Saprolegniosis in Amphibians: An Integrated Overview of a Fluffy Killer Disease
Abstract
:1. Introduction
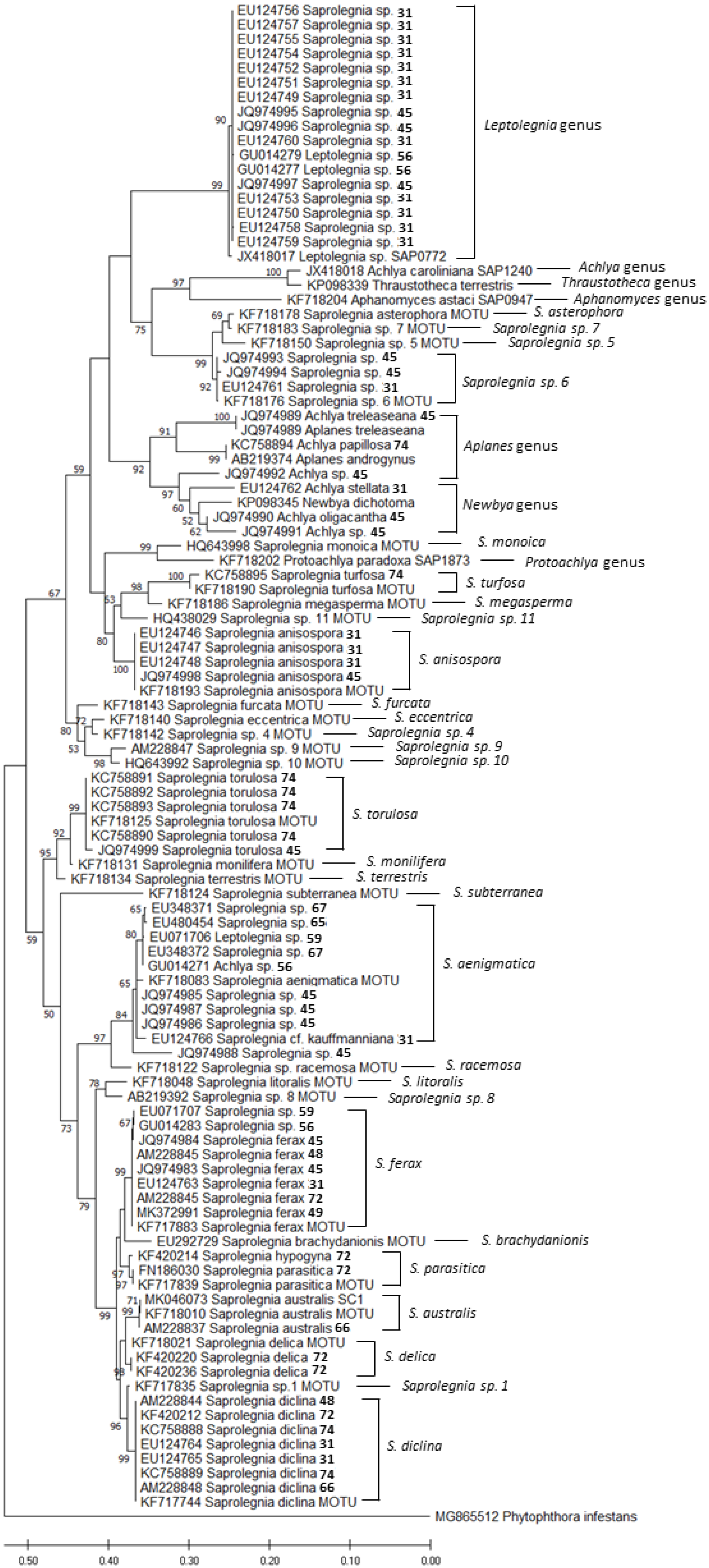
2. Amphibian-Related Oomycetes Phylogeny, Taxonomy, and Genomics
3. Saprolegniosis in Amphibians: From Natural Populations to Laboratory Assays
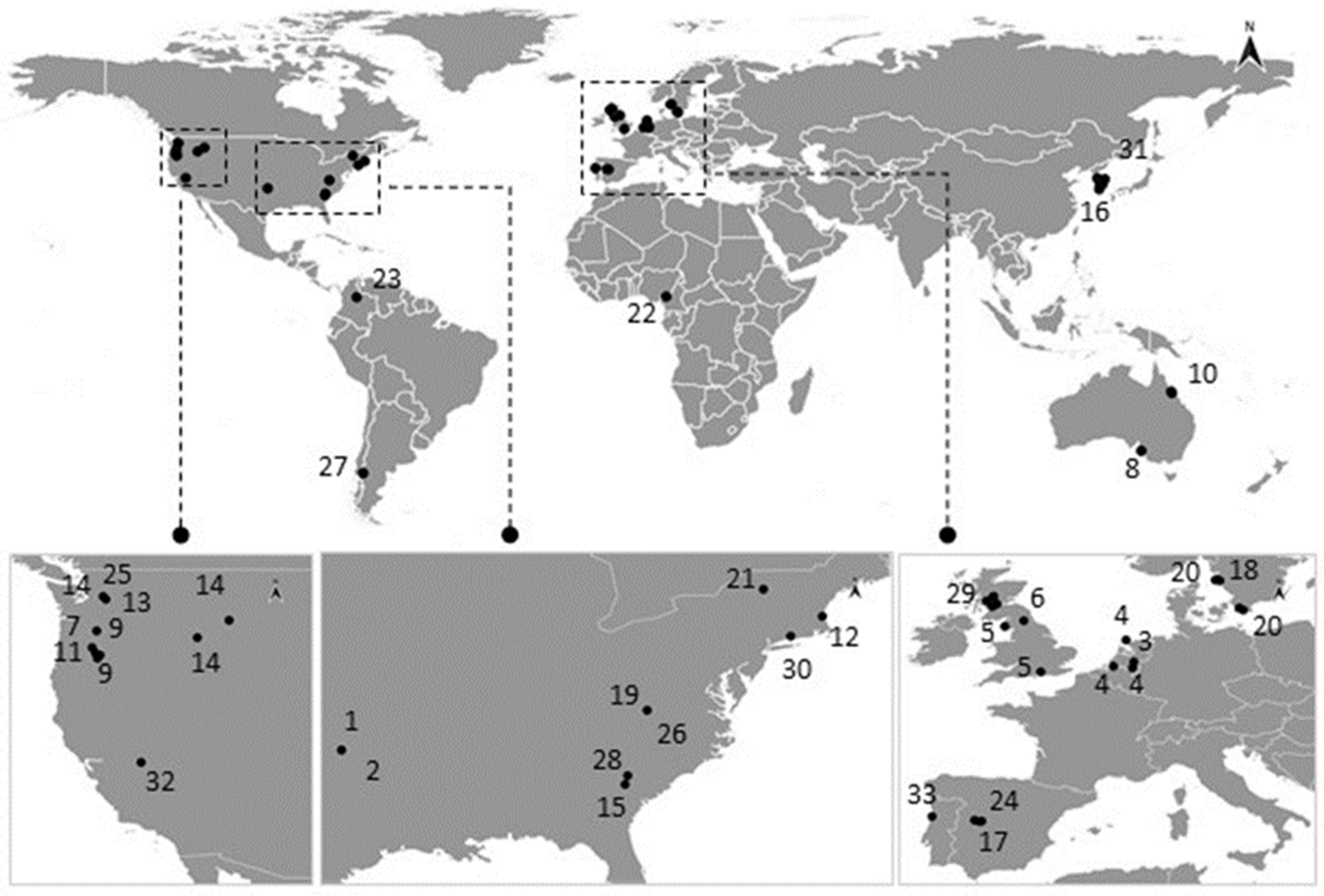
3.1. Occurrence in Natural Populations
3.2. Laboratory Assays
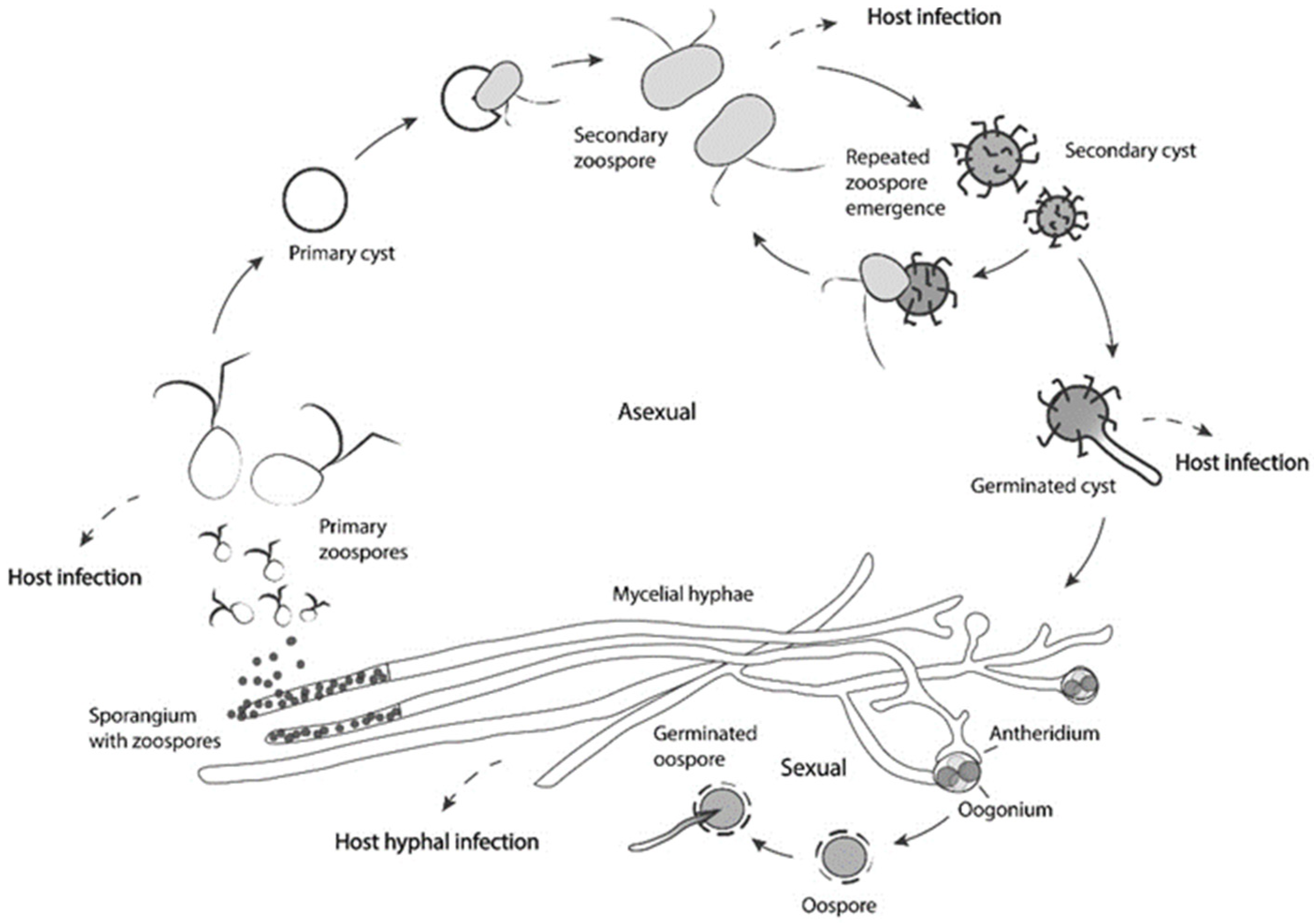
4. An Oomycete Called Saprolegnia
Saprolegnia: A Primary or Opportunistic Pathogen?
5. Factors That Can Influence Saprolegniosis
5.1. Saprolegnia sp. as Infectious Agent
5.2. The Amphibian Hosts
6. Pathogenesis and Clinical Signs
7. Treatment and New Therapies
7.1. Treatment
7.2. New Therapies
8. Future Needs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallana, M.; Ryser-Degiorgis, M.-P.; Wahli, T.; Segner, H. Climate change and infectious diseases of wildlife: Altered interactions between pathogens, vectors and hosts. Curr. Zool. 2013, 59, 427–437. [Google Scholar] [CrossRef]
- Wilmers, C.C.; Post, E.; Peterson, R.O.; Vucetich, J.A. Predator disease out-break modulates top-down, bottom-up and climatic effects on herbivore population dynamics. Ecol. Lett. 2006, 9, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.F.; Sax, D.F.; Lafferty, K.D. Evidence for the role of infectious disease in species extinction and endangerment. Conserv. Biol. 2006, 20, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Lips, K.R.; Brem, F.; Brenes, R.; Reeve, J.D.; Alford, R.A.; Voyles, J.; Carey, C.; Livo, L.; Pessier, A.P.; Collins, J.P. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc. Natl. Acad. Sci. USA 2006, 103, 3165–3170. [Google Scholar] [CrossRef] [Green Version]
- Daszak, P.; Berger, L.; Cunningham, A.; Hyatt, A.D.; Green, E.; Speare, R. Emerging Infectious Diseases and Amphibian Population Declines. Emerg. Infect. Dis. 1999, 5, 735–748. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.; Hyatt, A.D. Infectious disease and amphibian population declines. Divers. Distrib. 2003, 9, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef]
- Waddle, J.H.; Grear, D.A.; Mosher, B.A.; Grant, E.H.C.; Adams, M.J.; Backlin, A.R.; Barichivich, W.J.; Brand, A.B.; Bucciarelli, G.M.; Calhoun, D.L.; et al. Batrachochytrium salamandrivorans (Bsal) not detected in an intensive survey of wild North American amphibians. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Martel, A.; Spitzen-Van Der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M.C.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F.; et al. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc. Natl. Acad. Sci. USA 2013, 110, 15325–15329. [Google Scholar] [CrossRef] [Green Version]
- OIE OIE-Listed Diseases, Infections and Infestations in Force in 2020. Available online: https://www.oie.int/animal-health-in-the-world/oie-listed-diseases-2020/ (accessed on 1 January 2020).
- Sarowar, M.N.; Hossain, M.J.; Nasrin, T.; Naznin, T.; Hossain, Z.; Rahman, M.M. Molecular identification of oomycete species affecting aquaculture in Bangladesh. Aquac. Fish. 2019, 4, 105–113. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Paull, S.H. The ecology and emergence of diseases in fresh waters. Freshw. Biol. 2011, 56, 638–657. [Google Scholar] [CrossRef]
- Kiesecker, J.M.; Blaustein, A.R. Synergism between UV-B radiation and a pathogen magnifies amphibian embryo mortality in nature. Proc. Natl. Acad. Sci. USA 1995, 92, 11049–11052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiesecker, J.M.; Blaustein, A.R.; Miller, C.L. Transfer of a Pathogen from Fish to Amphibians. Conserv. Biol. 2001, 15, 1064–1070. [Google Scholar] [CrossRef]
- Sarowar, M.N.; van den Berg, A.H.; McLaggan, D.; Young, M.R.; van West, P. Saprolegnia strains isolated from river insects and amphipods are broad spectrum pathogens. Fungal Biol. 2014, 118, 579–590, Reprint in Fungal Biol. 2013, 117, 752–763. [Google Scholar] [CrossRef]
- Lafferty, K.D. The ecology of climate change and infectious diseases. Ecology 2009, 90, 888–900. [Google Scholar] [CrossRef]
- Grogan, L.F.; Berger, L.; Rose, K.; Grillo, V.; Cashins, S.D.; Skerratt, L.F. Surveillance for Emerging Biodiversity Diseases of Wildlife. PLoS Pathog. 2014, 10, e1004015. [Google Scholar] [CrossRef]
- Thines, M.; Kamoun, S. Oomycete–plant coevolution: Recent advances and future prospects. Curr. Opin. Plant Biol. 2010, 13, 427–433. [Google Scholar] [CrossRef]
- Pessier, A.P. An overview of amphibian skin disease. Semin. Avian Exot. Pet Med. 2002, 11, 162–174. [Google Scholar] [CrossRef]
- Densmore, C.L.; Green, D.E. Diseases of amphibians. ILAR J. 2007, 48, 235–254. [Google Scholar] [CrossRef] [Green Version]
- Strijbosch, H. Habitat Selection of Amphibians during Their Aquatic Phase. Oikos 1979, 33, 363. [Google Scholar] [CrossRef]
- Leuven, R.S.E.W.; den Hartog, C.; Christiaans, M.M.C.; Heijligers, W.H.C. Effects of water acidification on the distribution pattern and the reproductive success of amphibians. Experientia 1986, 42, 495–503. [Google Scholar] [CrossRef]
- Banks, B.; Beebee, T.J.C. Reproductive Success of Natterjack Toads Bufo calamita in Two Contrasting Habitats. J. Anim. Ecol. 1988, 57, 475. [Google Scholar] [CrossRef]
- Blackburn, D.C.; Evans, B.J.; Pessier, A.P.; Vredenburg, V.T. An enigmatic mortality event in the only population of the critically endangered cameroonian frog Xenopus longipes. Afr. J. Herpetol. 2010, 59, 111–122. [Google Scholar] [CrossRef]
- Chowdry, P.; Eng, C.; Recchio, I.; Wiedner, E. Saprolegniasis in a Chinese Giant Salamander (Andrias davidianus). J. Herpetol. Med. Surg. 2012, 21, 43. [Google Scholar] [CrossRef]
- Michaels, C.J. Comparison of methods for controlling Saprolegnia-like infection in the egg sacks of Asiatic salamanders (Hynobius). Herpetol. Bull. 2018, 140, 25–27. [Google Scholar]
- Ghirardi, R.; Cazenave, J.; López, J.A.; Antoniazzi, C.E.; Perotti, M.G. Water mould exposure induces enzymatic antioxidant defences in embryos of the Two-colored Oval Frog (Elachistocleis bicolor) (Anura: Microhylidae). Can. J. Zool. 2020, 98, 411–416. [Google Scholar] [CrossRef]
- Magray, A.R.; Lone, S.A.; Ganai, B.A.; Ahmad, F.; Dar, G.J.; Dar, J.S.; Rehman, S. Comprehensive, classical and molecular characterization methods of Saprolegnia (Oomycota; Stramnipila), an important fungal pathogen of fish. Fungal Biol. Rev. 2019, 33, 166–179. [Google Scholar] [CrossRef]
- van den Berg, A.H.; McLaggan, D.; Diéguez-Uribeondo, J.; van West, P. The impact of the water moulds Saprolegnia diclina and Saprolegnia parasitica on natural ecosystems and the aquaculture industry. Fungal Biol. Rev. 2013, 27, 33–42. [Google Scholar] [CrossRef]
- Diéguez-Uribeondo, J.; Fregeneda-Grandes, J.M.; Cerenius, L.; Pérez-Iniesta, E.; Aller-Gancedo, J.M.; Tellería, M.T.; Söderhäll, K.; Martín, M.P. Re-evaluation of the enigmatic species complex Saprolegnia diclina-Saprolegnia parasitica based on morphological, physiological and molecular data. Fungal Genet. Biol. 2007, 44, 585–601. [Google Scholar] [CrossRef]
- Johnson, J.E.; Belmont, S.F.; Wagner, R.S. DNA barcoding as a means to identify organisms associated with amphibian eggs. Herpetol. Conserv. Biol. 2008, 3, 116–127. [Google Scholar]
- Sandoval-Sierra, J.V.; Diéguez-Uribeondo, J. A Comprehensive Protocol for Improving the Description of Saprolegniales (Oomycota): Two Practical Examples (Saprolegnia aenigmatica sp. nov. and Saprolegnia racemosa sp. nov.). PLoS ONE 2015, 10, e0132999. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.; Fitzpatrick, D.A. Recent advances in oomycete genomics. In Advances in Genetics; Academic Press Inc.: Cambridge, MA, USA, 2020; Volume 105, pp. 175–228. ISBN 9780128216859. [Google Scholar]
- Rocha, S.C.O.; Lopez-Lastra, C.C.; Marano, A.V.; de Souza, J.I.; Rueda-Páramo, M.E.; Pires-Zottarelli, C.L.A. New phylogenetic insights into Saprolegniales (Oomycota, Straminipila) based upon studies of specimens isolated from Brazil and Argentina. Mycol. Prog. 2018, 17, 691–700. [Google Scholar] [CrossRef]
- Hulvey, J.P.; Padgett, D.E.; Bailey, J.C. Species boundaries within Saprolegnia (Saprolegniales, Oomycota) based on morphological and DNA sequence data. Mycologia 2007, 99, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Sierra, J.V.; Martín, M.P.; Diéguez-Uribeondo, J. Species identification in the genus Saprolegnia (Oomycetes): Defining DNA-based molecular operational taxonomic units. Fungal Biol. 2014, 118, 559–578. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Sadinski, W.; Gallant, A.L.; Cleaver, J.E. Climate’s cascading effects on disease, predation, and hatching success in Anaxyrus canorus, the threatened Yosemite toad. Glob. Ecol. Conserv. 2020, 23, e01173. [Google Scholar] [CrossRef]
- Prada-Salcedo, L.D.; Franco-Correa, M.; Acosta-Galvis, A.R. Primer registro de Saprolegnia sp. en una población de anfibios en Colombia. Univ. Sci. 2011, 16, 234. [Google Scholar] [CrossRef] [Green Version]
- Czeczuga, B.; Muszyńska, E.; Krzemińska, A. Aquatic fungi growing on the spawn of certain amphibians. Amphibia-Reptilia 1998, 19, 239–251. [Google Scholar] [CrossRef]
- Gomez-Mestre, I.; Touchon, J.C.; Warkentin, K.M. Amphibian embryo and parental defenses and a larval predator reduce egg mortality from water mold. Ecology 2006, 87, 2570–2581. [Google Scholar] [CrossRef]
- Touchon, J.C.; Gomez-Mestre, I.; Warkentin, K.M. Hatching plasticity in two temperate anurans: Responses to a pathogen and predation cues. Can. J. Zool. 2006, 84, 556–563. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Grant Hokit, D.; O’Hara, R.K.; Holt, R.A. Pathogenic fungus contributes to amphibian losses in the pacific northwest. Biol. Conserv. 1994, 67, 251–254. [Google Scholar] [CrossRef]
- Kiesecker, J.M.; Blaustein, A.R. Influences of Egg Laying Behavior on Pathogenic Infection of Amphibian Eggs. Conserv. Biol. 1997, 11, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Romansic, J.M.; Higashi, E.M.; Diez, K.A.; Blaustein, A.R. Susceptibility of newly-metamorphosed frogs to a pathogenic water mould (Saprolegnia sp.). Herpetol. J. 2007, 17, 161–166. [Google Scholar] [CrossRef]
- Ault, K.K.; Johnson, J.E.; Pinkart, H.C.; Wagner, R.S. Genetic comparison of water molds from embryos of amphibians Rana cascadae, Bufo boreas and Pseudacris regilla. Dis. Aquat. Organ. 2012, 99, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.; Griffiths, R.; Jeffries, P. Susceptibility of frog (Rana temporaria) and toad (Bufo bufo) eggs to invasion by Saprolegnia. Amphibia-Reptilia 2003, 24, 261–268. [Google Scholar] [CrossRef]
- Fernández-Benéitez, M.J.; Ortiz-Santaliestra, M.E.; Lizana, M.; Diéguez-Uribeondo, J. Saprolegnia diclina: Another species responsible for the emergent disease ‘Saprolegnia infections’ in amphibians. FEMS Microbiol. Lett. 2008, 279, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Benéitez, M.J.; Ortiz-Santaliestra, M.E.; Lizana, M.; Diéguez-Uribeondo, J. Differences in susceptibility to Saprolegnia infections among embryonic stages of two anuran species. Oecologia 2011, 165, 819–826. [Google Scholar] [CrossRef]
- Groffen, J.; Oh, S.Y.; Kwon, S.; Jang, Y.; Borzee, A. High mortality in Bufo gargarizans eggs associated with an undescribed Saprolegnia ferax strain in the Republic of Korea. Dis. Aquat. Organ. 2019, 137, 89–99. [Google Scholar] [CrossRef]
- Berger, L.; Speare, R.; Thomas, A.; Hyatt, A. Mucocutaneous fungal disease in tadpoles of Bufo marinus in Australia. J. Herpetol. 2001, 35, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Williamson, I.; Bull, C. Population ecology of the Australian frog Crinia signifera: Egg-laying patterns and egg mortality. Wildl. Res. 1994, 21, 621. [Google Scholar] [CrossRef]
- Martín-Torrijos, L.; Sandoval-Sierra, J.V.; Muñoz, J.; Diéguez-Uribeondo, J.; Bosch, J.; Guayasamin, J.M. Rainbow trout (Oncorhynchus mykiss) threaten Andean amphibians. Neotrop. Biodivers. 2016, 2, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Kiesecker, J.M.; Blaustein, A.R. Pathogen reverses competition between larval amphibians. Ecology 1999, 80, 2442–2448. [Google Scholar] [CrossRef]
- Romansic, J.; Diez, K.; Higashi, E.; Blaustein, A. Effects of nitrate and the pathogenic water mold Saprolegnia on survival of amphibian larvae. Dis. Aquat. Organ. 2006, 68, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bragg, A.N. Saprolegnia on tadpoles again in Oklahoma. Southwest. Nat. 1962, 7, 79–80. [Google Scholar] [CrossRef]
- Ruthig, G.; Provost-Javier, K. Multihost saprobes are facultative pathogens of bullfrog Lithobates catesbeianus eggs. Dis. Aquat. Organ. 2012, 101, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Ghirardi, R.; Cazenave, J.; Lopez, J.A.; Antoniazzi, C.E.; Perotti, M.G. Evaluation of stress responses to water molds in embryos of Physalaemus albonotatus. Herpetol. Conserv. Biol. 2018, 13, 216–223. [Google Scholar]
- Perotti, M.G.; Basanta, M.D.; Steciow, M.M.; Sandoval-Sierra, J.V.; Diéguez-Uribeondo, J. Early breeding protects anuran eggs from Saprolegnia infection. Austral Ecol. 2013, 38, 672–679. [Google Scholar] [CrossRef]
- Ruthig, G. Water molds of the genera Saprolegnia and Leptolegnia are pathogenic to the North American frogs Rana catesbeiana and Pseudacris crucifer, respectively. Dis. Aquat. Organ. 2009, 84, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Petrisko, J.E.; Pearl, C.A.; Pilliod, D.S.; Sheridan, P.P.; Williams, C.F.; Peterson, C.R.; Bury, R.B. Saprolegniaceae identified on amphibian eggs throughout the Pacific Northwest, USA, by internal transcribed spacer sequences and phylogenetic analysis. Mycologia 2008, 100, 171–180. [Google Scholar] [CrossRef]
- Romansic, J.; Diez, K.; Higashi, E.; Johnson, J.; Blaustein, A. Effects of the pathogenic water mold Saprolegnia ferax on survival of amphibian larvae. Dis. Aquat. Organ. 2009, 83, 187–193. [Google Scholar] [CrossRef]
- Sagvik, J.; Uller, T.; Stenlund, T.; Olsson, M.; Tobias, A.E.; Ae, U.; Ae, T.S.; Olsson, M. Intraspecific variation in resistance of frog eggs to fungal infection. Evol. Ecol. 2008, 22, 193–201. [Google Scholar] [CrossRef]
- Sagvik, J.; Uller, T.; Olsson, M. A genetic component of resistance to fungal infection in frog embryos. Proc. R. Soc. Lond. B Biol. Sci. 2008, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uller, T.; Sagvik, J.; Olsson, M. Pre-hatching exposure to water mold reduces size at metamorphosis in the moor frog. Oecologia 2009, 160, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Karraker, N.E.; Ruthig, G.R. Effect of road deicing salt on the susceptibility of amphibian embryos to infection by water molds. Environ. Res. 2009, 109, 40–45. [Google Scholar] [CrossRef]
- Kim, S.; Eom, A.-H.; Park, D.; Ra, N.-Y. Detection of infectious fungal diseases of frogs inhabiting in Korea. Mycobiology 2008, 36, 10. [Google Scholar] [CrossRef] [Green Version]
- Ruthig, G.R. The influence of temperature and spatial distribution on the susceptibility of southern leopard frog eggs to disease. Oecologia 2008, 156, 895–903. [Google Scholar] [CrossRef]
- Beattie, R.C.; Aston, R.J.; Milner, A.G.P. A field study of fertilization and embryonic development in the common frog (Rana temporaria) with particular reference to acidity and temperature. J. Appl. Ecol. 1991, 28, 346–357. [Google Scholar] [CrossRef]
- Muir, A.; Kilbride, E.; Mable, B. Spatial variation in species composition of Saprolegnia, a parasitic oomycete of amphibian eggs, in Scotland. Herpetol. J. 2015, 25, 257–263. [Google Scholar]
- Bragg, A.N.; Bragg, W.N. Parasitism of spadefoot tadpoles by Saprolegnia. Herpetologica 1957, 14, 34. [Google Scholar]
- Ford, T.R.; Dillehay, D.L.; Mook, D.M. Cutaneous acariasis in the African clawed frog (Xenopus laevis). Comp. Med. 2004, 54, 713–717. [Google Scholar]
- Walls, S.C.; Jaeger, R.G. Aggression and exploitation as mechanisms of competition in larval salamanders. Can. J. Zool. 1987, 65, 2938–2944. [Google Scholar] [CrossRef]
- Urban, M.C.; Lewis, L.A.; Fučíková, K.; Cordone, A. Population of origin and environment interact to determine oomycete infections in spotted salamander populations. Oikos 2015, 124, 274–284. [Google Scholar] [CrossRef]
- Croshaw, D.A. Singly laid mole salamander (Ambystoma talpoideum) eggs resist mortality from water mold infection. Behaviour 2014, 151, 125–136. [Google Scholar] [CrossRef]
- Lefcort, H.; Hancock, K.A.; Maur, K.M.; Rostal, D.C. The Effects of Used Motor Oil, Silt, and the Water Mold Saprolegnia parasitica on the Growth and Survival of Mole Salamanders (Genus Ambystoma). Arch. Environ. Contam. Toxicol. 1997, 32, 383–388. [Google Scholar] [CrossRef]
- Terry, J.; Taguchi, Y.; Dixon, J.; Kuwabara, K.; Takahashi, M.K. Preoviposition paternal care in a fully aquatic giant salamander: Nest cleaning by a den master. J. Zool. 2019, 307, 36–42. [Google Scholar] [CrossRef]
- Green, A.J. Implications of pathogenic fungi for life-history evolution in amphibians. Funct. Ecol. 1999, 13, 573–575. [Google Scholar] [CrossRef]
- AmphibiaWeb. Available online: https://amphibiaweb.org/index.html (accessed on 18 February 2022).
- Jiang, R.H.Y.; de Bruijn, I.; Haas, B.J.; Belmonte, R.; Löbach, L.; Christie, J.; van den Ackerveken, G.; Bottin, A.; Bulone, V.; Díaz-Moreno, S.M.; et al. Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen Saprolegnia parasitica. PLoS Genet. 2013, 9, e1003272. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, V.; Rezinciuc, S.; Bulone, V. Quantitative proteomic analysis of four developmental stages of Saprolegnia parasitica. Front. Microbiol. 2018, 8, 2658. [Google Scholar] [CrossRef]
- QGIS.org QGIS. 2020. Available online: https://qgis.org/en/site/ (accessed on 11 January 2020).
- Cramp, R.L.; Franklin, C.E. Exploring the link between ultraviolet B radiation and immune function in amphibians: Implications for emerging infectious diseases. Conserv. Physiol. 2018, 6. [Google Scholar] [CrossRef]
- Palen, W.J.; Schindler, D.E.; Adams, M.J.; Pearl, C.A.; Bruce Bury, R.; Diamond, S.A. Optical characteristics of natural waters protect amphibians from UV-B in the U.S. Pacific Northwest. Ecology 2004, 83, 2951–2957. [Google Scholar] [CrossRef]
- Savory, F.; Leonard, G.; Richards, T.A. The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes. PLOS Pathog. 2015, 11, e1004805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masigol, H.; Khodaparast, S.A.; Woodhouse, J.N.; Rojas-Jimenez, K.; Fonvielle, J.; Rezakhani, F.; Mostowfizadeh-Ghalamfarsa, R.; Neubauer, D.; Goldhammer, T.; Grossart, H. The contrasting roles of aquatic fungi and oomycetes in the degradation and transformation of polymeric organic matter. Limnol. Oceanogr. 2019, 64, 2662–2678. [Google Scholar] [CrossRef] [Green Version]
- Money, N.P.; Davis, C.M.; Ravishankar, J.P. Biomechanical evidence for convergent evolution of the invasive growth process among fungi and oomycete water molds. Fungal Genet. Biol. 2004, 41, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Songe, M.M.; Willems, A.; Wiik-Nielsen, J.; Thoen, E.; Evensen, Ø.; van West, P.; Skaar, I. Saprolegnia diclina IIIA and S. parasitica employ different infection strategies when colonizing eggs of Atlantic salmon, Salmo salar L. J. Fish Dis. 2016, 39, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bly, J.E.; Lawson, L.A.; Dale, D.J.; Szalai, A.J.; Durborow, R.M.; Clem, L.W. Winter saprolegniosis in channel catfish. Dis. Aquat. Organ. 1992, 13, 155–164. [Google Scholar] [CrossRef]
- Rezinciuc, S.; Sandoval-Sierra, J.V.; Ruiz-León, Y.; Van West, P.; Diéguez-Uribeondo, J. Specialized attachment structure of the fish pathogenic oomycete Saprolegnia parasitica. PLoS ONE 2018, 13, e0190361. [Google Scholar] [CrossRef]
- Diéguez-Uribeondo, J.; Cerenius, L.; Söderhäll, K. Repeated zoospore emergence in Saprolegnia parasitica. Mycol. Res. 1994, 98, 810–815. [Google Scholar] [CrossRef]
- Smith, S.N.; Armstrong, R.A.; Rimmer, J.J. Influence of environmental factors on zoospores of Saprolegnia diclina. Trans. Br. Mycol. Soc. 1984, 82, 413–421. [Google Scholar] [CrossRef]
- El-Feki, M.; Hatai, K.; Hussein, M.M.A. Chemotactic and chemokinetic activities of Saprolegnia parasitica toward different metabolites and fish tissue extracts. Mycoscience 2003, 44, 159–162. [Google Scholar] [CrossRef]
- Rand, T.G.; Munden, D. Chemotaxis of zoospores of two fish-egg-pathogenic strains of saprolegnia diclina (oomycotina: Saprolegniaceae) toward salmonid egg chorion extracts and selected amino acids and sugars. J. Aquat. Anim. Health 1993, 5, 240–245. [Google Scholar] [CrossRef]
- McGowan, J.; Fitzpatrick, D.A. Genomic, Network, and Phylogenetic Analysis of the Oomycete Effector Arsenal. mSphere 2017, 2, e00408-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleason, F.H.; Chambouvet, A.; Sullivan, B.K.; Lilje, O.; Rowley, J.J.L. Multiple zoosporic parasites pose a significant threat to amphibian populations. Fungal Ecol. 2014, 11, 181–192. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A. Amphibian immunity–stress, disease, and climate change. Dev. Comp. Immunol. 2017, 66, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Koeypudsa, W.; Phadee, P.; Tangtrongpiros, J.; Hatai, K. Influence of pH, Temperature and Sodium Chloride Concentration on Growth Rate of Saprolegnia sp. J. Sci. Res. Chula. Univ. 2005, 30, 123–130. [Google Scholar]
- Barnes, M.; Gabel, A.; Durben, D.; Hightower, T.; Berger, T. Changes in Water Hardness Influence Colonization of Saprolegnia diclina. N. Am. J. Aquac. 2004, 66, 222–227. [Google Scholar] [CrossRef]
- Wright, K.M.; Whitaker, B.R. Amphibian Medicine and Captive Husbandry; Krieger Publishing Company: Malabar, FL, USA, 2001; ISBN 9783642253874. [Google Scholar]
- Blaustein, A.R.; Walls, S.C.; Bancroft, B.A.; Lawler, J.J.; Searle, C.L.; Gervasi, S.S. Direct and Indirect Effects of Climate Change on Amphibian Populations. Diversity 2010, 2, 281–313. [Google Scholar] [CrossRef]
- Benard, M.F. Warmer winters reduce frog fecundity and shift breeding phenology, which consequently alters larval development and metamorphic timing. Glob. Chang. Biol. 2015, 21, 1058–1065. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Holt, R.D. How should environmental stress affect the population dynamics of disease? Ecol. Lett. 2003, 6, 654–664. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.H.; Walke, J.B.; Cikanek, S.; Savage, A.E.; Mattheus, N.; Santiago, C.N.; Minbiole, K.P.C.; Harris, R.N.; Belden, L.K.; Gratwicke, B. Composition of symbiotic bacteria predicts survival in Panamanian golden frogs infected with a lethal fungus. Proc. R. Soc. B Biol. Sci. 2015, 282. [Google Scholar] [CrossRef] [Green Version]
- Carbajal-González, M.T.; Fregeneda-Grandes, J.M.; Suárez-Ramos, S.; Rodríguez Cadenas, F.; Aller-Gancedo, J.M. Bacterial skin flora variation and in vitro inhibitory activity against Saprolegnia parasitica in brown and rainbow trout. Dis. Aquat. Organ. 2011, 96, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Antwis, R.E.; Haworth, R.L.; Engelmoer, D.J.P.; Ogilvy, V.; Fidgett, A.L.; Preziosi, R.F. Ex situ Diet Influences the Bacterial Community Associated with the Skin of Red-Eyed Tree Frogs (Agalychnis callidryas). PLoS ONE 2014, 9, e85563. [Google Scholar] [CrossRef]
- Costa, S.; Lopes, I.; Proença, D.N.; Ribeiro, R.; Morais, P.V. Diversity of cutaneous microbiome of Pelophylax perezi populations inhabiting different environments. Sci. Total Environ. 2016, 572, 995–1004. [Google Scholar] [CrossRef]
- Belmonte, R.; Wang, T.; Duncan, G.J.; Skaar, I.; Mélida, H.; Bulone, V.; van West, P.; Secombes, C.J. Role of pathogen-derived cell wall carbohydrates and prostaglandin E2 in immune response and suppression of fish immunity by the oomycete Saprolegnia parasitica. Infect. Immun. 2014, 82, 4518–4529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pessier, A.P. Edematous Frogs, Urinary Tract Disease, and Disorders of Fluid Balance in Amphibians. J. Exot. Pet Med. 2009, 18, 4–13. [Google Scholar] [CrossRef]
- National Research Council (US) Subcommittee on Amphibian Standards. Amphibians—Guidelines for the Breeding, Care and Management of Laboratory Animals; National Academies Press (US): Cambridge, MA, USA, 1974. [Google Scholar]
- Walker, I.D.F.; Whitaker, B.R. Amphibian Therapeutics. Vet. Clin. N. Am. Exot. Anim. Pract. 2000, 3, 239–255. [Google Scholar] [CrossRef]
- Rosado, D.; Xavier, R.; Severino, R.; Tavares, F.; Cable, J.; Pérez-Losada, M. Effects of disease, antibiotic treatment and recovery trajectory on the microbiome of farmed seabass (Dicentrarchus labrax). Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Poll, C.P. Wound Management in Amphibians: Etiology and Treatment of Cutaneous Lesions. J. Exot. Pet Med. 2009, 18, 20–35. [Google Scholar] [CrossRef]
- Wright, K. Amphibian Husbandry and Medicine; Reptile Me; Mader, D.R., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1996. [Google Scholar]
- Smith, S.A. Compendium of Drugs and Compounds Used in Amphibians. ILAR J. 2007, 48, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Crawshaw, G.J.; Kirk, R. Amphibian Medicine; Kirk’s Cur.; Kirk, R., Bonagura, J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 1992. [Google Scholar]
- Ewert, J.; Cooper, J.E.; Langton, T.; Matz, G.; Reilly, K.; Schwantje, H. Background information On the species-specific proposals for amphibians. In Proceedings of the 8th Meeting of the Working Party, Strasbourg, France, 22–24 September 2004; 2004; 123. [Google Scholar]
- Raphael, B.L. Amphibians. Vet. Clin. N. Am.-Small Anim. Pract. 1993, 23, 1271–1286. [Google Scholar] [CrossRef]
- Ali, S.E.; Evensen, Ø.; Skaar, I. Recent advances in the mitigation of Saprolegnia infections in freshwater fish and their eggs. Battle Against Microb. Pathog. Basic Sci. Technol. Adv. Educ. Prog. 2015, 692–697. [Google Scholar]
- Ali, S.E.; Thoen, E.; Evensen, O.; Skaar, I. Boric Acid inhibits germination and colonization of Saprolegnia spores in vitro and in vivo. PLoS ONE 2014, 9, e91878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warrilow, A.G.S.; Hull, C.M.; Rolley, N.J.; Parker, J.E.; Nes, W.D.; Smith, S.N.; Kelly, D.E.; Kelly, S.L. Clotrimazole as a Potent Agent for Treating the Oomycete Fish Pathogen Saprolegnia parasitica through Inhibition of Sterol 14α-Demethylase (CYP51). Appl. Environ. Microbiol. 2014, 80, 6154–6166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, H.S.; Choi, T.J. The efficacy of Virkon-S for the control of saprolegniasis in common carp, Cyprinus carpio L. PeerJ 2018, 2018, e5706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampieri, M.P.; Galuppi, R.; Carelle, M.S.; Macchioni, F.; Cioni, P.L.; Morelli, I. Effect of selected essential oils and pure compounds on Saprolegnia parasitica. Pharm. Biol. 2003, 41, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.M.; Saeed Mirzargar, S.; Mousavi, H.E.; Omidbaigi, R.; Khosravi, A.; Bahonar, A. Antifungal and toxicity effects of new combined essential oils on Oncorhynchus mykiss in comparison with malachite green. Iran. J. Vet. Sci. Technol. 2012, 4, 1–8. [Google Scholar] [CrossRef]
- Mostafa, A.A.-F.; Al-Askar, A.A.; Taha Yassin, M. Anti-saprolegnia potency of some plant extracts against Saprolegnia diclina, the causative agent of saprolengiasis. Saudi J. Biol. Sci. 2020, 27, 1482–1487. [Google Scholar] [CrossRef]
- Miljanović, A.; Grbin, D.; Pavić, D.; Dent, M.; Jerković, I.; Marijanović, Z.; Bielen, A. Essential oils of sage, rosemary, and bay laurel inhibit the life stages of oomycete pathogens important in aquaculture. Plants 2021, 10, 1676. [Google Scholar] [CrossRef]
- Madrid, A.; Godoy, P.; González, S.; Zaror, L.; Moller, A.; Werner, E.; Cuellar, M.; Villena, J.; Montenegro, I. Chemical characterization and anti-oomycete activity of Laureliopsis philippianna essential oils against Saprolegnia parasitica and S. australis. Molecules 2015, 20, 8033–8047. [Google Scholar] [CrossRef] [Green Version]
- Tedesco, P.; Beraldo, P.; Massimo, M.; Fioravanti, M.L.; Volpatti, D.; Dirks, R.; Galuppi, R. Comparative Therapeutic Effects of Natural Compounds Against Saprolegnia spp. (Oomycota) and Amyloodinium ocellatum (Dinophyceae). Front. Vet. Sci. 2020, 7, 83. [Google Scholar] [CrossRef]
- Adel, M.; Dadar, M.; Zorriehzahra, M.J.; Elahi, R.; Stadtlander, T. Antifungal activity and chemical composition of Iranian medicinal herbs against fish pathogenic fungus, Saprolegnia parasitica. Iran. J. Fish. Sci. 2020, 19, 3239–3254. [Google Scholar] [CrossRef]
- Campbell, C.R.; Voyles, J.; Cook, D.I.; Dinudom, A. Frog skin epithelium: Electrolyte transport and chytridiomycosis. Int. J. Biochem. Cell Biol. 2012, 44, 431–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voyles, J.; Berger, L.; Young, S.; Speare, R.; Webb, R.; Warner, J.; Rudd, D.; Campbell, R.; Skerratt, L. Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis. Dis. Aquat. Organ. 2007, 77, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Wright, K. Important clinical aspects of amphibian physiology. In Proceedings of the the North American Veterinary Conference-Exotics—Reptiles and Amphibians, Orlando, FL, USA, 7–11 January 2006; Volume 20. [Google Scholar]
- Fort, D.J.; Fort, T.D.; Mathis, M.B.; Ball, R.W. Boric Acid Is Reproductively Toxic to Adult Xenopus laevis, but Not Endocrine Active. Toxicol. Sci. 2016, 154, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laposata, M.M.; Dunson, W.A. Effects of Boron and Nitrate on Hatching Success of Amphibian Eggs. Arch. Environ. Contam. Toxicol. 1998, 35, 615–619. [Google Scholar] [CrossRef]
- Shi, H.; Sun, Z.; Liu, Z.; Xue, Y. Effects of clotrimazole and amiodarone on early development of amphibian (Xenopus tropicalis). Toxicol. Environ. Chem. 2012, 94, 128–135. [Google Scholar] [CrossRef]
- Choi, M.J.; Kim, S.C.; Kim, A.N.; Kwon, H.B.; Ahn, R.S. Effect of endocrine disruptors on the oocyte maturation and ovulation in amphibians, Rana dybowskii. Integr. Biosci. 2007, 11, 1–8. [Google Scholar] [CrossRef]
- Gyllenhammar, I.; Eriksson, H.; Söderqvist, A.; Lindberg, R.H.; Fick, J.; Berg, C. Clotrimazole exposure modulates aromatase activity in gonads and brain during gonadal differentiation in Xenopus tropicalis frogs. Aquat. Toxicol. 2009, 91, 102–109. [Google Scholar] [CrossRef]
- Schmidt, B.R.; Geiser, C.; Peyer, N.; Keller, N.; Von Rütte, M. Assessing whether disinfectants against the fungus Batrachochytrium dendrobatidis have negative effects on tadpoles and zooplankton. Amphib. Reptil. 2009, 30, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Hangartner, S.; Laurila, A. Effects of the disinfectant Virkon S on early life-stages of the moor frog (Rana arvalis). Amphib. Reptil. 2012, 33, 349–353. [Google Scholar] [CrossRef]
- Tavares-Dias, M. Current knowledge on use of essential oils as alternative treatment against fish parasites. Aquat. Living Resour. 2018, 31, 13. [Google Scholar] [CrossRef] [Green Version]
- Goulet, F.; Vachon, P.; Hélie, P. Evaluation of the toxicity of eugenol at anesthetic doses in African clawed frogs (Xenopus laevis). Toxicol. Pathol. 2011, 39, 471–477. [Google Scholar] [CrossRef]
- Hernández, S.E.; Sernia, C.; Bradley, A.J. The effect of three anaesthetic protocols on the stress response in cane toads (Rhinella marina). Vet. Anaesth. Analg. 2012, 39, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.A. Anesthetic Considerations for Amphibians. J. Exot. Pet Med. 2009, 18, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Salbego, J.; Maia, J.L. dos S.; Toni, C.; Rodrigues, A.S.S.; Sousa, E.M.O.; Silva, L.V.F. da; Mourão, R.H.V.; Barata, L.E.S.; Heinzmann, B.M.; Baldisserotto, B. Anesthesia and sedation of map treefrog (Hypsiboas geographicus) tadpoles with essential oils. Ciência Rural 2017, 47. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Gómez, O.; Byrne, A.Q.; Gunderson, A.R.; Jenkinson, T.S.; Noss, C.F.; Rothstein, A.P.; Womack, M.C.; Rosenblum, E.B. Invasive vegetation affects amphibian skin microbiota and body condition. PeerJ 2020, 2020. [Google Scholar] [CrossRef]
- Gozlan, R.E.; Britton, J.R.; Cowx, I.; Copp, G.H. Current knowledge on non-native freshwater fish introductions. J. Fish Biol. 2010, 76, 751–786. [Google Scholar] [CrossRef]
- Sandoval-Sierra, J.V.; Latif-Eugenin, F.; Martín, M.P.; Zaror, L.; Diéguez-Uribeondo, J. Saprolegnia species affecting the salmonid aquaculture in Chile and their associations with fish developmental stage. Aquaculture 2014, 434, 462–469. [Google Scholar] [CrossRef]
| Host Species | IUCN Red List Category (Last Assessment) | Population Trend | Life Stage | Pathogen | Country | Reference | GenBank Accession Number |
|---|---|---|---|---|---|---|---|
| Anura | |||||||
| Anaxyrus canorus | EN, 2004 | Decreasing | Eggs (w) | Saprolegnia diclina | USA | [38] | |
| Atelopus mittermeieri | EN, 2017 | Decreasing | Adult (w) | Saprolegnia sp. | Colombia | [39] | |
| Bombina bombina | LC, 2009 | Decreasing | Eggs (l) | Saprolegnia ferax Saprolegnia parasitica Aphanomyces stellatus Dictyuchus sterilis Leptomitus lacteus (*) | Poland | [40] | |
| Bufo americanus1 | LC, 2015 | Stable | Eggs (w) (l) | Saprolegnia sp. Achlya sp. | USA | [41] | |
| Bufo americanus1 | LC, 2015 | Stable | Embryo (l) | Achlya sp. Saprolegnia sp. | USA | [42] | |
| Bufo boreas 2 | LC, 2015 | Decreasing | Eggs (w) | Saprolegnia ferax | USA | [43] | |
| Bufo boreas 2 | LC, 2015 | Decreasing | Eggs (l) | Saprolegnia ferax | USA | [13] | |
| Bufo boreas 2 | LC, 2015 | Decreasing | Eggs (w) (l) | Saprolegnia ferax | USA | [44] | |
| Bufo boreas 2 | LC, 2015 | Decreasing | Embryos (w) (l) | Saprolegnia ferax | USA | [14] | |
| Bufo boreas 2 | LC, 2015 | Decreasing | Metamorphs (l) | Saprolegnia sp. | USA | [45] | |
| Bufo boreas 2 | LC, 2015 | Decreasing | Egg (w) | Saprolegnia anisospora (s) S. diclina (s) Saprolegnia. sp. 1 (s) Saprolegnia sp. 2 (s) | USA | [31] | EU124746 to EU124748 EU124759 to EU124753 EU124761 |
| Bufo boreas 2 (*) | LC, 2015 | Decreasing | Eggs (w) | Saprolegnia spp. (s) Achlya spp.(s) | USA | [46] | JQ974984 JQ974986 to JQ974990, JQ974992 to JQ974999 |
| Bufo bufo3 | LC, 2009 | Stable | Eggs (w) | Saprolegnia (Molding) | Netherlands | [21] | |
| Bufo bufo3 | LC, 2009 | Stable | Eggs (w) | Saprolegniaceae | Netherlands | [22] | |
| Bufo bufo3 | LC, 2009 | Stable | Eggs (l) | Saprolegnia ferax Saprolegnia parasitica Aphanomyces stellatus Dictyuchus sterilis Leptomitus lacteus (*) | Poland | [40] | |
| Bufo bufo3 | LC, 2009 | Stable | Eggs (l) | “Saprolegnia diclina-S. parasitica complex” | UK | [47] | |
| Bufo bufo3 | LC, 2009 | Stable | Eggs (n.d) | Saprolegnia diclina (s) | Spain | [36] | KF717747 KF717750 |
| Bufo calamita4 | LC, 2009 | Decreasing | Eggs (w) | Saprolegnia (Molding) | Netherlands | [21] | |
| Bufo calamita4 | LC, 2009 | Decreasing | Eggs (w) | Saprolegniaceae | Netherlands | [22] | |
| Bufo calamita4 | LC, 2009 | Decreasing | Eggs (w) | Saprolegnia “infestation” (**) | UK | [23] | |
| Bufo calamita4 | LC, 2009 | Decreasing | Eggs (w) | Saprolegnia diclina | Spain | [48] | |
| Bufo calamita4 | LC, 2009 | Decreasing | Embryo (w) (l) | Saprolegnia diclina (s) | Spain | [49] | AM228844 |
| Bufo calamita4 | LC, 2009 | Decreasing | Eggs (n.d) | Saprolegnia diclina (s) | Spain | [36] | KF717752 |
| Bufo gargarizans | LC, 2018 | Stable | Eggs (w) | Saprolegnia ferax (s) | Korea | [50] | MK372991 |
| Bufo marinus5 | LC, 2008 | Increasing | Tadpoles (w) | Aphanomyces sp. | Australia | [51] | |
| Crinia signifera | LC, 2004 | Stable | Eggs (w) | Water mold infections | Australia | [52] | |
| Elachistocleis bicolor | LC, 2004 | Stable | Embryo (l) | Saprolegnia-like sp. | Argentina | [27] | |
| Engystomops petersi | LC, 2017 | Stable | Embryo (l) | Saprolegnia diclina (s) | Ecuador | [53] | |
| Hyla molleri | LC, 2008 | Decreasing | Eggs (n.d) | Saprolegnia diclina (s) | Spain | [36] | KF717749 |
| Hyla regilla6 | LC, 2004 | Stable | Eggs (l) | Saprolegnia ferax | USA | [13] | |
| Hyla regilla6 | LC, 2004 | Stable | Eggs (l)(w) | Saprolegnia ferax | USA | [44] | |
| Hyla regilla6 | LC, 2004 | Stable | Embryos (l) | Saprolegnia ferax | USA | [54] | |
| Hyla regilla6 | LC, 2004 | Stable | Embryos (w) | Saprolegnia ferax | USA | [14] | |
| Hyla regilla6 | LC, 2004 | Stable | Larvae (l) | Saprolegnia sp. | USA | [55] | |
| Hyloscirtus alytolylax | NT, 2004 | Decreasing | Eggs (n.d) | Saprolegnia sp. 2 (s) | Ecuador | [36] | KF718069 |
| Lithobates berlandieri7 | LC, 2008 | Stable | Tadpoles (w) | Saprolegnia sp. | USA | [56] | |
| Lithobatescatesbeiana8 | LC, 2015 | Increasing | Eggs (w) | Leptolegnia sp. (s) | USA | [57] | GU014271 |
| Pelobates cultripes | VU, 2020 | Decreasing | Eggs (l) (w) | Saprolegnia. diclina (s) | Spain | [49] | AM228845 |
| Pelobates cultripes | VU, 2020 | Decreasing | Eggs (n.d) | Saprolegnia diclina (s) Saprolegnia sp. 2 (s) | Spain | [36] | KF717745/ KF717746 KF717748 KF717753 KF717754 KF717770 KF718050 to KF718054 |
| Pelobates fuscus | LC, 2009 | Decreasing | Eggs (w) | Saprolegnia (Molding) | Netherlands | [21] | |
| Pelophylax perezi | LC, 2020 | Decreasing | Eggs (n.d) | Saprolegnia diclina (s) | Spain | [36] | KF717751 |
| Pelophylax perezi | LC, 2020 | Decreasing | Eggs (w) Tadpoles (l) | Saprolegnia australis (s) | Portugal | Costa et al. (in prep.) | MK046073 |
| Physalaemus albonotatus | LC, 2004 | Stable | Embryo (l) | Saprolegnia sp. | Argentina | [58] | |
| Pleurodema thaul | LC, 2015 | Stable | Eggs (l) (w) | Saprolegnia ferax Saprolegnia sp. Saprolegnia diclina | Argentina | [59] | |
| Pleroderma thaul | LC, 2015 | Stable | Eggs (n.d) | Saprolegnia diclina (s) S. australis (s) S. anisospora (s) | Argentina | [36] | KF717781 to KF717785 KF717972 KF718194 |
| Pseudacris crucifer | LC, 2015 | Stable | Eggs (w) (l) | Leptolegnia sp. (s) | USA | [60] | EU071706 |
| Pseudacris crucifer | LC, 2015 | Stable | Eggs (w) Adult (w) | Saprolegnia sp. (s) Achlya sp. (s) | USA | [57] | GU014279 GU014283 |
| Pseudacrisregilla | LC, 2004 | Stable | Metamorphs (l) | Saprolegnia sp. | USA | [45] | |
| Pseudacris regilla | LC, 2004 | Stable | Egg (w) | Saprolegnia. sp. 1 (s) S. cf kauffmaniana (s) Achlya stellata (s) | USA | [31] | EU124762 EU124766 EU124754 to EU124757 |
| Pseudacris regilla | LC, 2004 | Stable | Eggs (w) | Saprolegnia diclina (s) Achlya sp.(s) | USA | [61] | |
| Pseudacris regilla | LC, 2004 | Stable | Larvae (l) | Saprolegnia ferax | USA | [62] | |
| Pseudacris streckeri | LC. 2015 | Unknown | Tadpoles (w) | Saprolegnia sp. | USA | [56] | |
| Rana arvalis | LC, 2009 | Stable | Eggs (w) | Saprolegnia (Molding) | Netherlands | [21] | |
| Rana arvalis | LC, 2009 | Stable | Eggs (w) | Saprolegniaceae | Netherlands | [22] | |
| Rana arvalis | LC, 2009 | Stable | Eggs (l) | Saprolegnia ferax Saprolegnia parasitica Aphanomyces stellatus Dictyuchus sterilis Leptomitus lacteus (*) | Poland Ukraine | [40] | |
| Rana arvalis | LC, 2009 | Stable | Eggs (l) (w) | Saprolegnia spp. | Sweden | [63,64] | |
| Rana arvalis | LC, 2009 | Stable | Eggs (l) (w) | Saprolegnia sp. | Sweden | [65] | |
| Rana aurora | LC, 2015 | Decreasing | Embryos (w) | Saprolegnia ferax | USA | [14] | |
| Rana aurora | LC, 2015 | Decreasing | Larvae (l) | Saprolegnia sp. | USA | [55] | |
| Rana aurora | LC, 2015 | Decreasing | Metamorphs (l) | Saprolegnia sp. | USA | [45] | |
| Rana aurora | LC, 2015 | Decreasing | Eggs (w) | Saprolegnia spp. (s) Achlya sp.(s) | USA | [61] | |
| Rana aurora | LC, 2015 | Decreasing | Larvae (l) | Saprolegnia ferax | USA | [62] | |
| Rana cascadae | NT, 2004 | Decreasing | Eggs (l) | Saprolegnia ferax | USA | [13] | |
| Rana cascadae | NT, 2004 | Decreasing | Eggs (w) (l) | Saprolegnia ferax | USA | [44] | |
| Rana cascadae | NT, 2004 | Decreasing | Embryos (l) | Saprolegnia ferax | USA | [54] | |
| Rana cascadae | NT, 2004 | Decreasing | Embryos (w) | Saprolegnia ferax | USA | [14] | |
| Rana cascadae | NT, 2004 | Decreasing | Metamorphs (l) | Saprolegnia sp. | USA | [45] | |
| Rana cascadae | NT, 2004 | Decreasing | Eggs (w) | Saprolegnia sp. (s) Achlya sp.(s) Leptolegnia sp. (s) | USA | [61] | |
| Rana cascadae | NT, 2004 | Decreasing | Eggs (w) | Saprolegnia sp. (s) Saprolegnia ferax (s) | USA | [46] | JQ974983, JQ974985, JQ974991 |
| Rana catesbeiana | LC, 2015 | Increasing | Eggs (w) (l) | Saprolegnia sp. (s) | USA | [60] | EU071707 |
| Rana clamitans | LC, 2015 | Stable | Eggs (w) (l) Embryos (w) (l) | Saprolegnia (s) | USA | [66] | EU480454 |
| Rana dalmatina | LC, 2009 | Decreasing | Eggs (l) | Saprolegnia ferax Saprolegnia parasitica Aphanomyces stellatus Dictyuchus sterilis Leptomitus lacteus (*) | Ukraine | [40] | |
| Rana esculenta9 | Eggs (w) | Saprolegniaceae | Netherlands | [22] | |||
| Rana esculenta9 | Eggs (w) | Saprolegnia (Molding) | Netherlands | [21] | |||
| Rana kl. esculenta10 | Eggs (l) | Saprolegnia ferax Saprolegnia parasitica Aphanomyces stellatus Dictyuchus sterilis Leptomitus lacteus (*) | Poland | [40] | |||
| Rana huanrenensis | LC, 2018 | Decreasing | Adult (w) | Saprolegnia diclina (s) | Korea | [67] | AM228848 |
| Rana lessonae11 | LC, 2008 | Decreasing | Eggs (l) | Saprolegnia ferax Saprolegnia parasitica Aphanomyces stellatus Dictyuchus sterilis Leptomitus lacteus (*) | Poland | [40] | |
| Rana luteiventris | LC, 2015 | Decreasing | Eggs (w) | Saprolegnia sp. (s) Achlya sp.(s) Leptolegnia sp. (s) | USA | [61] | |
| Rana plancyi chosenica12 | VU, 2020 | Decreasing | Tadpoles (c) | Saprolegnia australis (s) | Korea | [67] | AM228837 |
| Rana pretiosa | VU, 2004 | Decreasing | Eggs (w) | Saprolegnia sp. (s) Achlya sp.(s) Leptolegnia sp. (s) | USA | [61] | |
| Rana ridibunda13 | LC, 2009 | Increasing | Eggs (l) | Saprolegnia ferax Saprolegnia parasitica Aphanomyces stellatus Dictyuchus sterilis Leptomitus lacteus (*) | Poland | [40] | |
| Ranasphenocephala | LC, 2021 | Stable | Eggs (w) (l) | Saprolegnia (s) | USA | [68] | EU348371 EU348372 |
| Rana sylvatica | LC, 2015 | Stable | Eggs (w) (l) | Saprolegnia sp. Achlya sp. | USA | [41] | |
| Rana sylvatica | LC, 2015 | Stable | Embryo (l) | Achlya sp. Saprolegnia sp. | USA | [42] | |
| Rana sylvatica | LC, 2015 | Stable | Eggs (w) (l) Embryos (w) (l) | Saprolegnia (s) | USA | [66] | EU480454 |
| Rana temporaria | LC, 2008 | Stable | Eggs (w) | Saprolegnia (Molding) | Netherlands | [21] | |
| Rana temporaria | LC, 2008 | Stable | Eggs (w) | Saprolegniaceae | Netherlands | [22] | |
| Rana temporaria | LC, 2008 | Stable | Eggs (w) | Saprolegnia sp. | UK | [69] | |
| Rana temporaria | LC, 2008 | Stable | Eggs (l) | Saprolegnia ferax Saprolegnia parasitica Aphanomyces stellatus Dictyuchus sterilis Leptomitus lacteus (*) | Poland | [40] | |
| Rana temporaria | LC, 2008 | Stable | Eggs (l) | “Saprolegnia diclina-S. parasitica complex” | UK | [47] | |
| Rana temporaria | LC, 2008 | Stable | Eggs (w) | Saprolegnia spp. (s) | Scotland | [70] | |
| Scinax garbei | LC, 2004 | Stable | Eggs (n.d) | Saprolegnia sp. 2 (s) | Ecuador | [36] | KF718071 KF718072 |
| Spea bombifrons | LC, 2015 | Stable | Tadpoles (w) | Saprolegnia sp. | USA | [71] | |
| Xenopus laevis | LC, 2020 | Increasing | Adult (c) | Saprolegnia sp. | USA | [72] | |
| Xenopus laevis | LC, 2020 | Increasing | Embryo (l) | S. hypogyna (s) S. delica (s) S. diclina (s) S. parasitica (N12) (s) S. ferax (SAP442) (s) | Scotland | [15] | KF420214 KF420220 KF420212/KF420236 FN186030 AM228845 |
| Xenopus longipes | CR, 2017 | Decreasing | Adults (w) | “Saprolegniosis” | Cameroon | [24] | |
| Urodela | |||||||
| Ambystoma gracile | LC, 2015 | Stable | Larvae (l) | Saprolegnia sp. | USA | [55] | |
| Ambystoma gracile | LC, 2015 | Stable | Egg (w) | Saprolegnia ferax (s) Saprolegnia sp. (s) | USA | [31] | EU124763 EU124752 |
| Ambystoma macrodactylum | LC, 2015 | Stable | Eggs (w) | Saprolegnia sp. (s) Leptolegnia sp. (s) | USA | [61] | |
| Ambystoma maculatum | LC, 2015 | Stable | Eggs (w) (l) | Saprolegnia sp. Achlya sp. | USA | [41] | |
| Ambystoma maculatum | LC, 2015 | Stable | Eggs (w) (l) Embryos (w) (l) | Saprolegnia (s) | USA | [66] | EU480454 |
| Ambystoma maculatum | LC, 2015 | Stable | Larvae (l) | Saprolegnia sp. | USA | [73] | |
| Ambystoma maculatum | LC, 2015 | Stable | Eggs (w) (l) | Saprolegnia torulosa (s) S. diclina (s) S. turfosa (s) Achlya papilosa (s) | USA | [74] | KC758888 to KC758895 |
| Ambystoma talpoideum | LC, 2004 | Stable | Eggs (w) (l) | “Water mold infection” | USA | [75] | |
| Ambystoma tigrinum | LC, 2015 | Stable | Larvae (l) | Saprolegnia | USA | [76] | |
| Andrias davidianus | CR, 2004 | Decreasing | Adult (c) | Saprolegniosis (**) | USA | [25] | |
| Andrias japonicus | CR, 2004 | Decreasing | Eggs (c) | “Water mold infection” | Japan | [77] | |
| Hynobius dunni | VU, 2021 | Decreasing | Eggs (c) | Saprolegniosis (**) | UK | [26] | |
| Notophthalmus viridescens | LC, 2015 | Stable | Adult (w) | Achlya sp. (s) | USA | [57] | GU014277 |
| Triturus vulgaris14 | LC, 2009 | Stable | Eggs (l) | Saprolegnia ferax Saprolegnia parasitica Aphanomyces stellatus Dictyuchus sterilis Leptomitus lacteus (*) | Poland | [40] | |
| Triturus vulgaris14 | LC, 2009 | Stable | Eggs (l) Adults (l) | Saprolegnia sp. | UK | [78] |
| Treatment Agent | Amphibians | Reference | |
|---|---|---|---|
| Malachite green †1 | 0.2 mg/L as a bath for 1 h daily 67 mg/L for 15 s, once daily for 2–3 days | [114] [100] | |
| Formalin †2 | 1.5 mL/L of a 10% formalin solution dip for 10 min (48 h) | [115,116] | |
| Methylene blue | 50 mg/mL 10-s dip, <2 mg/mL for tadpoles | [114] [115] | |
| Copper sulphate | 500 mg/L for 2 min, once daily for 5 days, then once weekly until healed) | [100] | |
| Sea salt | Bath:10–25 g/L for 5–30 min | [114] | |
| Sodium chloride | Bath:10–25 g/L for 5–30 min | [114] | |
| Calcium propionate | Solution of 2–3% calcium propionate for 1 min, daily | [117] | |
| Potassium permanganate | 200 mg/L for 5 min 1:5000 for 5 min | [100] [118] | |
| Mercurochrome | 2% solution, topical painting | [117] | |
| Benzalkonium chloride | 2 mg/L as a 60-min bath (24 h) 0.25 mg/L, 3 times a week | [100] [116] | |
| New therapies | |||
| Treatment agent | Species tested | In vitro | Reference |
| Boric acid | S. parasitica S. diclina | 800 mg/L (complete inhibition) >200 mg/L ↓ spore activity and mycelial growth | [119,120] |
| Clotrimazole | S. parasitica S. diclina S. ferax | MIC100 of 1 to 2 mg/L | [121] |
| Virkon-S | S. parasitica | Spore and mycelial growth inhibition >4 mg/L | [122] |
| Essential oils | S. parasitica S. diclina S. ferax S. australis | Essential oils and pure compounds (See references) | [123,124,125,126,127,128,129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, S.; Lopes, I. Saprolegniosis in Amphibians: An Integrated Overview of a Fluffy Killer Disease. J. Fungi 2022, 8, 537. https://doi.org/10.3390/jof8050537
Costa S, Lopes I. Saprolegniosis in Amphibians: An Integrated Overview of a Fluffy Killer Disease. Journal of Fungi. 2022; 8(5):537. https://doi.org/10.3390/jof8050537
Chicago/Turabian StyleCosta, Sara, and Isabel Lopes. 2022. "Saprolegniosis in Amphibians: An Integrated Overview of a Fluffy Killer Disease" Journal of Fungi 8, no. 5: 537. https://doi.org/10.3390/jof8050537
APA StyleCosta, S., & Lopes, I. (2022). Saprolegniosis in Amphibians: An Integrated Overview of a Fluffy Killer Disease. Journal of Fungi, 8(5), 537. https://doi.org/10.3390/jof8050537







