Abstract
Fungi bioaccumulation of heavy metals is a promising approach to remediate polluted soil and water. Boletus griseus could accumulate high amounts of Cd, even in a natural habitat with low Cd contents. This study found a symbiotic association of B. griseus with a fungus. The symbiotic fungus was isolated and identified as Hypomyces chrysospermus. The isolated strain had a strong ability to tolerate Cd. The minimum inhibitory concentration of Cd of fungal growth was 200 mg·L−1. The Cd bioaccumulation capacity of the fungus reached 10.03 mg·g−1. The biomass production of the fungus was promoted by 20 mg·L−1 Cd. However, high concentrations of Cd suppressed fungal growth and significantly altered the morphology and fine texture of fungal hyphae and chlamydospores. The immobilization effects of the cell wall and acid compounds and antioxidant enzymes were employed by the fungus to alleviate the toxic effects of Cd. The results not only demonstrate a new insight into the Cd bioconcentration mechanisms of B. griseus but also provide a potential bioremediation fungus for Cd contamination.
1. Introduction
Boletus griseus is a common wild-grown edible mushroom in Yunnan Province, and it is a member of the genus Boletus in the family Boletaceae [1]. Studies have confirmed that B. griseus can accumulate high amounts of Cd in fruiting bodies, even from natural habitats with low Cd contents in the soil matrix [2,3,4]. The Cd contents of 153 samples of B. griseus were found to range from 1.61–42.67 mg·kg−1 while those in soil ranged from 0.03–0.57 mg·kg−1. The bioconcentration factors of B. griseus for Cd were 24–386 [4]. The factors affecting Cd migration from soil to B. griseus were investigated. Principal component analysis elucidated that the soil physical-chemical properties, such as the Cd content in soil, electrical conductivity, total carbon, total nitrogen, pH, and dissolved organic carbon, were factors that affected the Cd accumulation of B. griseus [4]. Macrofungi are known to effectively accumulate higher concentrations of metals and metalloids than vascular plants [5]. Therefore, the accumulation of elements in mushrooms has attracted significant research attention [6,7,8]. Considering the natural values of Cd in mushrooms, B. griseus showed significantly higher Cd contents than most reported mushrooms, except for the family Agaricaceae [9].
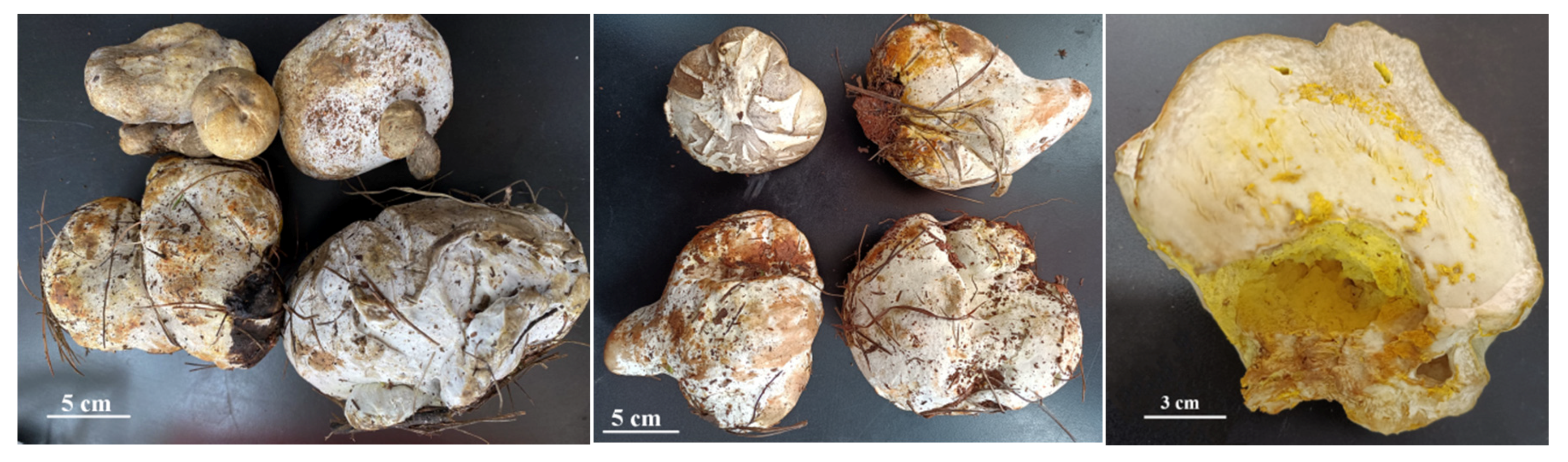
B. griseus is a kind of ectomycorrhizal mushroom, and is symbiotically associated with a large number of trees and shrubs. It can be found along the forest edge of pine-oak mixed forests in Yunnan Province [10]. When sampling, two phenotypes of the sporocarps of B. griseus were found, namely, normal-developed sporocarps (Figure 1) and symbiotic fruiting bodies of B. griseus–mycoparasitic fungus (Figure 2). Owing to the geographical location and ecological, climatic, topographic, and geological factors, Yunnan Province is rich in fungal biodiversity, with more than 882 edible species and an average annual yield of 500,000 tons. Many studies have focused on the resource survey, annual yield, bioactivities and bioactive components, and food safety of wild edible mushrooms [2,11,12,13,14,15,16]. However, few reports have studied the fungicolous fungi and host–parasite relationships in wild edible mushrooms, except for the genus Hypomyces [17,18].

Figure 1.
Normal-developed sporocarps of B. griseus.

Figure 2.
The symbiotic fruiting bodies of B. griseus–mycoparasitic fungus and their vertical section.
Hypomyces is a genus in the family Hypocreaceae with more than 150 species, including the most characteristic mycoparasites of diverse fungal hosts of agarics, boletes, russules, thelephores, and polypores [17]. Most species of Hypomyces are obligatory parasites, growing only on a specific host. The parasites of Hypomyces usually cause systematic infection and mummification of the host fruiting bodies [18]. Douhan and Rizzo collected 22 isolates from 21 infected bolete and one infected agaric in California oak woodlands, among which 20 isolates from individual bolete hosts were identified as H. microspermus and H. chrysospermus [17]. Kaygusuz et al. described H. chrysospermus isolated from infected Suillus luteus as a new record in Turkey [18]. Thus, the genus Hypomyces exclusively comprises boleticolous species.
Up to now, few studies about the B. griseus–mycoparasitic fungus association have been carried out. The symptoms of mycoparasitic fungus infection of B. griseus in this study differed from those of other bolete mushrooms, as studies found that infections of the genus Hypomyces led to total necrosis of bolete hosts [18]. Considering the strong Cd bioaccumulation capacity of B. griseus, even in natural habitats without Cd contamination, the unique biological strategy of B. griseus to take up Cd from the soil matrix was assumed, such as being parasitized by fungicolous fungi and forming a symbiote. Thus, this study aimed (1) to test and analyze the Cd contents in normal-developed sporocarps and symbiotic fruiting bodies of B. griseus–mycoparasitc fungus, (2) to isolate and identify the mycoparasitc fungus from normal-developed sporocarps (Figure 1) and symbiotic fruiting bodies of B. griseus–mycoparasitc fungus (Figure 2), and (3) to assess the Cd tolerance and Cd-accumulating traits of isolated mycoparasitic fungus. This study could help to exploit the possible mechanism of Cd bioaccumulation of B. griseus, from the perspective of the roles of the fungi-associated fungi in Cd uptake.
2. Material and Methods
2.1. Sampling
Samples of normal-developed sporocarps and symbiotic fruiting bodies of B. griseus–mycoparasitic fungus (marked as deformed sporocarps) were collected from four locations separated by approximately 20–40 km, being Guishan Town, Shilin Town, Banqiao Town, and Changhu Town in Shilin City, Yunnan Province, China, in June and July of 2020 and 2021. Five to six areas that were at a distance from the communication routes and other sources of environmental pollution and rich in B. griseus were selected from each location. During the sampling, attention was paid to make sure that the normal-developed and deformed sporocarps were collected from the same area (within approximately 10 ha−1) and at the same time (a single day). In total, 22 samples of normal-developed sporocarps and 22 samples of deformed sporocarps were collected from 22 studied areas. From each of the studied areas, 10–15 fruiting bodies were collected and pooled for each sample.
The samples were brought to Kunming University of Science and Technology, Wild Edible Mushroom Research Laboratory, where the samples were photographed. Then, three normal-developed sporocarps and one deformed sporocarp were randomly selected for the isolation and identification of mycoparasitic fungus. The rest of the sporocarps were cleaned to remove forest debris and washed successively with running water and distilled water. The cleaned sporocarps were freeze dried and ground into a fine powder (40 meshes) and stored in sealed polyethylene bugs in a vacuum dryer for Cd determination.
2.2. Cd Determination
The Cd contents of normal-developed and deformed sporocarps were determined by atomic absorption spectrometry [2]. Briefly, 0.1 g samples were soaked overnight in open polytetrafluoroethylene (PTFE) vessels with 8 mL of concentrated nitric acid (65%). Then, after pre-digestion was conducted using a digestion apparatus at 120 °C for 60 min, the PTFE vessels were closed and heated in a microwave oven (MARS6, CEM Corp., Charlotte, NC, USA). The digestion conditions were set at 1.6 kW power by a 3-step heating program, being 8 min ramp, temperature of 120 °C, and 5 min hold; 5 min of ramp, temperature of 150 °C, and 5 min hold; 8 min ramp, temperature of 190 °C, and 25 min hold. The digest was diluted and filtered on 0.45 μm polyethersulfone membrane filters. Then, the solution was determined by an atomic absorption spectrophotometer (AAS400G, Analytik Jena AG, Jena, Germany) with a deuterium background corrector. The analytical wavelengths were determined at 228.8 nm. The pyrolysis and atomization temperatures were 350 and 1300 °C, respectively. Signals were measured as the peak area. The assurance quality of the analytical method was investigated through analysis of the certified reference materials of GBW10025 (CRM spirulina) supplied by Geophysiochemistry Prospecting Institute of Academy of Geological Science of China. The certified value for Cd of CRM spirulina was 0.37 mg·kg−1. CRM was analyzed for Cd with each analytical batch of samples in triplicate, and the recovery of Cd was 92.5–106.04%. Three replicates were conducted for the Cd determination of the samples.
2.3. Isolation of Mycoparasitic Fungus
All selected normal and deformed sporocarps from Section 2.1 were cleaned to remove forest debris, washed with running water to remove dirt, subjected to surface sterilization sequentially with 75% ethanol for 90 s and 5% NaCl 3 times, and then rinsed in sterile water 3 times.
For normal sporocarps, small pieces (0.5 cm × 0.5 cm × 0.5 cm) from under the epidermis were taken from the base of the stipes. The pieces were placed on potato dextrose agar (PDA) plates and incubated at 28 °C in the dark for 5 days.
For deformed sporocarps, a flame-sterilized loop was used to pick out spores (Figure 2) on PDA plates. The plates were incubated at 28 °C in the dark for 5 days.
After 5 days, the leading edges of the fungal colonies was transferred to PDA plates and incubated at 28 °C in the dark for 5 days for growth and isolation.
2.4. Morphological Identification and Taxonomic Analyses of Mycoparasitic Fungus
The morphological characteristics were recorded, including the colony shape, size, color, surface state, and edge. The microstructures of the endophyte mycelium and spores were examined using a light microscope after being stained bright blue with gossypol blue dye.
The genomic DNA of the isolated fungus was extracted using a Trelief Plant Genomic DNA Kit (Tsingke Biotechnology Co., Ltd., Beijing, China). The ITS1-ITS4 region was amplified using PCR primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) [19]. Electrophoresis was carried out using agarose gel. The PCR products were purified using PCR purification kits (Tsingke Biotechnology Co., Ltd., Beijing, China) and sequenced using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA). Raw sequences were aligned using ContigExpress (Vector NTI Suite8.0; Invitrogen) and BLAST searched for the best match in NCBI (blast.ncbi.nlm.nih.gov accessed on 2 March 2019).
2.5. Determination of the Cd Resistance of Mycoparasitic Fungus
The Cd resistance of the isolated mycoparasitic fungus was examined using the PDA plates with gradually increasing concentrations of CdCl2, with Cd2+ of 0 (control), 80, 120, 160, and 400 mg·L−1. Six replicates were conducted for each Cd treatment. The plates were incubated at 28 °C in the dark for 5 days. The concentration of Cd2+ at which no visible fungal growth was observed was considered the minimum inhibitory concentration (MIC) of the mycoparasitic fungus. The Cd2+ level just below the MIC was considered as the highest Cd2+ concentration tolerated by the mycoparasitic fungus. Then, the inhibition percentage (IP, %) of Cd2+ of the fungus was measured by the ratio of the colony diameter with Cd2+ treatment to the colony diameter of the control [20]. EC50 refers to the Cd2+ concentration required to inhibit 50% of the fungal growth [21].
2.6. Effects of Cd2+ on the Morphology of Mycoparasitic Fungus
Scanning electron microscopy (SEM) was employed to investigate the micromorphology of the mycelia from the colonies of the 0–160 mg·L−1 Cd2+ treatments [22]. Briefly, the colonies of the mycoparasitic fungus from PDA plates with 0–160 mg·L−1 Cd2+ were collected, and the mycelia was fixed and used as the samples. Images of the mycelia under different Cd2+ concentrations were obtained via SEM analysis (VEGA3-SBH, Tescan) under the following analytical conditions: HW = 25.0 kW, WD = 14.77 mm, and SignalA = SE.
2.7. Effects of Cd2+ on the Growth of Mycoparasitic Fungus
In accordance with the highest Cd2+ concentration tolerated by the fungus, the effects of Cd2+ on the growth of the fungus were examined using shake flasks with 100 mL potato dextrose broth (PDB) added with gradually increased concentrations of CdCl2, with Cd2+ of 0 (control), 20, 40, 60, 80, 100, 120, 140, and 160 mg·L−1. Six replicates were conducted for each Cd2+ treatment. Cultures were incubated at 28 °C and 120 rpm for 7 days. Then, the pH values of the fermentation mixtures were determined using a pH meter. The mixture was filtered, and the mycelia were collected and washed three times with deionized water. The fresh mycelia from the three fermentation mixtures were freeze dried. The dried mycelia were accurately weighed to determine the effects of Cd2+ on the biomass yields. The fresh mycelia from the rest of the three fermentation mixtures was pooled for the investigation of the contents of soluble protein and the activities of antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) [22].
2.8. Cd2+ Absorption by Mycoparasitic Fungus
The dried mycelia from Section 2.7 were digested, and the Cd contents in the mycelia were determined, as described in Section 2.2. The values of Cd2+ absorption by the mycoparasitic fungus during the incubation were calculated as mg Cd per g dried mycelia.
2.9. Data Analysis
Data were analyzed by SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The differences between means were analyzed using Duncan’s multiple range test or Student’s t-test at the 0.05 probability level. Graphical work was conducted on Excel 2007 (Microsoft, Redmond, WA, USA). The results were expressed as mean ± SD.
3. Results and Discussion
3.1. Cd Contents in Normal and Deformed Sporocarps of B. griseus
As shown in Table 1, the Cd contents in 22 normal-developed sporocarps of B. griseus ranged from 13.71–43.40 mg·kg−1, with an average of 24.45 mg·kg−1. According to a previous study [2], the Cd contents in 227 Boletaceae mushrooms from Yunnan Province ranged from not detected in B. aereus to 25.01 mg·kg−1 DW in B. griseus. The results of the present paper are consistent with those of the previous study [2]. Researchers have reported the contents of Cd in Boletaceae mushrooms, such as 1.2 mg·kg−1 in B. edulis [23]; 2.77 mg·kg−1 in B. edulis [13]; 0.58–1.31 mg·kg−1 in different parts of B. aereus, B. aestivalis, B. edulis, and B. pinophilius [24]; 1.44–2.01 mg·kg−1 in different parts of B. badius [25]; 0.24 mg·kg−1 in B. aereus [26]; and 0.28 mg·kg−1 in Neoboletus erythropus [27].

Table 1.
Cadmium contents of 22 samples of normal and deformed sporocarps of B. griseus (mg·kg−1 DW).
The Cd contents in 22 deformed sporocarps of B. griseus ranged from 6.35–49.29 mg·kg−1, with an average of 26.75 mg·kg−1. All the normal-developed and deformed sporocarps of B. griseus significantly accumulated high amounts of Cd. According to Student’s t-test, among the 22 samples, 13 deformed sporocarps had significantly higher Cd contents than the normal-developed sporocarps.
In the last decade, extensive studies have shown that symbiotic association, such as mycorrhizas of fungi and plants, could enhance the tolerance and accumulation abilities of hosts regarding heavy metals (HMs) [28]. Mutualistic symbiotic fungi, such as ectomycorrhizal fungi (EMF), arbuscular mycorrhizal fungi (AMF), dark septate endophytes (DSEs), and endophytic fungi, are considered to be the remarkable factors of metal-accumulating plant species [20,29,30,31,32]. Co-culturing of fungi–fungi was recently established to produce new secondary metabolites. Wang et al. reported that mycelial pellets (Aspergillus fumigatus) and Synechocystis sp. PCC6803 comprised a fungus–microalgae symbiotic system, assisting microalgae flocculation and immobilization and the adsorption behavior for HMs [33]. The co-cultivation of microalgae with filamentous fungi is a superior method to efficiently accumulate and harvest the total biomass, and to remove pollutants from water [34]. However, the interactions of fungi associated with fungi have not yet been fully investigated, except for morphological and anatomical studies and molecular phylogenetic analyses of mycoparasitic fungi [35].
3.2. Isolation and Identification of Mycoparasitic Fungus
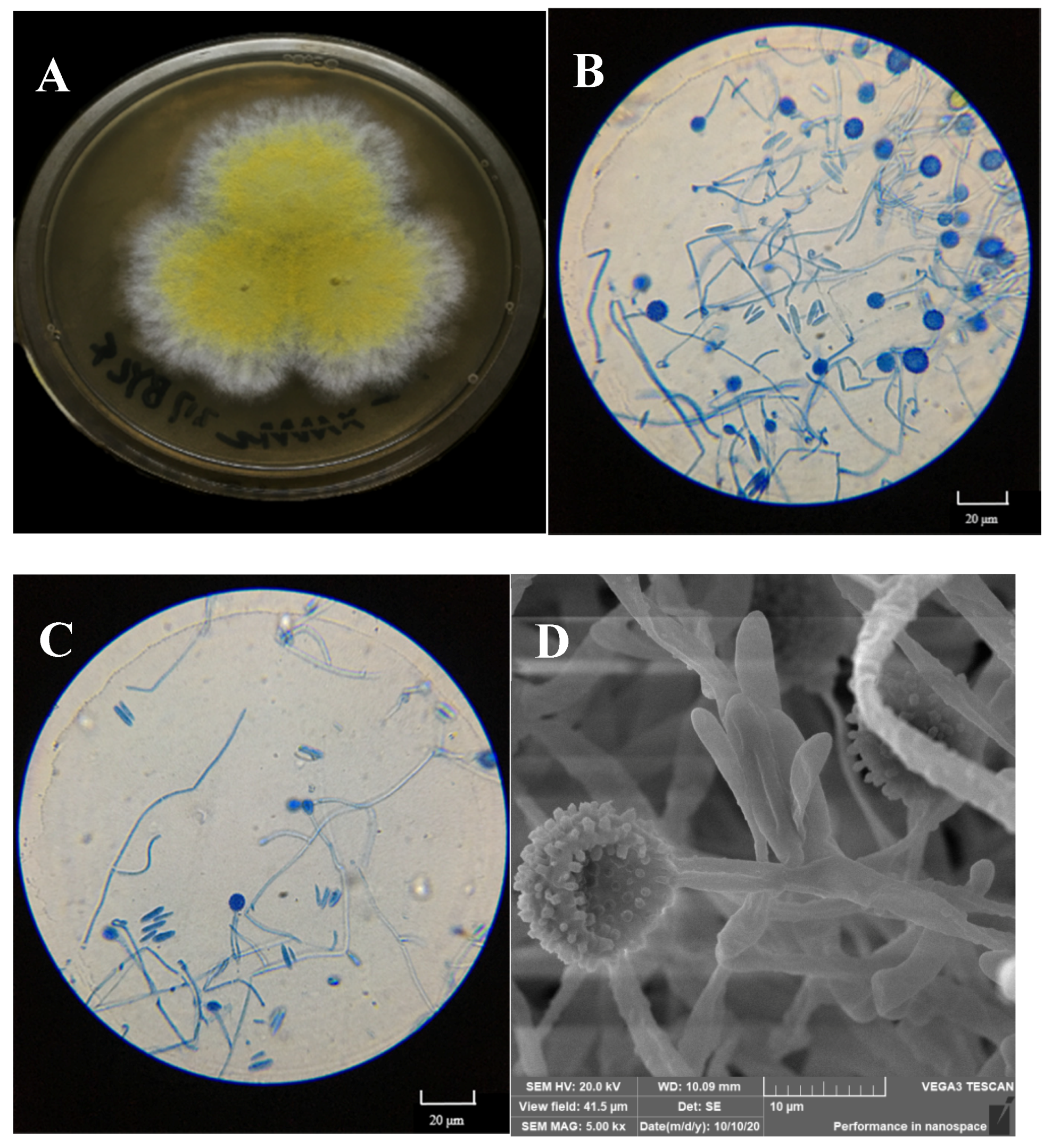
Fungal outgrowths from the surface-sterilized tissues of normal sporocarps and the spores of deformed sporocarps were observed after incubation on PDA plates. Only one filamentous fungal colony was isolated on the basis of unique phenotypic characteristics, as shown in Figure 3A.
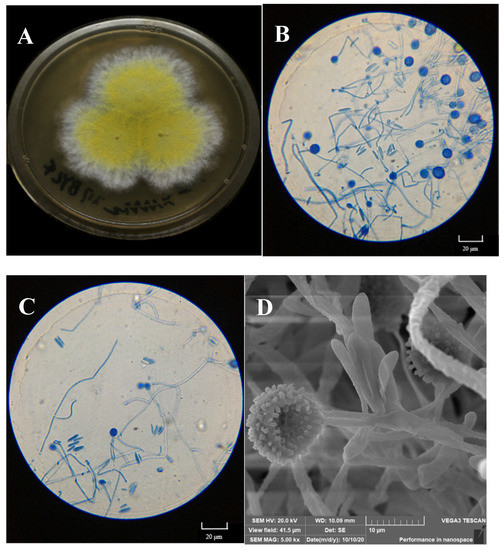
Figure 3.
Macroscopic features of the isolated mycoparasitic fungus, Hypomyces chrysospermus. (A) Colonial morphology on PDA, (B,C): Optical microscopy images of the morphology of mycelia and conidiophore and chlamydospores; (D) scanning electron micrographs of mycelia and chlamydospores).
The colony on PDA medium was white, and it had a flat appearance with a regular edge. Then, the mycelia differentiated into yellow spores after approximately 3 days. As shown in Figure 3B,C, the mycelium was thin-walled and hyaline. Conidia were observed to be 10–20 × 3–8 μm in size, elliptical, aseptate, and single-celled, with smooth and thin walls, and hyaline. Globular tissues were observed to be approximately 10 μm in diameter under optical microscopy. SEM showed that the globular tissues were chlamydospores with thick walls and were prominently verrucose (Figure 3D).
According to the sequence of the ITS1-5.8S-ITS4 region, the isolated fungus belonged to the Ascomycota (classes: Sordariomycetes, Hypocreales, Hypocreaceae, and Hypomyces), and it was identified as Hypomyces chrysospermus based on a blast analysis in the NCBI GenBank database (MK560123.1).
H. chrysospermus is a cosmopolitan parasite of many boletes. According to Kaygusuz et al. [18], Hypomyces is easy to identify as the species in this genus usually produce bright-colored perithecia, conidiophores, and chlamydospores, and infection of H. chrysospermus causes decay of the host. However, H. chrysospermus seems to play an essential role in the development of sporocarps of B. griseus. H. chrysospermus-infected or uninfected B. griseus could develop normal fruiting bodies that had a distinguished gray color and were cap- and stipe-shaped. Meanwhile, under certain environmental conditions (temperature and relative humidity), deformed fruiting bodies with a significantly bigger size and weight usually formed, being a symbiont of B. griseus–H. chrysospermus. The symbiont had no shape features of bolete mushrooms, and it was usually spherical with a gray-white appearance, as shown in Figure 2.
3.3. Cd Tolerance of H. chrysospermus
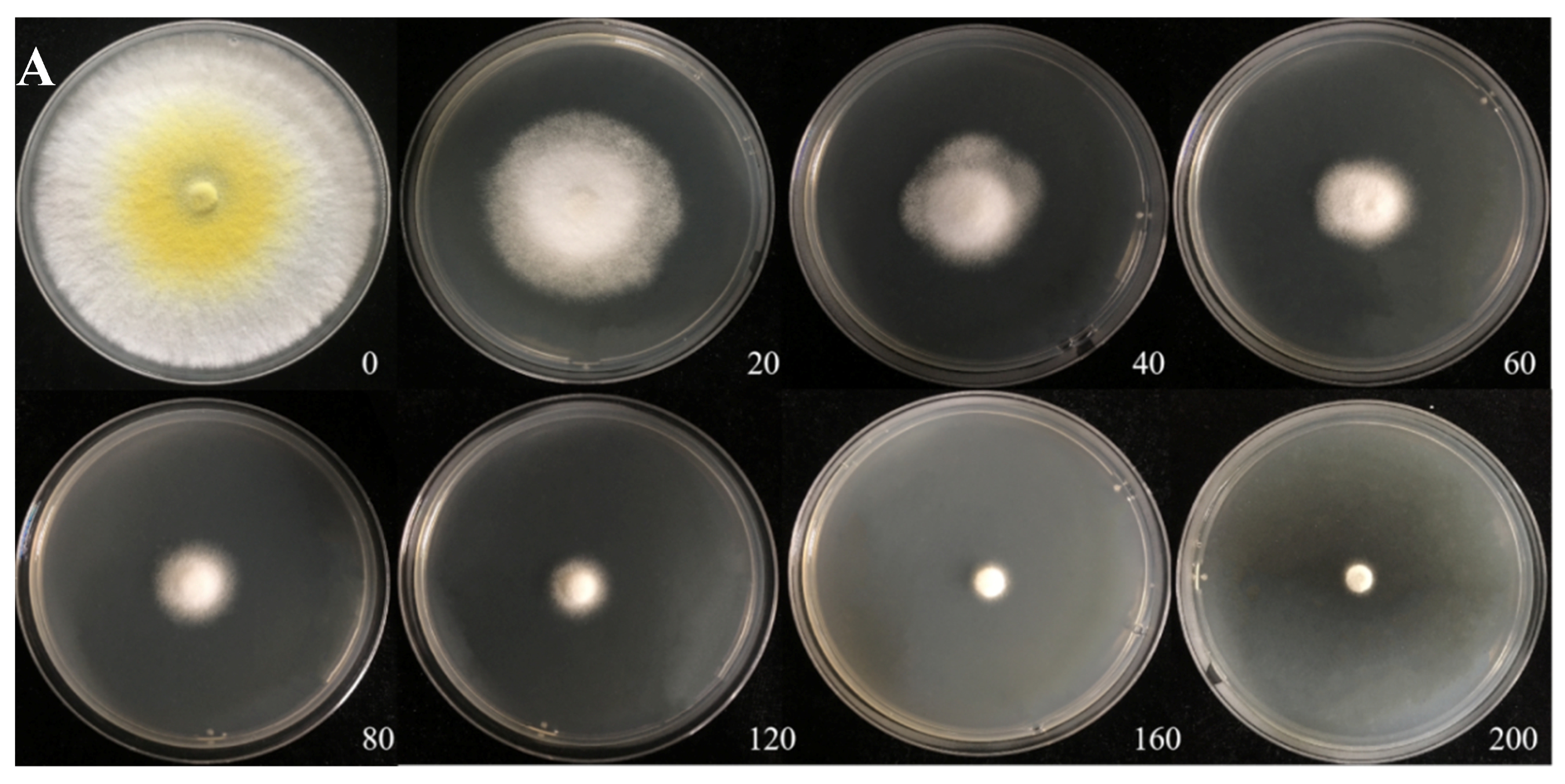
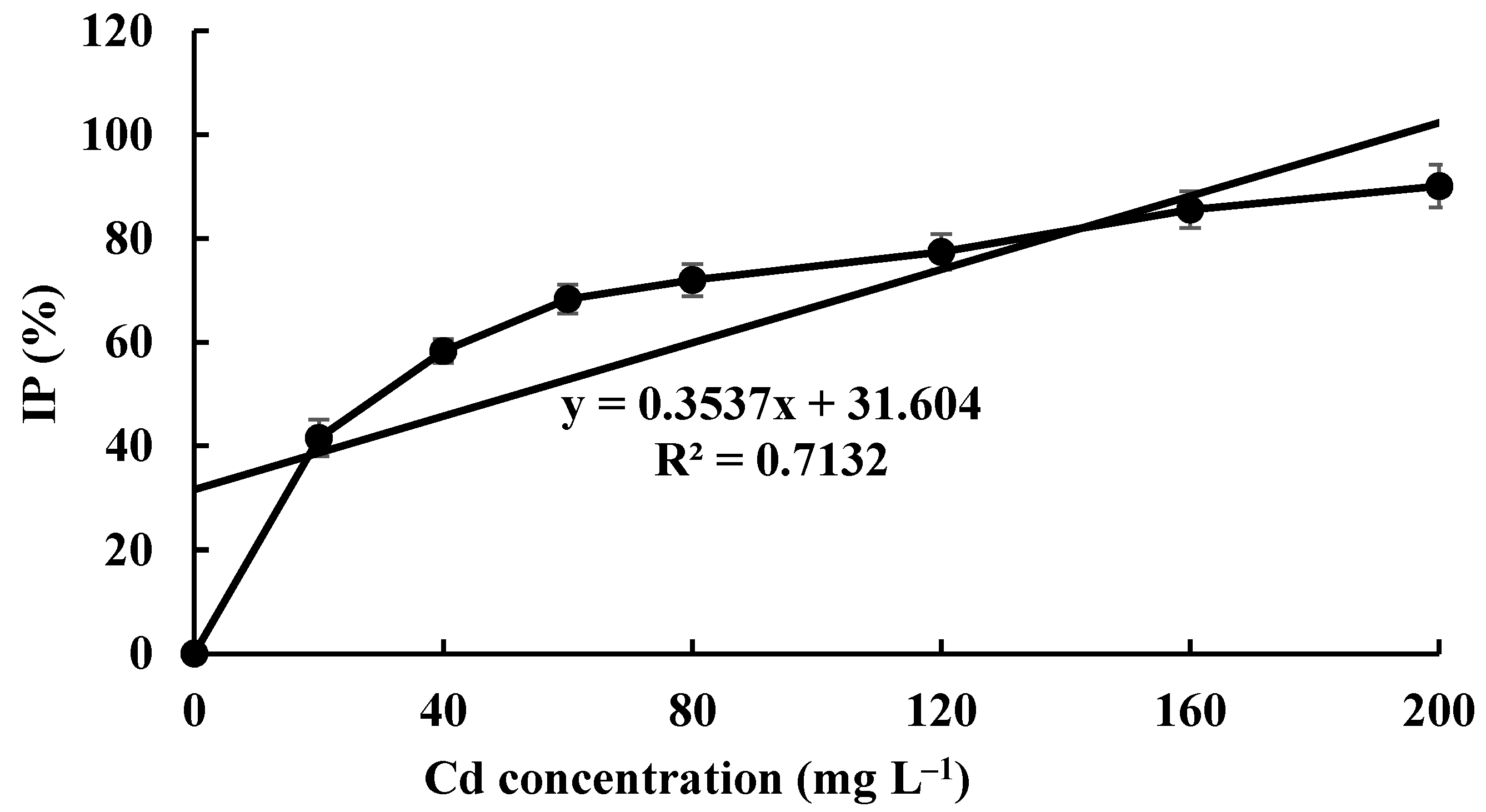
The Cd tolerance of H. chrysospermus was determined on Cd-enriched PDA plates. As shown in Figure 4, H. chrysospermus grew very rapidly when cultured on PDA. When cultured for 5 days on PDA without Cd2+, the diameter of the colonies was 7 cm on average (Figure 4A). Cd significantly inhibited H. chrysospermus. Colonies showed an inverse relationship with the Cd2+ concentrations, and the inhibition percentage showed a direct positive correlation with the Cd2+ concentrations, with R2 being 0.7132. The highest Cd2+ concentration tolerated by H. chrysospermus was 160 mg·L−1. The MIC value of Cd2+ to H. chrysospermus was determined to be 200 mg·L−1, and the EC50 was calculated to be 52 mg·L−1, according to the IP of Cd to H. chrysospermus (Figure 5). However, when cultured for a prolonged time, growing mycelium could be observed on the PDA of 200 mg·L−1, as shown in Figure 4B. Traxler et al. described a similar phenomenon; that is, the growth of Schizophyllum commune was still visible even at the highest metal concentrations used in PDA medium cultivated for 14 days [36].
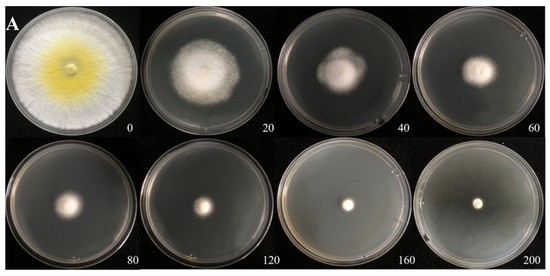
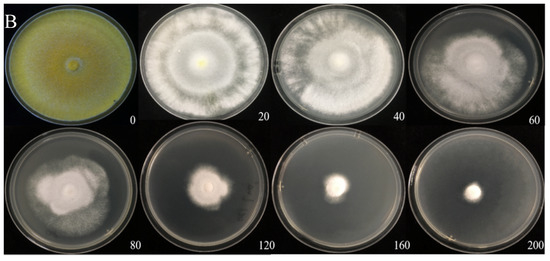
Figure 4.
Cadmium tolerance of H. chrysospermus on PDA plates. (A) 5 days; (B) 10 days. Data in the figure indicate Cd2+ concentrations (mg·L−1) in PDA.
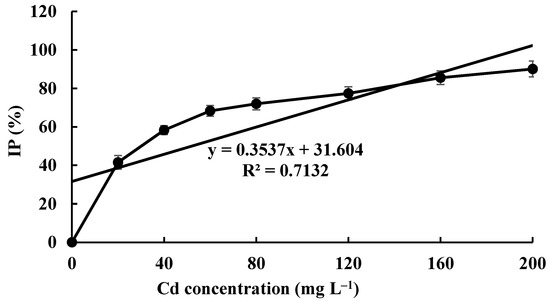
Figure 5.
The inhibition percentage of Cd2+ at different concentrations on H. chrysospermus.
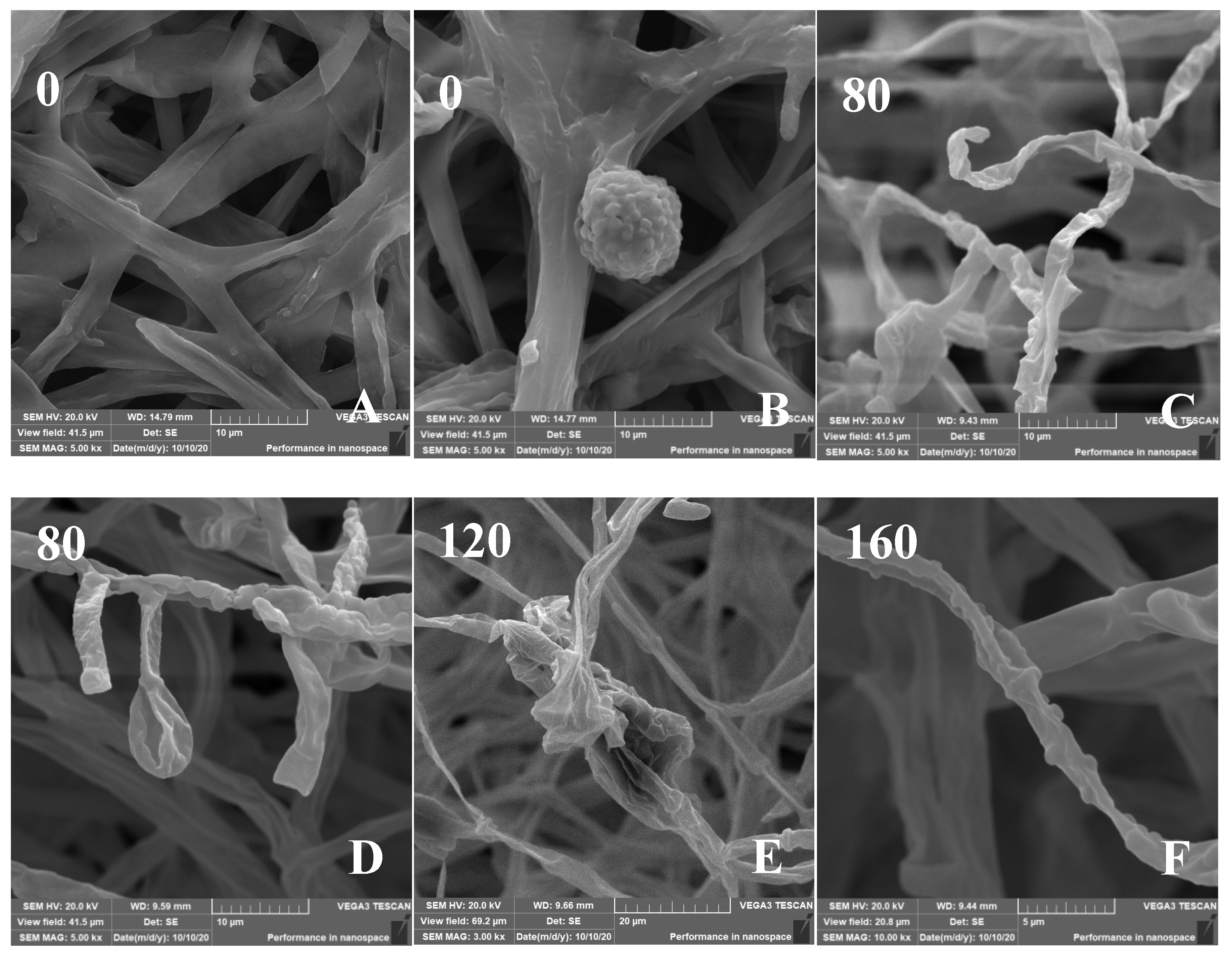
The effect of Cd on the micromorphology of the mycelia was investigated (Figure 6). The SEM images of control H. chrysospermus showed long and rope-like fungal hyphae, which were highly branched and intertwined with one another, and no physical damage was observed (Figure 6A). Meanwhile, fully developed chlamydospores with thick walls and prominent verrucose features were observed, as shown in Figure 3D and Figure 6B. The presence of Cd resulted in locally twisted (Figure 6C) and deformed chlamydospores (Figure 6D). The increased Cd treatment distorted and shrunk the cell walls of the fungal mycelium (Figure 6E,F). The mycelia were partly swollen when treated with 160 mg·L−1 of Cd (Figure 6F). This finding may be due to the immobilization effect of the cell wall via Cd2+ binding by functional groups, such as carboxyl (-COOH), hydroxyl (-OH), carbonyl (-COH), and amino (-NH2), to form precipitation on the cell surface [37,38]. Studies have shown that HMs have various effects on the composition of microbial cell components. The uptake and accumulation of HMs often causes significant damage at the morphological, cellular, physiological, and molecular levels. Cd is one of the most toxic metals among HMs. Sharma et al. reported that the fungal mycelium was slightly broken with some visual deformities [39]. However, the shape was distinguishable and well-regulated in the presence of Pb and Ni, whereas the presence of Cd distorted and shrunk the cell walls of Phlebia brevispora.
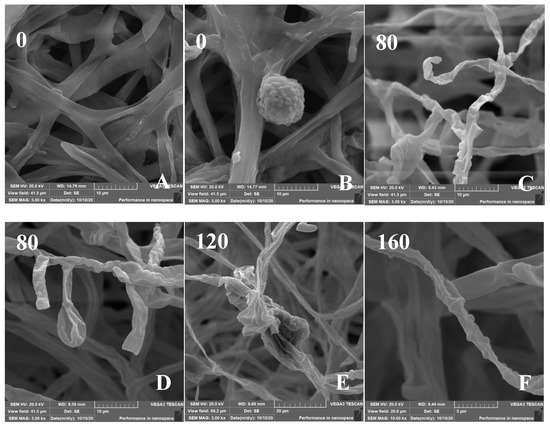
Figure 6.
(A–F) Scanning electron micrographs of H. chrysospermums grown for 7 days on PDA with gradually increasing concentrations of Cd2+. Data of 0–160 in the figures indicate the Cd2+ concentrations in PDA (mg·L−1).
Deng et al. characterized the features of a Cd-, Pb-, and Zn-resistant endophytic fungus Lasiodiplodia sp. MXSF31 from metal-accumulating Portulaca oleracea, and the strain was resistant to 5 mM Cd [40]. Mohammadian et al. studied different filamentous fungi isolated from contaminated mining soil and showed different profiles [41]. Among them, Trichoderma harzianum showed the maximum MIC value for Cd, being 35 mg·L−1. Albert et al. evaluated the tolerance of the soil fungus Absidia cylindrospora against three trace metals, namely, Cd, Cu, and Pb, before considering a possible use to treat contaminated soils. The concentration that inhibited 50% of fungal growth (IC50) was 100 mg·L−1 for Cd [21].
Cd is toxic to cells, and organisms show visible toxicity symptoms under Cd stress higher than 5 mg·kg−1. However, mushrooms are characterized by a high trace element accumulation capacity, especially in the case of Cd, Pb, Cu, and Zn [24,42]. Numerous papers showed that the contents of many trace elements, especially Cd and Hg, increased in mushrooms from polluted areas compared with those from unpolluted rural sites [24]. Some plant species, such as Solanum nigrum, Solanum photeinocarpum, and Siegesbeckia orientalis, were studied as Cd hyperaccumulators and accumulators, which could be used for phytoremediation of Cd-contaminated soil. These plant species were usually found in different ecotypes with quite different morphology and Cd accumulation, including the mining ecotype (accumulating ecotype) and the farmland ecotype (non-accumulating ecotype) [43,44]. However, B. griseus showed a ubiquitous Cd-accumulating capacity, which seemed unaffected by the natural habitat. Considering the Cd tolerance of H. chrysospermus isolated from normal and deformed sporocarps of B. griseus, the Cd absorption of H. chrysospermus could be assumed.
3.4. Cd Absorption by H. chrysospermus
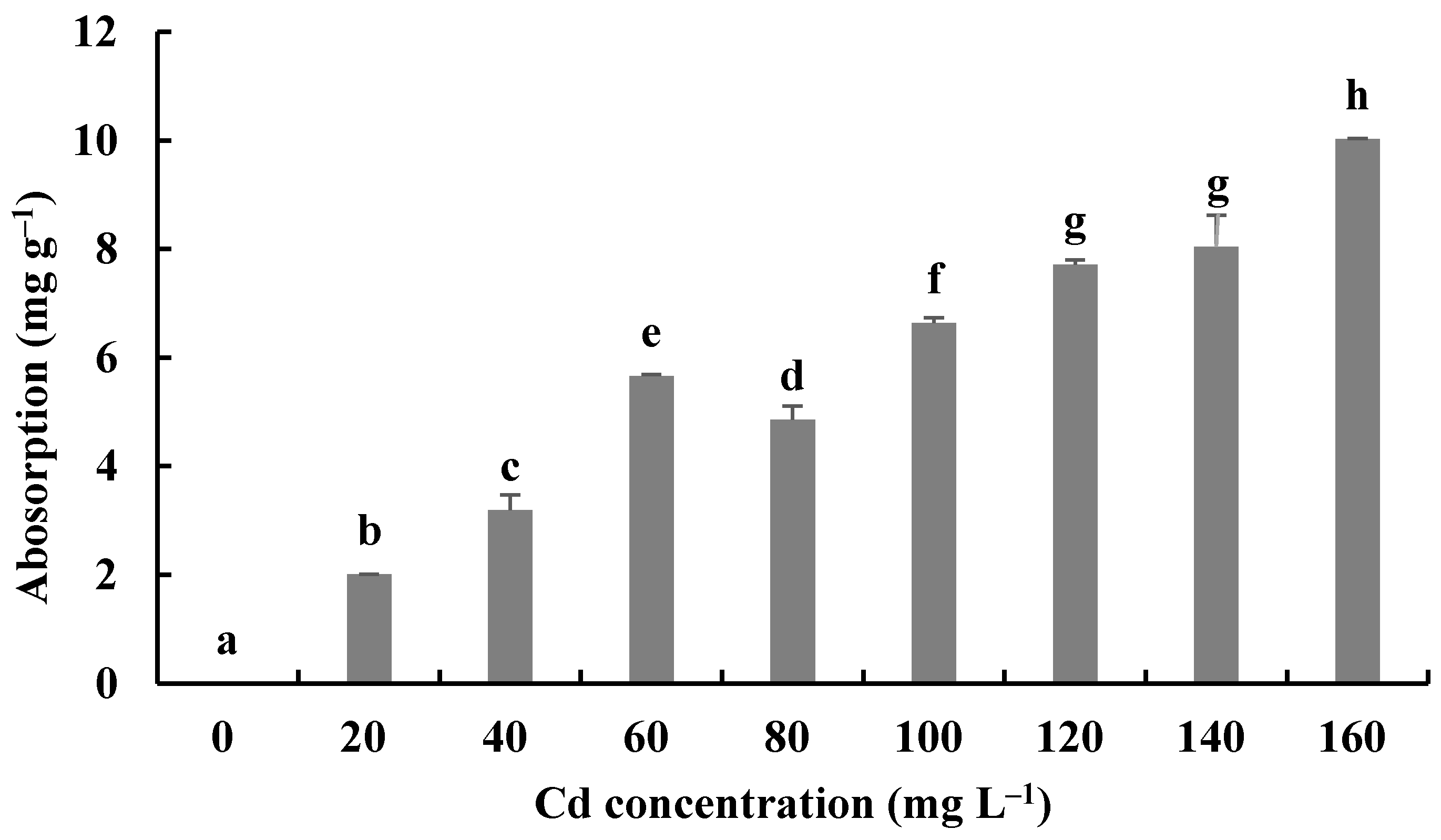
The Cd bioaccumulation of H. chrysospermus was assessed in relation to the initial Cd2+ concentrations (Figure 7). The Cd contents in dried biomass of H. chrysospermus increased with rising Cd2+ concentrations in PDB medium. The maximum uptake value was 10.03 mg·g−1. Li et al. described a similar phenomenon and reported that the Cd concentrations that accumulated in the hyphae of Pleurotus ostreatus HAU-2 increased with increasing concentrations of Cd in the liquid culture [22].
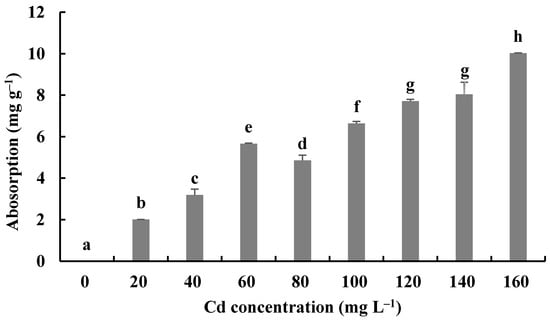
Figure 7.
Cd2+ absorption by H. chrysospermus. Different letters indicated significant difference (p < 0.05).
In comparison, the maximum values of Cd uptake by fungi in the liquid culture have been reported to be 4.6 × 104 mg·kg−1 for Lasiodiplodia sp. MXSF31 [40], 1.89 mg·g−1 for P. ostreatus [22], 9 μg·mg−1 for A. cylindrospora [21], and 2.3−11.9 mg·g−1 for 5 fungal strains, with the highest Cd tolerance shown by soybean and barley [45]. During the experiment described here, H. chrysospermus isolated from B. griseus showed a significant absorption capacity for Cd2+, and it may be a promising Cd bioabsorbent for bioremediation.
3.5. Effects of Cd on the Growth of H. chrysospermus
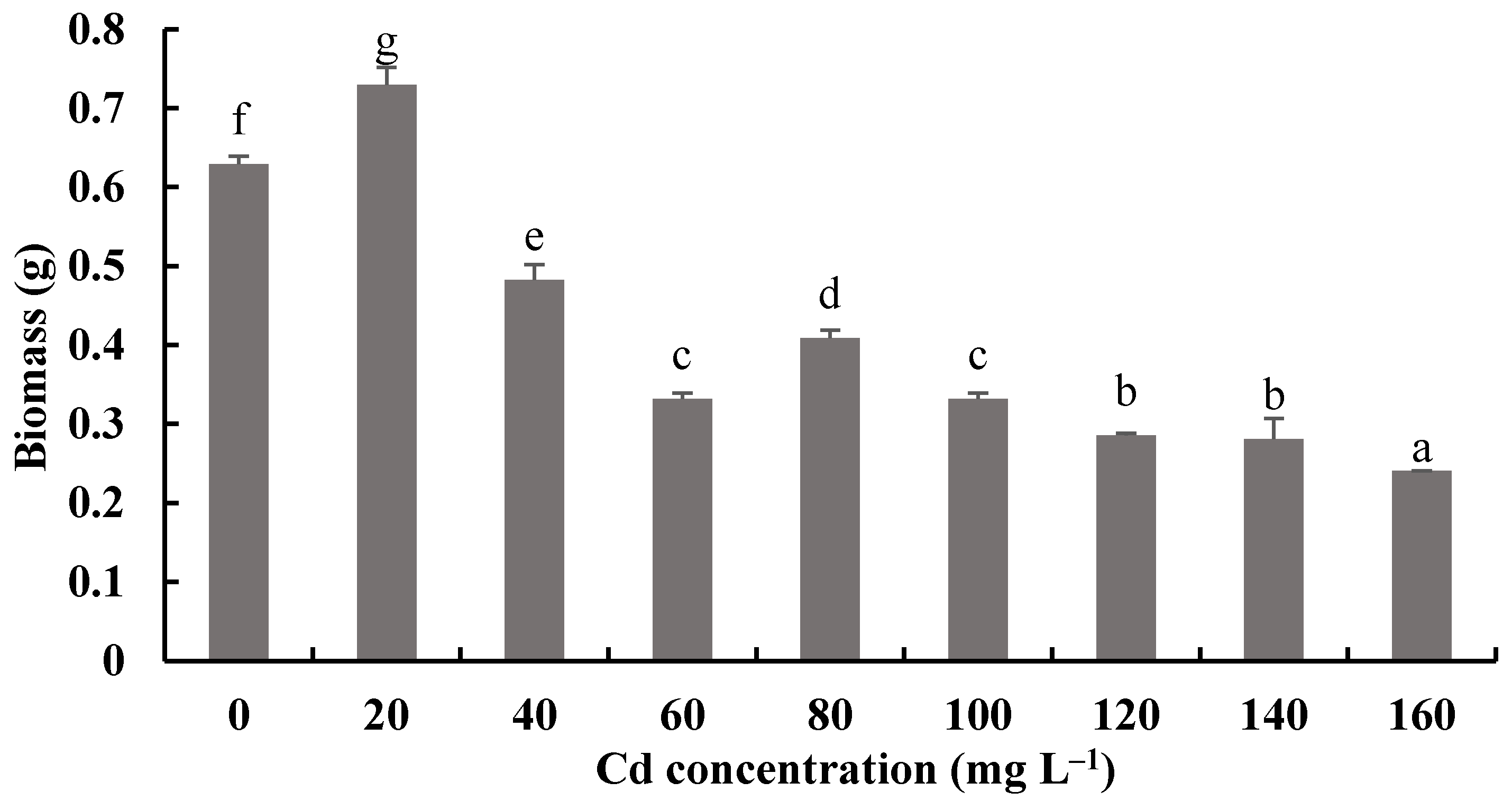
Figure 8 and Figure 9 show the biomass production and pH measurements of H. chrysospermus cultured for 7 days in PDB with different Cd2+ concentrations. When the Cd2+ concentration in the PDB medium increased from 0 to 20 mg·L−1, the biomass of H. chrysospermus increased significantly, being 0.73 mg DW. Then, the biomass decreased with increased Cd2+ concentrations up to 160 mg·L−1. Li et al. revealed that low-concentration Cd (<20 mg·L−1) and Cr (<150 mg·L−1) did not notably suppress the hypha growth of P. ostreatus HAU-2, but higher concentrations of Cd and Cr evidently suppressed it [22]. Sharma et al. demonstrated that an enhancement in the fungal biomass of P. brevispora was observed with an increased metal concentration up to 1, 4, and 10 μmol·L−1 for Cd, Ni, and Pb, respectively, which then declined with a further increase in the metal concentrations [39].
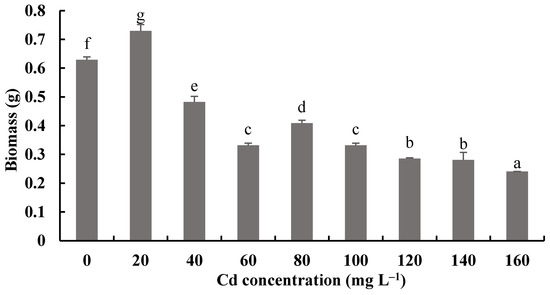
Figure 8.
Effects of different concentrations of Cd2+ on the biomass of H. chrysospermus. Different letters indicated significant difference (p < 0.05).
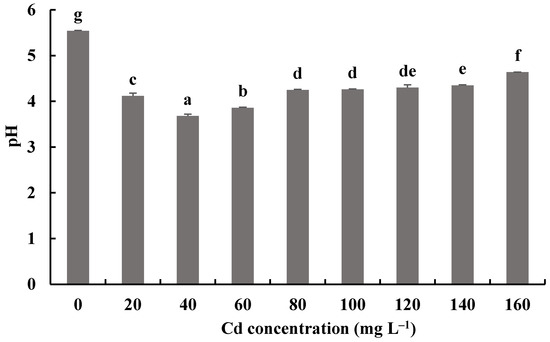
Figure 9.
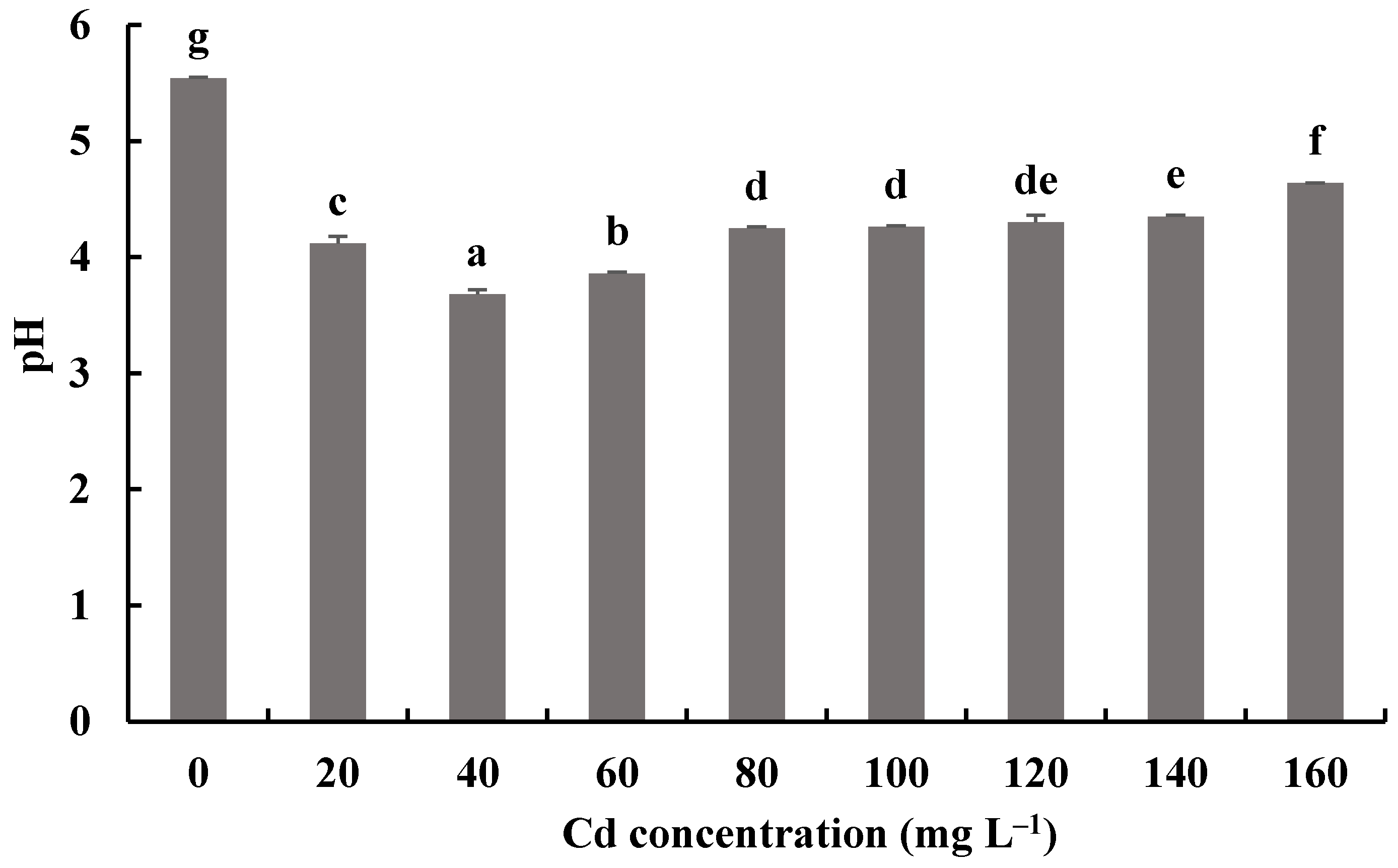
Effects of different concentrations of Cd2+ on the pH of a fermentation mixture of H. chrysospermus. Different letters indicated significant difference (p < 0.05).
The pH values of fermentation mixtures of H. chrysospermus with and without Cd2+ all decreased compared with the initial pH of 6.19. Meanwhile, the pH values of fermentation mixtures with Cd2+ were significantly lower than those of the control. The lowest pH value of 3.68 was shown by the fermentation mixtures with 40 mg·L−1 Cd2+, a decrease by 2.51. Fungi can secrete inorganic acid and organic acid compounds to alleviate the toxic effects of HMs [46]. Wang et al. showed that P. ostreatus ISS-1 secreted oxalic acid, citric acid, and formic acid regardless of Pb exposure, but the oxalic acid content was significantly higher under Pb stress than that in the control [47]. Organic acids (citric acid and oxalic acid) chelate with toxic metal ions and form precipitation.
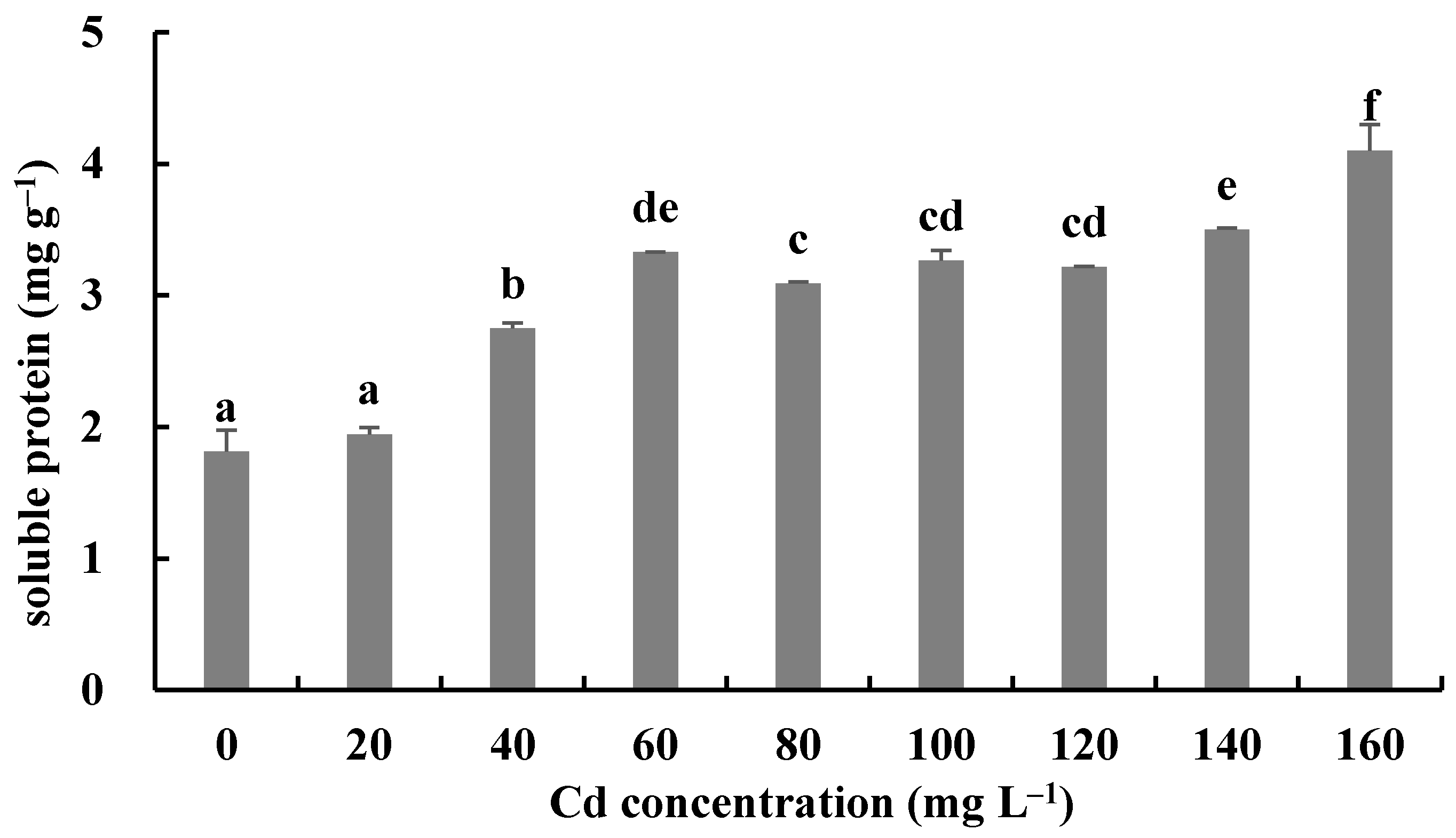
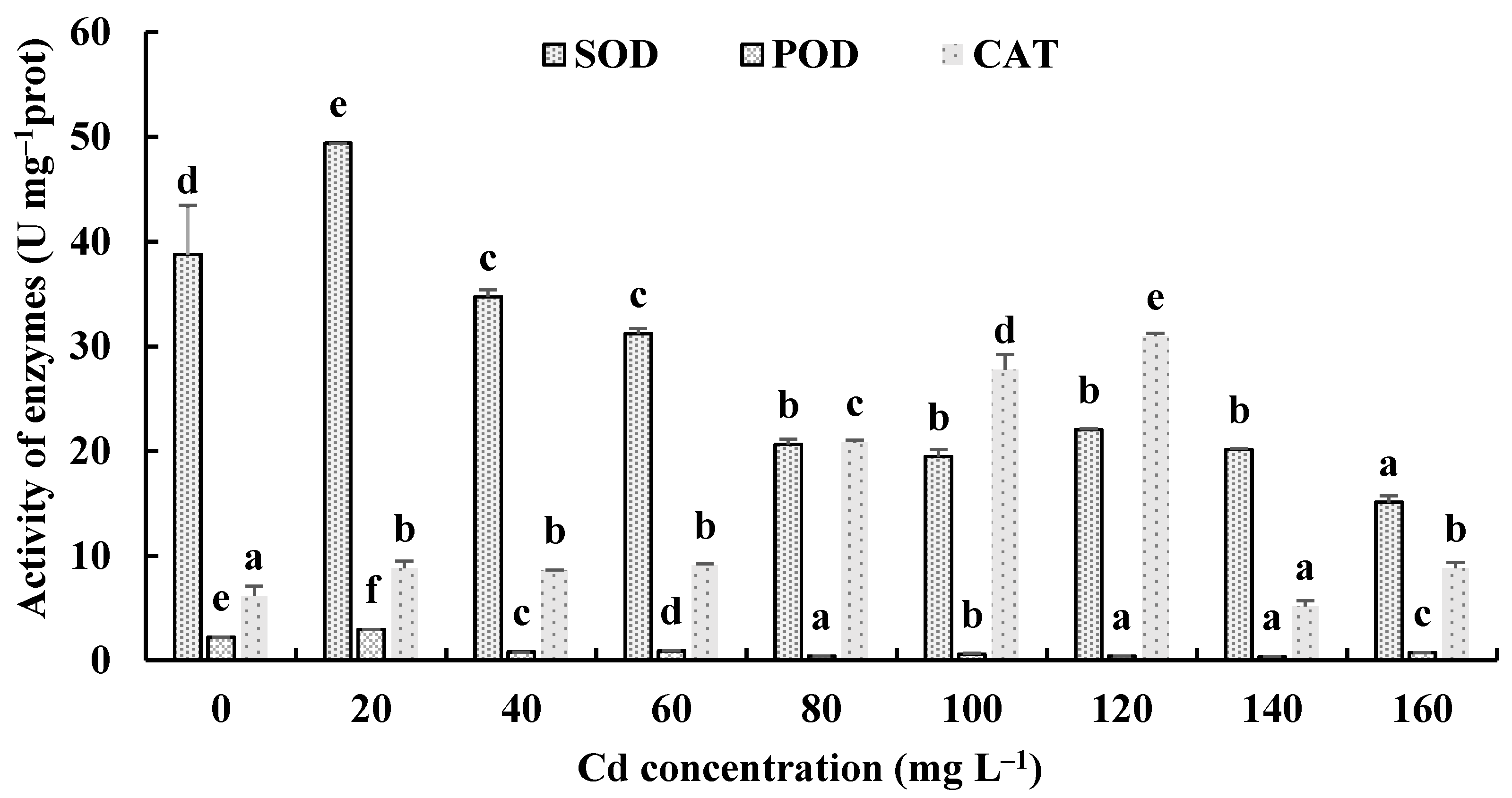
The responses of the soluble protein and antioxidant enzymes of the fungus with different Cd2+ concentrations were assayed to further investigate the tolerance factors of H. chrysospermus to Cd2+. As shown in Figure 10, the soluble protein contents of H. chrysospermus significantly increased with increased Cd2+ concentrations. Figure 11 shows the effects of different concentrations of Cd2+ on the activities of the antioxidant enzymes of H. chrysospermus. The SOD activity was sensitive to Cd2+ treatment, showing a significant increasing tendency for 20 mg·L−1 Cd2+, but it decreased with the increasing Cd2+ concentration. H. chrysospermus in this study showed a significantly lower POD activity [22], which was suppressed by Cd2+ treatments. The CAT activities under Cd stress were higher than those in the control group. The CAT activity reached a maximum value of 30.97 U·mg−1 prot with a Cd2+ concentration of 120 mg·L−1. The exposure of fungal species to HMs induces stress conditions, resulting in the production of reaction oxygen species, such as superoxide, peroxides, and hydroxyl radicals, that damage fungal cell and organelle structures and alter metabolism [22,46]. Thus, the fungi of HM-resistant species need to adopt strategies to resist oxidative stress. The cellular immune system is the basic strategy employed to resist metal toxicity. Oxidized enzyme species, such as SOD, POD, and CAT, are important components of the cellular immune system, and they exert a significant effect on the removal of cellular active oxygen. Li et al. revealed that the concentrations of these three enzymes first increased and then decreased in the presence of Cd and Cr, indicating that oxidized enzyme species could be induced by relatively low concentrations of Cd or Cr, thus playing a role in the removal of active oxygen [22]. However, overly high concentrations of HMs may severely damage cells, suppressing enzymes.
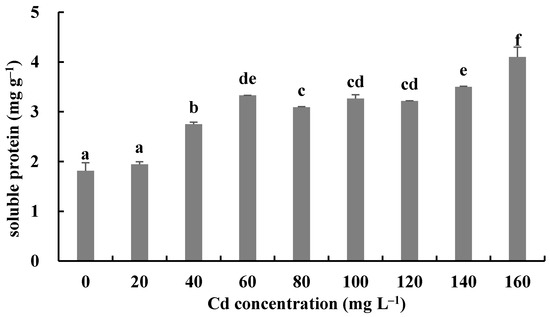
Figure 10.
Effects of different concentrations of Cd2+ on soluble protein of H. chrysospermus. Different letters indicated significant difference (p < 0.05).
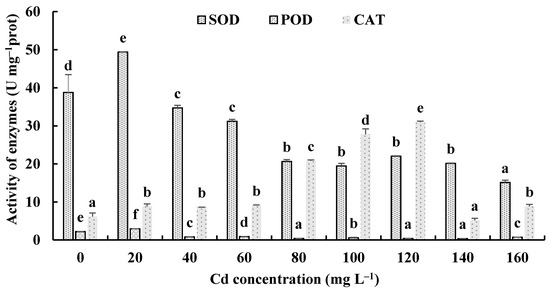
Figure 11.
Effects of different concentrations of Cd2+ on the activities of antioxidant enzymes of H. chrysospermus. Different letters indicated significant difference (p < 0.05).
4. Conclusions
To the authors’ best knowledge, this work is the first study to identify the symbiotic association of B. griseus and H. chrysospermus and elucidate the Cd-resistant characteristics of the isolated strain of H. chrysospermus from B. griseus. This study further confirms the Cd bioaccumulation capacity of B. griseus. The isolated strain of H. chrysospermus from B. griseus showed a strong ability to tolerate Cd, and the maximum Cd uptake reached 10.03 mg·g−1. In general, the time required for fruiting body differentiation, including induction, development, and maturation, in mushroom-forming fungi is about 4–12 days. Considering the strong Cd bioaccumulation capacity of B. griseus, even in natural habitats without Cd contamination, the symbiotic association of H. chrysospermus might represent the biological strategy adopted by B. griseus to promptly and efficiently take up Cd from the soil matrix. The MIC of Cd of the isolated strain of H. chrysospermus was 200 mg·L−1. However, the growth of the strain was still visible after prolonged culturing. The immobilization effects of the cell wall and acid compounds and antioxidant enzymes were employed by the fungi to alleviate the toxic effects of Cd. Thus, this study not only provides new insight into the Cd bioconcentration mechanisms of B. griseus but also provides a potential bioremediation fungus for Cd contamination.
Author Contributions
Conceptualization, Z.T. and L.S.; Methodology, Z.T., Y.Z. and L.S.; Formal analysis, Z.T. and Y.W.; Investigation, C.M. and Y.S.; Project administration, Y.Z. and L.S.; Software, Z.T.; Validation, Y.Z. and L.S.; Writing—original draft, Z.T. and L.S.; Writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financially supported by the National Natural Science Foundation of China (31860421, 21767014).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mao, X.L. The Macromycetes of China; Science: Beijing, China, 2009; p. 816. [Google Scholar]
- Sun, L.; Chang, W.; Bao, C.; Zhuang, Y. Metal contents, bioaccumulation, and health risk assessment in wild edible Boletaceae mushrooms. J. Food Sci. 2017, 82, 1500–1508. [Google Scholar] [CrossRef]
- Bao, C.J.; Chang, W.D.; Zhuang, Y.L.; Sun, L.P. Nutritional characteristics and protein composition of fruiting bodies of Boletus griseus. J. Food Sci. 2017, 38, 83–89. [Google Scholar] [CrossRef]
- Xiao, J.J.; Li, P.C.; Zhuang, Y.L.; Sun, L.P.; Sun, Y. Multivariate statistics for investigation of factors affecting migrating of cadmium from soil matrix to Boletus griseus. Chin. J. Anal. Chem. 2021, 2, 301–308. [Google Scholar] [CrossRef]
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef] [Green Version]
- Seeger, R. Cadmium in Pilzen. Z. Lebensm. Unters. Forsch. 1978, 166, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Drewnowska, M.; Falandysz, J. Investigation on mineral composition and accumulation by popula redible mushroom common chanterelle (Cantharellus cibarius). Ecotox. Environ. Safe 2015, 113, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Borovička, J.; Braeuer, S.; Walenta, M.; Hršelová, H.; Leonhardt, T.; Sácký, J.; Kaňa, A.; Goessler, W. A new mushroom hyperaccumulator: Cadmium and arsenic in the ectomycorrhizal basidiomycete. Thelephora penicillata. Sci. Total Environ. 2022, 826, 154227. [Google Scholar] [CrossRef] [PubMed]
- Borovička, J.; Braeuer, S.; Sáckýd, J.; Kameník, J.; Goessler, W.; Trubač, J.; Strnad, L.; Rohovec, J.; Leonhardt, T.; Kotrba, P. Speciation analysis of elements accumulated in Cystoderma carcharias from clean and smelter-polluted sites. Sci. Total Environ. 2019, 648, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.Q.; Liu, P.G. Species diversity of wild edible mushrooms from Pinus yunnanensis forests and conservation strategies. Biodivers. Sci. 2005, 13, 58–69. [Google Scholar] [CrossRef]
- Liu, Y.T.; Sun, J.; Luo, Z.Y.; Rao, S.Q.; Su, Y.J.; Xu, R.R.; Yang, Y.J. Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem. Toxicol. 2012, 50, 1238–1244. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, J.; Wu, L.H.; Zhao, Y.L.; Li, T.; Li, J.Q.; Wang, Y.Z.; Liu, H.G. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chem. 2014, 151, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Huang, Q.; Cai, H.; Guo, X.; Wang, T.; Gui, M. Study of heavy metal concentrations in wild edible mushrooms in Yunnan Province, China. Food Chem. 2015, 188, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, M.; Sobhi, H.R.; Esrafili, A.; FarzadKia, M.; Yeganeh, M. Heavy metals content in edible mushrooms: A systematic review, meta-analysis and health risk assessment. Trends Food Sci. Technol. 2021, 109, 527–535. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, T.; Fan, J.; Zhuang, Y.; Sun, L. Protective effects of a polysaccharide from Boletus aereus on S180 tumor-bearing mice and its structural characteristics. Int. J. Biol. Macromol. 2021, 188, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, L.; Liu, Y.; Xiang, S.; Zhang, H.; Yi, L.; Shang, Y.; Xu, W. Identification techniques and detection methods of edible fungi species. Food Chem. 2022, 374, 131803. [Google Scholar] [CrossRef]
- Douhan, G.W.; Rizzo, D.M. Host-parasite relationships among bolete infecting Hypomyces species. Mycol. Res. 2003, 107, 1342–1349. [Google Scholar] [CrossRef]
- Kaygusuz, O.; Çolak, Ö.F.; Türkekul, İ. A new genus record for the macrofungi of Turkey of a fungicolous and mycoparasitic species, Hypomyces chrysospermus Tul. & C. Tul. (Hypocreaceae De Not.). Feddes Repert. 2018, 129, 241–246. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogeentics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Khan, A.R.; Waqas, M.; Ullah, I.; Khan, A.L.; Khan, M.A.; Lee, I.-J.; Shin, J.-H. Culturable endophytic fungal diversity in the cadmium hyperaccumulator Solanum nigrum L. and their role in enhancing phytoremediation. Environ. Exp. Bot. 2017, 135, 126–135. [Google Scholar] [CrossRef]
- Albert, Q.; Leleyter, L.; Lemoine, M.; Heutte, N.; Rioult, J.P.; Sage, L.; Baraud, F.; Garon, D. Comparison of tolerance and biosorption of three trace metals (Cd, Cu, Pb) by the soil fungus Absidia cylindrospora. Chemosphere 2018, 196, 386–392. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Pan, Y.; Yu, H.; Zhang, X.; Shen, Y.; Jiao, S.; Wu, K.; La, G.; Yuan, Y.; et al. Mechanisms of Cd and Cr removal and tolerance by macrofungus Pleurotus ostreatus HAU-2. J. Hazard. Mater. 2017, 330, 1–8. [Google Scholar] [CrossRef]
- Vinichuk, M.M. Copper, zinc, and cadmium in various fractions of soil and fungi in a Swedish forest. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2013, 48, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.J.; Alonso, J.; Garcia, M.A. Cadmium in edible mushrooms from NW Spain: Bioconcentration factors and consumer health implications. Food Chem. Toxicol. 2016, 88, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Proskura, N.; Podlasinska, J.; Skopicz-Radkiewicz, L. Chemical composition and bioaccumulation ability of Boletus badius (Fr.) Fr. collected in western Poland. Chemosphere 2017, 168, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Liu, G.; Wang, L. Assessment of potential human health risk of trace element in wild edible mushroom species collected from Yunnan Province, China. Environ. Sci. Pollut. Res. Int. 2020, 27, 29218–29227. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Yildiz, D.; Akata, I.; Tepe, B. Evaluation of the metal concentrations of wild mushroom species with their health risk assessments. Environ. Sci. Pollut. Res. Int. 2021, 28, 21437–21454. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Tang, Y.; Shi, L.; Zhong, K.; Shen, Z.; Chen, Y. Ectomycorrhizal fungi may not act as a barrier inhibiting host plant absorption of heavy metals. Chemosphere 2019, 215, 115–123. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Wang, J.; Zhang, X.; Shen, Z.; Shi, L.; Chen, Y. The great potential for phytoremediation of abandoned tailings pond using ectomycorrhizal Pinus sylvestris. Sci. Total Environ. 2020, 719, 137475. [Google Scholar] [CrossRef]
- Yu, P.; Sun, Y.; Huang, Z.; Zhu, F.; Sun, Y.; Jiang, L. The effects of ectomycorrhizal fungi on heavy metals’ transport in Pinus massoniana and bacteria community in rhizosphere soil in mine tailing area. J. Hazard. Mater. 2020, 381, 121203. [Google Scholar] [CrossRef]
- Su, Z.Z.; Dai, M.D.; Zhu, J.N.; Liu, X.H.; Li, L.; Zhu, X.M.; Wang, J.Y.; Yuan, Z.L.; Lin, F.C. Dark septate endophyte Falciphora oryzae-assisted alleviation of cadmium in rice. J. Hazard. Mater. 2021, 419, 126435. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, R.; Fan, L.; Cui, L.; Zhang, Y.; Cheng, J.; Wu, X.; Zeng, W.; Tian, Q.; Shen, L. Construction of fungi-microalgae symbiotic system and adsorption study of heavy metal ions. Sep. Purif. Technol. 2021, 268, 118689. [Google Scholar] [CrossRef]
- Chu, R.; Li, S.; Zhu, L.; Yin, Z.; Hu, D.; Liu, C.; Mo, F. A review on co-cultivation of microalgae with filamentous fungi: Efficient harvesting, wastewater treatment and biofuel production. Renew. Sustain. Energy Rev. 2021, 139, 110689. [Google Scholar] [CrossRef]
- Liu, J.W.; Ge, Z.W.; Horak, E.; Vizzini, A.; Halling, R.E.; Pan, C.L.; Yang, Z.L. Squamanitaceae and three new species of Squamanita parasitic on Amanita basidiomes. IMA Fungus 2021, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Traxler, L.; Shrestha, J.; Richter, M.; Krause, K.; Schafer, T.; Kothe, E. Metal adaptation and transport in hyphae of the wood-rot fungus Schizophyllum commune. J. Hazard. Mater. 2022, 425, 127978. [Google Scholar] [CrossRef]
- Gu, S.; Lan, C.Q. Biosorption of heavy metal ions by green alga Neochloris oleoabundans: Effects of metal ion properties and cell wall structure. J. Hazard. Mater. 2021, 418, 126336. [Google Scholar] [CrossRef]
- Soto-Ramirez, R.; Lobos, M.G.; Cordova, O.; Poirrier, P.; Chamy, R. Effect of growth conditions on cell wall composition and cadmium adsorption in Chlorella vulgaris: A new approach to biosorption research. J. Hazard. Mater. 2021, 411, 125059. [Google Scholar] [CrossRef]
- Sharma, K.R.; Giri, R.; Sharma, R.K. Lead, cadmium and nickel removal efficiency of white-rot fungus Phlebia brevispora. Lett. Appl. Microbiol. 2020, 71, 637–644. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, R.; Shi, Y.; Hu, L.; Tan, H.; Cao, L. Characterization of Cd-, Pb-, Zn-resistant endophytic Lasiodiplodia sp. MXSF31 from metal accumulating Portulaca oleracea and its potential in promoting the growth of rape in metal-contaminated soils. Environ. Sci. Pollut. Res. Int. 2014, 21, 2346–2357. [Google Scholar] [CrossRef]
- Mohammadian, E.; Babai Ahari, A.; Arzanlou, M.; Oustan, S.; Khazaei, S.H. Tolerance to heavy metals in filamentous fungi isolated from contaminated mining soils in the Zanjan Province, Iran. Chemosphere 2017, 185, 290–296. [Google Scholar] [CrossRef]
- Zsigmond, A.R.; Kantor, I.; May, Z.; Urak, I.; Heberger, K. Elemental composition of Russula cyanoxantha along an urbanization gradient in Cluj-Napoca (Romania). Chemosphere 2020, 238, 124566. [Google Scholar] [CrossRef]
- Wang, J.; Lin, L.; Luo, L.; Liao, M.; Lv, X.; Wang, Z.; Liang, D.; Xia, H.; Wang, X.; Lai, Y.; et al. The effects of abscisic acid (ABA) addition on cadmium accumulation of two ecotypes of Solanum photeinocarpum. Environ. Monit. Assess. 2016, 188, 182. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Liu, Y.; Li, M.; Li, J.; Luo, J.; Lux, A.; Kovac, J.; Yuan, S.; Li, B.; Li, Q.; et al. Cd-induced difference in root characteristics along root apex contributes to variation in Cd uptake and accumulation between two contrasting ecotypes of Sedum alfredii. Chemosphere 2020, 243, 125290. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, L.; Kistaubayeva, A.; Brazhnikova, Y.; Omirbekova, A.; Mukasheva, T.; Savitskaya, I.; Karpenyuk, T.; Goncharova, A.; Egamberdieva, D.; Sokolov, A. Characterization of cadmium-tolerant endophytic fungi isolated from soybean (Glycine max) and barley (Hordeum vulgare). Heliyon 2021, 7, e08240. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, E.; Priyadarshini, S.S.; Cousins, B.G.; Pradhan, N. Metal-Fungus interaction: Review on cellular processes underlying heavy metal detoxification and synthesis of metal nanoparticles. Chemosphere 2021, 274, 129976. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, B.; Sun, X.; Yu, L.; Wu, L.; Liu, W.; Wang, D.; Li, Y.; Jia, R.; Yu, H.; et al. Removal and tolerance mechanism of Pb by a filamentous fungus: A case study. Chemosphere 2019, 225, 200–208. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).