 1996, 25, 59–60. [Google Scholar]
- Singh, S.D.; Siradhana, B.S. Chemical control of late wilt of maize induced by Cephalosporium maydis. Indian J. Mycol. Plant Pathol. 1989, 19, 121–122. [Google Scholar]
- Spot, L.; Mahmoud, E.Y.; Hussien, Z.N.; Ibrahim, M.M.; Yousef, H. Current Science International Volume: 10| Issue: 01| Jan.-March| 2021. Available online: http://w.curresweb.com/csi/csi/2021/csi.2021.10.1.3.pdf (accessed on 17 March 2022).
- Mahmoud, E.E.-D.; Ibrahim, M.; Saleh, W.; Ahmed, M. Compatibility between antagonistic fungi and bacteria and their influence in controlling sunflower charcoal rot. Egypt J. Phytopathol. 2015, 43, 53–64. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and chitosan: Structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Bhaskara Reddy, M.V.; Arul, J.; Angers, P.; Couture, L. Chitosan treatment of wheat seeds induces resistance to Fusarium graminearum and improves seed quality. J. Agric. Food Chem. 1999, 47, 1208–1216. [Google Scholar] [CrossRef]
- Bhuvaneshwari, S.; Sivasubramanian, V. Comparative Studies for Chitosan Yield and Chelating Ability of Aspergillus niger and Rhizopus oryzae. Indian J. Biotechnol. 2013, 12, 429–431. [Google Scholar]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Hassan, O.; Chang, T. Chitosan for eco-friendly control of plant disease. Asian J. Plant Pathol. 2017, 11, 53–70. [Google Scholar] [CrossRef]
- Narrod, C.A.; Abbott, L. Agriculture, Food, and Water Nanotechnologies for the Poor: Opportunities and Constraints; International Food Policy Research Institute: Washington, DC, USA, 2011. [Google Scholar]
- Pérez-de-Luque, A.; Hermosín, M.C. Nanotechnology and Its Use in Agriculture. In Bio-Nanotechnology a Revolut Food, Biomed Health Science; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 383–398. [Google Scholar]
- Frewer, L.J.; Norde, W.; Fischer, A.; Kampers, F. Nanotechnology in the Agri-Food Sector: Implications for the Future; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 3527634800. [Google Scholar]
- Dubchak, S.; Ogar, A.; Mietelski, J.W.; Turnau, K. Influence of silver and titanium nanoparticles on arbuscular mycorrhiza colonization and accumulation of radiocaesium in Helianthus annuus. Span. J. Agric. Res. 2010, 8, 103–108. [Google Scholar] [CrossRef]
- Saharan, V.; Mehrotra, A.; Khatik, R.; Rawal, P.; Sharma, S.S.; Pal, A. Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int. J. Biol. Macromol. 2013, 62, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Samra, A.S.; Sabet, K.A.; Hingorani, M.K. A new wilt disease of Maize in Egypt. Plant Dis. Rep. 1962, 46, 481–483. [Google Scholar]
- Shoala, T.; Gehan, A.; Basma, H. Effects of Salicylic Acid in The Normal and Nano Form Against Selected Fungi That Infect Citrus Trees (Citrus sinensis). Int. J. Sustain. Dev. Sci. 2021, 4, 1–14. [Google Scholar]
- El-Shafey, H.A.; El-Shorbagy, F.A.; Khalil, I.I.; El-Assiuty, E.M. Additional sources of resistance to the late-wilt disease of maize caused by Cephalosporium maydis. Agric. Res. Rev. 1988, 66, 221–230. [Google Scholar]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [Green Version]
- Sedhom, S.A.; El-Badawy, M.E.M.; Hosary, A.A.E.; Abd El-Latif, M.S.; Rady, A.M.; Moustafa, M.M.; Mohamed, S.A.; Badr, O.A.; Abo-Marzoka, S.A.; Baiumy, K.A.; et al. Molecular markers and GGE biplot analysis for selecting higher-yield and drought-tolerant maize hybrids. Agron. J. 2021, 113, 3871–3885. [Google Scholar] [CrossRef]
- Abdelatty, A.M.; Mandouh, M.I.; Mohamed, S.A.; Busato, S.; Badr, O.A.M.; Bionaz, M.; Elolimy, A.A.; Moustafa, M.M.A.; Farid, O.A.A.; Al-Mokaddem, A.K. Azolla leaf meal at 5% of the diet improves growth performance, intestinal morphology and p70S6K1 activation, and affects cecal microbiota in broiler chicken. Animal 2021, 15, 100362. [Google Scholar] [CrossRef]
- Degani, O.; Dor, S.; Movshovitz, D.; Rabinovitz, O. Methods for studying Magnaporthiopsis maydis, the maize late wilt causal agent. Agronomy 2019, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Weller, S.A.; Elphinstone, J.G.; Smith, N.C.; Boonham, N.; Stead, D. Detection of Ralstonia solanacearum strains with a quantitative, multiplex, real-time, fluorogenic PCR (TaqMan) assay. Appl. Environ. Microbiol. 2000, 66, 2853–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SAS Institute Inc. SAS Procedure Guide Version 6.12 ED; SAS Institute Inc.: Cary, NC, USA, 2004. [Google Scholar]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Son: Hoboken, NJ, USA, 1984; ISBN 0471870927. [Google Scholar]
- Zeller, K.A.; Jurgenson, J.E.; El-Assiuty, E.M.; Leslie, J.F. Isozyme and amplified fragment length polymorphisms from Cephalosporium maydis in Egypt. Phytoparasitica 2000, 28, 121–130. [Google Scholar] [CrossRef]
- Demirci, F.; Bayraktar, H.; Babalioǧullu, I.; Dolar, F.S.; Maden, S. In vitro and in vivo effects of some fungicides against the chickpea blight pathogen, Ascochyta rabiei. J. Phytopathol. 2003, 151, 519–524. [Google Scholar] [CrossRef]
- Hide, G.A.; Read, P.J.; Hall, S.M. Resistance to thiabendazole in Fusarium species isolated from potato tubers affected by dry rot. Plant Pathol. 1992, 41, 745–748. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Nikunj, P.; Purvi, D.; Niti, P.; Anamika, J.; Gautam, H.K. Agronanotechnology for plant fungal disease management: A review. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 71–84. [Google Scholar]
- Li, Y.-T.; Hwang, S.-G.; Huang, Y.-M.; Huang, C.-H. Effects of Trichoderma asperellum on nutrient uptake and Fusarium wilt of tomato. Crop Prot. 2018, 110, 275–282. [Google Scholar] [CrossRef]
- Jiang, P.-D.; Zhu, Y.-G.; Wang, X.-L.; Wei, Z.H.U.; Zhang, X.-Q.; Hai-yan, X.I.E.; Wang, X.-D. Metabolism of reactive oxygen species in the cytoplasmic male-sterile cotton anther. Agric. Sci. China 2007, 6, 275–280. [Google Scholar] [CrossRef]
- Leuber-Metzger, G.; Meins, F., Jr. Functions and Regulation of Plant p”-1, 3-glucanasés (PR-2). In Pathog Proteins Plants; Datta, S.K., Muthukrishnan, S., Eds.; CRC Press: Boca Raton, FL, USA, 1999; Volume 45–76. [Google Scholar]
- van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saharan, V.; Sharma, G.; Yadav, M.; Choudhary, M.K.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P. Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. Int. J. Biol. Macromol. 2015, 75, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Rentel, M.C.; Lecourieux, D.; Ouaked, F.; Usher, S.L.; Petersen, L.; Okamoto, H.; Knight, H.; Peck, S.C.; Grierson, C.S.; Hirt, H. OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 2004, 427, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Shoala, T.; Edwards, M.G.; Knight, M.R.; Gatehouse, A.M.R. OXI1 kinase plays a key role in resistance of Arabidopsis towards aphids (Myzus persicae). Transgenic. Res. 2018, 27, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Petersen, L.N.; Ingle, R.A.; Knight, M.R.; Denby, K.J. OXI1 protein kinase is required for plant immunity against Pseudomonas syringae in Arabidopsis. J. Exp. Bot. 2009, 60, 3727–3735. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Primer | Sequence | References |
|---|---|---|
| AFLP H. maydis 200 bp specific fragment (A200a) | 5-CCGACGCCTAAAATACAGGA-3 | [38] |
| 5-GGGCTTTTTAGGGCCTTTTT-3 | ||
| Cytochrome c oxidase (Cox) | 5-GTATGCCACGTCGCATTCCAGA-3 | [39] |
| 5-CAACTACGGATATATAAGRRCCRRAACTG-3 |
| Treatment | DS% | Yield Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| Plant Height (cm) | Ear No/Plant | Rows No/Ear | Kernels No/Row | 100-Kernel Weight (g) | Grain Yield/Plant (g) | |||
| Giza 166 cv. | Chitosan 5 mM | 31.2 | 147.7 | 1.0 | 12.7 | 38.0 | 15.3 | 73.8 |
| Nano-chitosan 5 mM | 22.7 | 173.0 | 1.0 | 16.0 | 40.0 | 23.0 | 147.2 | |
| Premis Ultra 2.5% | 33.3 | 169.7 | 1.0 | 14.0 | 42.0 | 19.9 | 117.0 | |
| Control | 41.1 | 132.5 | 1.0 | 12.8 | 36.5 | 16.1 | 75.2 | |
| Giza 168 cv. | Chitosan 5 mM | 44.4 | 131.3 | 1.0 | 12.3 | 25.4 | 13.4 | 41.9 |
| Nano-chitosan 5 mM | 27.2 | 166.9 | 1.0 | 14.7 | 38.7 | 19.7 | 112.1 | |
| Premis Ultra 2.5% | 33.3 | 145.8 | 1.0 | 12.7 | 30.7 | 15.1 | 58.9 | |
| Control | 45.4 | 124.9 | 1.0 | 10.7 | 26.7 | 14.0 | 40.0 | |
| Giza 177 cv. | Chitosan 5 mM | 38.3 | 142.1 | 1.0 | 12.1 | 34.7 | 13.7 | 57.5 |
| Nano-chitosan 5 mM | 25.5 | 155.9 | 1.0 | 14.7 | 32.0 | 24.2 | 113.8 | |
| Premis Ultra 2.5% | 34.3 | 155.6 | 1.0 | 12.7 | 36.0 | 20.4 | 93.3 | |
| Control | 44.4 | 142.5 | 1.0 | 11.3 | 33.3 | 14.7 | 55.3 | |
| Giza 162 cv. | Chitosan 5 mM | 33.3 | 186.7 | 1.0 | 13.8 | 37.3 | 17.6 | 90.6 |
| Nano-chitosan 5 mM | 22.2 | 187.9 | 1.0 | 18.0 | 44.0 | 25.4 | 201.2 | |
| Premis Ultra 2.5% | 31.2 | 201.7 | 1.0 | 17.3 | 44.0 | 21.9 | 166.7 | |
| Control | 36.3 | 188.0 | 1.0 | 14.0 | 40.0 | 15.5 | 86.8 | |
| Giza 180 cv. | Chitosan 5 mM | 37.6 | 141.2 | 1.0 | 13.2 | 27.1 | 10.2 | 36.5 |
| Nano-chitosan 5 mM | 27.1 | 166.8 | 1.0 | 14.7 | 34.7 | 25.6 | 130.6 | |
| Premis Ultra 2.5% | 31.2 | 153.7 | 1.0 | 14.7 | 30.0 | 21.7 | 95.7 | |
| Control | 41.3 | 128.2 | 1.0 | 11.3 | 26.0 | 11.4 | 33.5 | |
| Giza 178 cv. | Chitosan 5 mM | 38.6 | 97.4 | 1.0 | 12.0 | 20.7 | 14.2 | 35.3 |
| Nano-chitosan 5 mM | 21.7 | 184.6 | 1.0 | 15.3 | 38.0 | 27.4 | 159.3 | |
| Premis Ultra 2.5% | 29.5 | 122.3 | 1.0 | 14.7 | 26.7 | 22.7 | 89.1 | |
| Control | 42.3 | 101.6 | 1.0 | 11.7 | 18.2 | 12.4 | 26.4 | |
| L.S.D at 0.05 | 9.71 | 6.97 | NS | 2.11 | 5.64 | 6.16 | 10.22 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, E.O.; Shoala, T.; Attia, A.M.F.; Badr, O.A.M.; Mahmoud, S.Y.M.; Farrag, E.S.H.; EL-Fiki, I.A.I. Chitosan and Nano-Chitosan for Management of Harpophora maydis: Approaches for Investigating Antifungal Activity, Pathogenicity, Maize-Resistant Lines, and Molecular Diagnosis of Plant Infection. J. Fungi 2022, 8, 509. https://doi.org/10.3390/jof8050509
Hassan EO, Shoala T, Attia AMF, Badr OAM, Mahmoud SYM, Farrag ESH, EL-Fiki IAI. Chitosan and Nano-Chitosan for Management of Harpophora maydis: Approaches for Investigating Antifungal Activity, Pathogenicity, Maize-Resistant Lines, and Molecular Diagnosis of Plant Infection. Journal of Fungi. 2022; 8(5):509. https://doi.org/10.3390/jof8050509
Chicago/Turabian StyleHassan, Eman O., Tahsin Shoala, Amany M. F. Attia, Omnia A. M. Badr, Sabry Y. M. Mahmoud, Eman S. H. Farrag, and Ibrahim A. I. EL-Fiki. 2022. "Chitosan and Nano-Chitosan for Management of Harpophora maydis: Approaches for Investigating Antifungal Activity, Pathogenicity, Maize-Resistant Lines, and Molecular Diagnosis of Plant Infection" Journal of Fungi 8, no. 5: 509. https://doi.org/10.3390/jof8050509
APA StyleHassan, E. O., Shoala, T., Attia, A. M. F., Badr, O. A. M., Mahmoud, S. Y. M., Farrag, E. S. H., & EL-Fiki, I. A. I. (2022). Chitosan and Nano-Chitosan for Management of Harpophora maydis: Approaches for Investigating Antifungal Activity, Pathogenicity, Maize-Resistant Lines, and Molecular Diagnosis of Plant Infection. Journal of Fungi, 8(5), 509. https://doi.org/10.3390/jof8050509






