Organic and Conventional Bean Pesticides in Development of Autochthonous Trichoderma Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trichoderma Strains
2.2. Pesticides
2.3. In Vitro Evaluation
2.4. Statistical Analysis
3. Results
3.1. Acaricides
3.2. Insecticides
3.3. Fungicides
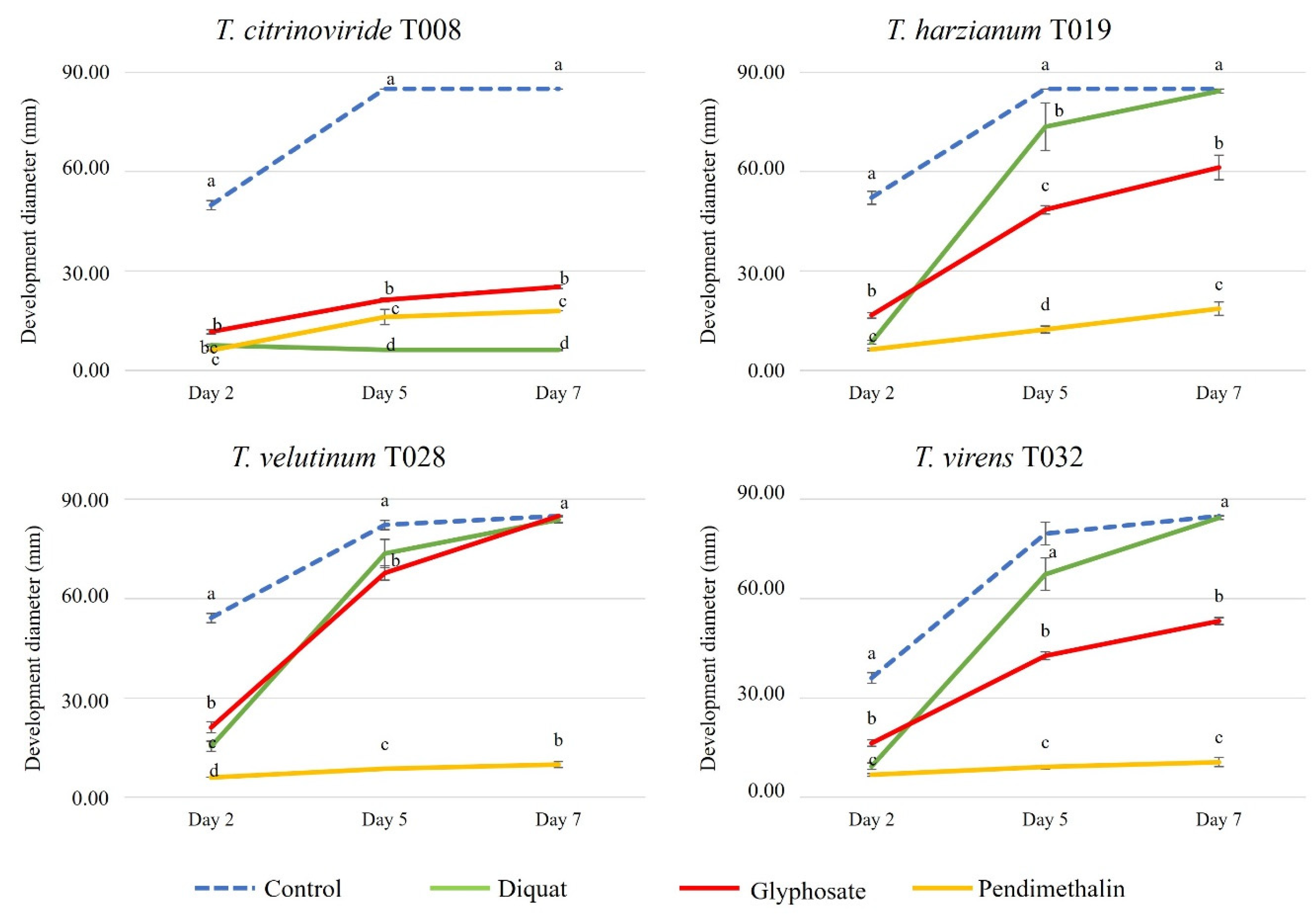
3.4. Herbicides
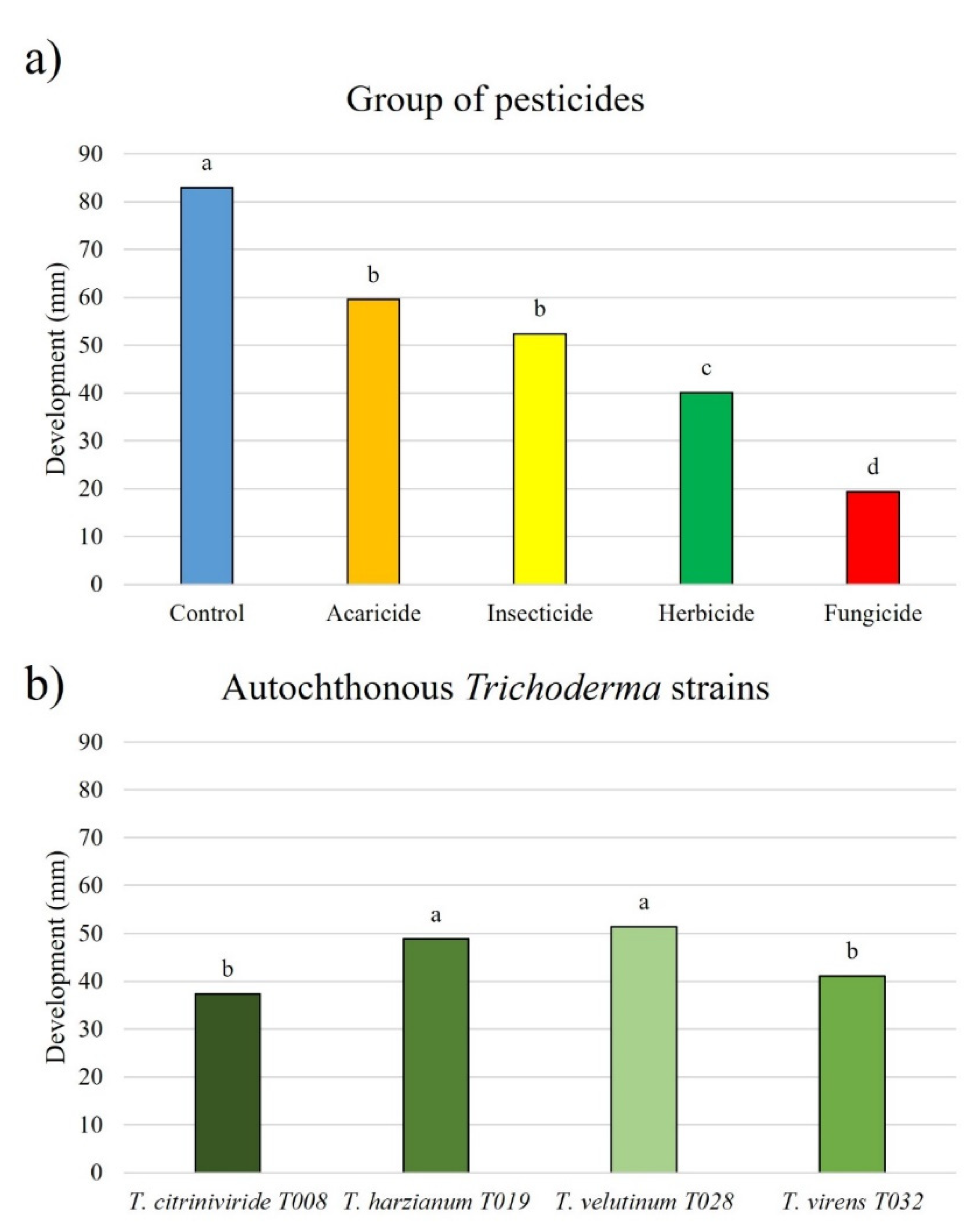
3.5. Trichoderma Development and Groups of Pesticides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat Sales of Pesticides by Type of Pesticide. Available online: https://ec.europa.eu/eurostat/databrowser/view/tai02/default/table?lang=en (accessed on 31 January 2022).
- EFSA. Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Diquat. EFSA (Eur. Food Saf. Auth.) J. 2015, 13, 4308. [Google Scholar] [CrossRef]
- EFSA. Statement on the Available Outcomes of the Human Health Assessment in the Context of the Pesticides Peer Review of the Active Substance Chlorpyrifos. EFSA (Eur. Food Saf. Auth.) J. 2019, 17, e05809. [Google Scholar] [CrossRef]
- EFSA; Abdourahime, H.; Anastassiadou, M.; Arena, M.; Auteri, D.; Barmaz, S.; Brancato, A.; Bura, L.; Carrasco Cabrera, L.; Chaideftou, E.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Mancozeb. EFSA (Eur. Food Saf. Auth.) J. 2020, 18, 5755. [Google Scholar] [CrossRef]
- Brimner, T.A.; Boland, G.J. A Review of the Non-Target Effects of Fungi Used to Biologically Control Plant Diseases. Agric. Ecosyst. Environ. 2003, 100, 3–16. [Google Scholar] [CrossRef]
- MAPA. Estadística Anual de Consumo de Productos Fitosanitarios y Estadística Quinquenal de Utilización de Productos Fitosanitarios en la Agricultura. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/estadisticas-medios-produccion/fitosanitarios.aspx (accessed on 20 January 2022).
- Harman, G.E.; Kubicek, C.P. Trichoderma and Gliocladium. In Volume 1: Basic Biology, Taxonomy and Genetics; CRC Press: London, UK, 2002; Volume 1. [Google Scholar]
- Mayo-Prieto, S.; Campelo, M.P.; Lorenzana, A.; Rodríguez-González, A.; Reinoso, B.; Gutiérrez, S.; Casquero, P.A. Antifungal Activity and Bean Growth Promotion of Trichoderma Strains Isolated from Seed vs Soil. Eur. J. Plant Pathol. 2020, 158, 817–828. [Google Scholar] [CrossRef]
- Abd-El-Khair, H.; Elshahawy, I.E.; Haggag, H.E.K. Field Application of Trichoderma Spp. Combined with Thiophanate-Methyl for Controlling Fusarium solani and Fusarium oxysporum in Dry Bean. Bull. Natl. Res. Cent. 2019, 43, 19. [Google Scholar] [CrossRef] [Green Version]
- Ruano-Rosa, D.; Arjona-Girona, I.; López-Herrera, C.J. Integrated Control of Avocado White Root Rot Combining Low Concentrations of Fluazinam and Trichoderma spp. Crop Prot. 2018, 112, 363–370. [Google Scholar] [CrossRef]
- Hefnawy, M.; Omima, A.; Res, M.N.-I.J.S. Impact of the Fungicide Rizolix T50% on the Antagonistic Activity of Trichoderma harzianum and Trichoderma koningii. Int. J. Sci. Res. 2014, 3, 1767–1773. [Google Scholar]
- Rodríguez-González, A.; Frontela, A.; Lorenzana, A.; da Silva, P.H.; Mayo, S.; González-López, O.; Gutiérrez, S.; Casquero, P.A. Evaluation of the Insecticidal Activity of Trichoderma harzianum against Xylotrechus arvicola and Acanthoscelides obtectus Inmature Stages. Planta Med. 2016, 82, S1–S381. [Google Scholar] [CrossRef]
- Rodríguez-González, Á.; Carro-Huerga, G.; Mayo-Prieto, S.; Lorenzana, A.; Gutiérrez, S.; Peláez, H.J.; Casquero, P.A. Investigations of Trichoderma spp. and Beauveria bassiana as Biological Control Agent for Xylotrechus arvicola, a Major Insect Pest in Spanish Vineyards. J. Econ. Entomol. 2018, 111, 2585–2591. [Google Scholar] [CrossRef] [Green Version]
- Mayo-Prieto, S.; Marra, R.; Vinale, F.; Rodríguez-González, Á.; Woo, S.L.; Lorito, M.; Gutiérrez, S.; Casquero, P.A. Effect of Trichoderma velutinum and Rhizoctonia solani on the Metabolome of Bean Plants (Phaseolus vulgaris L.). Int. J. Mol. Sci. 2019, 20, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-González, A.; Suárez-Villanueva, V.; Mayo, S.; Carro-Huerga, G.; Álvarez-García, S.; Casquero, P.A.; Gutiérrez, S. Control of Acanthoscelides obtectus (Coleoptera: Chrisomelidae: Bruchinae) Adults through Trichodiene Produced by Trichoderma harzianum. Phytopathol. Mediterr. 2017, 56, 340. [Google Scholar]
- Mayo, S.; González-López, Ó.; Rodríguez-González, Á.; da Silva, P.H.; Campelo, M.P.; Lorenzana, A.; Gutiérrez, S.; Casquero, P.A. Influence of Inoculation Technic in the Biocontrol Capacity of Trichoderma harzianum against Fusarium oxysporum from Protected Geographical Indication (PGI) “Alubia de La Bañeza-León”. In Proceedings of the XVIII International Plant Protection Congress, Berlín, Germany, 24–28 August 2015; p. 515. [Google Scholar]
- Mayo, S.; Gutierrez, S.; Malmierca, M.G.; Lorenzana, A.; Campelo, M.P.; Hermosa, R.; Casquero, P.A. Influence of Rhizoctonia solani and Trichoderma spp. in Growth of Bean (Phaseolus vulgaris L.) and in the Induction of Plant Defense-Related Genes. Front. Plant Sci. 2015, 6, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayo, S.; Cominelli, E.; Sparvoli, F.; González-López, O.; Rodríguez-González, A.; Gutiérrez, S.; Casquero, P.A. Development of a QPCR Strategy to Select Bean Genes Involved in Plant Defense Response and Regulated by the Trichoderma velutinum—Rhizoctonia solani Interaction. Front. Plant Sci. 2016, 7, 1109. [Google Scholar] [CrossRef]
- EFSA; Arena, M.; Auteri, D.; Barmaz, S.; Bellisai, G.; Brancato, A.; Brocca, D.; Bura, L.; Byers, H.; Chiusolo, A.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Chlorothalonil. EFSA (Eur. Food Saf. Auth.) J. 2018, 16, e05126. [Google Scholar] [CrossRef]
- EFSA; Arena, M.; Auteri, D.; Barmaz, S.; Bellisai, G.; Brancato, A.; Brocca, D.; Bura, L.; Byers, H.; Chiusolo, A.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Thiophanate-Methyl. EFSA J. 2018, 16, e05133. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Peer Review of the Pesticide Risk Assessment of the Active Substance Thiram. EFSA (Eur. Food Saf. Auth.) J. 2017, 15, e04700. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. Optimizing Biological Control Agents for Controlling Nematodes of Tomato in Egypt. Egypt. J. Biol. Pest Control 2020, 30, 1–10. [Google Scholar] [CrossRef]
- Wilson, M.J.; Jackson, T.A. Progress in the Commercialisation of Bionematicides. BioControl 2013, 58, 715–722. [Google Scholar] [CrossRef]
- Roberts, T.R.; Hutson, D.H.; Lee, P.W.; Nicholls, P.H.; Plimmer, J.R. Metabolic Pathways of Agrochemicals: Part 2: Insecticides and Fungicides; Royal Society of Chemistry: Cambridge, UK, 1999; ISBN 978-0-85404-499-3. [Google Scholar]
- Zhu, H.; Selim, H.M. Retention and Mobility of Deltamethrin in Soils: 1. Adsorption-Desorption. Soil Sci. 2002, 167, 513–523. [Google Scholar] [CrossRef]
- Selim, H.M.; Zhu, H. Retention and Mobility of Deltamethrin in Soils: 2. Transport. Soil Sci. 2002, 167, 580–589. [Google Scholar] [CrossRef]
- Ruzo, L.O.; Holmstead, R.L.; Casida, J.E. Pyrethroid Photochemistry: Decamethrin. J. Agric. Food Chem. 1977, 25, 1385–1394. [Google Scholar] [CrossRef]
- Baicu, T. Toxicity of Some Pesticides to Trichoderma viride Pers. Crop Prot. 1982, 1, 349–358. [Google Scholar] [CrossRef]
- Bayoumi, Y.; Taha, N.; Shalaby, T.; Alshaal, T.; El-Ramady, H. Sulfur Promotes Biocontrol of Purple Blotch Disease via Trichoderma spp. and Enhances the Growth, Yield and Quality of Onion. Appl. Soil Ecol. 2019, 134, 15–24. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Radwan, M.A. Impact of Pesticides on Trichoderma harzianum and on Its Possible Antagonistic Activity against Fusarium oxysporum under in Vitro Conditions. Asian J. Agric. Biol. 2017, 5, 291–302. [Google Scholar]
- Jebakumar, R.S.; Anandaraj, M.; Sarma, Y.R. Compatibility of Phorate and Chlorpyriphos with Trichoderma harzianum (Rifai.) Applied for Integrated Disease Management in Black Pepper (Piper nigrum L.). J. Spices Aromat. Crops 2000, 9, 111–115. [Google Scholar]
- Suseela Bhai, R.; Thomas, J. Compatibility of Trichoderma harzianum (Ritai.) with Fungicides, Insecticides and Fertilizers. Indian Phytopathol. 2010, 63, 145–148. [Google Scholar]
- Widenfalk, A.; Svensson, J.M.; Goedkoop, W. Effects of the Pesticides Captan, Deltamethrin, Isoproturon, and Pirimicarb on the Microbial Community of a Freshwater Sediment. Environ. Toxicol. Chem. 2004, 23, 1920–1927. [Google Scholar] [CrossRef] [Green Version]
- Bhadouria, R.; Das, S.; Kumar, A.; Singh, R.; Singh, V.K. Mycoremediation of Agrochemicals. In Agrochemicals Detection, Treatment and Remediation; Narasimha Vara Prasad, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 593–620. ISBN 978-0-08-103017-2. [Google Scholar]
- Romeh, A.A.A. Biodegradation of Carbosulfan, Pirimicarb and Diniconazole Pesticides by Trichoderma spp. J. Environ. Res. 2001, 27, 1747–1754. [Google Scholar]
- Gentz, M.C.; Murdoch, G.; King, G.F. Tandem Use of Selective Insecticides and Natural Enemies for Effective, Reduced-Risk Pest Management. Biol. Control 2010, 52, 208–215. [Google Scholar] [CrossRef]
- Bonmatin, J.M.; Marchand, P.A.; Charvet, R.; Moineau, I.; Bengsch, E.R.; Colin, M.E. Quantification of Imidacloprid Uptake in Maize Crops. J. Agric. Food Chem. 2005, 53, 5336–5341. [Google Scholar] [CrossRef] [PubMed]
- Colin, M.E.; Bonmatin, J.M.; Moineau, I.; Gaimon, C.; Brun, S.; Vermandere, J.P. A Method to Quantify and Analyze the Foraging Activity of Honey Bees: Relevance to the Sublethal Effects Induced by Systemic Insecticides. Arch. Environ. Contam. Toxicol. 2004, 47, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Koppenhöfer, A.M.; Kaya, H.K. Synergism of Imidacloprid and an Entomopathogenic Nematode: A Novel Approach to White Grub (Coleoptera: Scarabaeidae) Control in Turfgrass. J. Econ. Entomol. 1998, 91, 618–623. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Grewal, P.S.; Kaya, H.K. Synergism of Imidacloprid and Entomopathogenic Nematodes against White Grubs: The Mechanism. Entomol. Exp. Appl. 2000, 94, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Purwar, J.P.; Sachan, G.C. Synergistic Effect of Entomogenous Fungi on Some Insecticides against Bihar Hairy Caterpillar Spilarctia obliqua (Lepidoptera: Arctiidae). Microbiol. Res. 2006, 161, 38–42. [Google Scholar] [CrossRef]
- Barelli, L.; Moonjely, S.; Behie, S.W.; Bidochka, M.J. Fungi with Multifunctional Lifestyles: Endophytic Insect Pathogenic Fungi. Plant Mol. Biol. 2016, 90, 657–664. [Google Scholar] [CrossRef]
- Daza, F.F.F.; Roman, G.R.; Rodriguez, M.V.; Vargas, I.A.G.; Heano, H.C.; Cereda, M.P.; Mulet, R.A.C. Spores of Beauveria bassiana and Trichoderma lignorum as a Bioinsecticide for the Control of Atta Cephalotes. Biol. Res. 2019, 52, 51. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Liang, M.; Li, L.; Sun, L.; Xu, Y.; Gao, J.; Wang, L.; Hou, Y.; Huang, S. Synergistic Effects of the Combined Application of Bacillus subtilis H158 and Strobilurins for Rice Sheath Blight Control. Biol. Control 2018, 117, 182–187. [Google Scholar] [CrossRef]
- Pinto, A.P.; Teixeira, D.M.; Caldeira, A.T.; Rodrigues, S.C. Biodegradation of Pesticides by Adapted Fungi. Potential Use on Biopurification Systems. In Agrochemicals Detection, Treatment and Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–23. [Google Scholar]
- Malandrakis, A.; Daskalaki, E.R.; Skiada, V.; Papadopoulou, K.K.; Kavroulakis, N. A Fusarium solani Endophyte vs Fungicides: Compatibility in a Fusarium oxysporum f.sp. radicis-lycopersici—Tomato Pathosystem. Fungal Biol. 2018, 122, 1215–1221. [Google Scholar] [CrossRef]
- Soesanto, L.; Mugiastuti, E.; Rahayuniati, R.F.; Manan, A.; Dewi, R.S. Compatibility Test of Four Trichoderma spp. Isolates on Several Synthetic Pesticides. Agrivita J. Agric. Sci. 2018, 40, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Wang, P.; Pu, Z.; Lu, L.; Chen, G.; Hu, X.; Fayyaz, A.; Gai, Y. Effects of Mancozeb on Citrus Rhizosphere Bacterial Community. Microb. Pathog. 2021, 154, 104845. [Google Scholar] [CrossRef] [PubMed]
- Roberti, R.; Badiali, F.; Pisi, A.; Veronesi, A.; Pancaldi, D.; Cesari, A. Sensitivity of Clonostachys rosea and Trichoderma spp. as Potential Biocontrol Agents to Pesticides. J. Phytopathol. 2006, 154, 100–109. [Google Scholar] [CrossRef]
- Banik, S.; Pérez-de-luque, A. In Vitro Effects of Copper Nanoparticles on Plant Pathogens, Beneficial Microbes and Crop Plants. Span. J. Agric. Res. 2017, 15, 1005. [Google Scholar] [CrossRef] [Green Version]
- Lal, H.C.; Mohan, R.; Kumar, P.; Ekka, S.; Kumar, B.; Kumar, N. Management of Wilt Complex in Brinjal Using Chemical, Biocontrol Agent and Soil Amendment Alone and in Combinations. Indian Phytopathol. 2017, 70, 220–223. [Google Scholar] [CrossRef] [Green Version]
- Vinale, F.; D’Ambrosio, G.; Abadi, K.; Scala, F.; Marra, R.; Turrà, D.; Woo, S.L.; Lorito, M. Application of Trichoderma harzianum (T22) and Trichoderma atroviride (P1) as Plant Growth Promoters, and Their Compatibility with Copper Oxychloride. J. Zhejiang Univ. Sci. 2004, 30, 425. [Google Scholar] [CrossRef]
- Muñoz-Leoz, B.; Ruiz-Romera, E.; Antigüedad, I.; Garbisu, C. Tebuconazole Application Decreases Soil Microbial Biomass and Activity. Soil Biol. Biochem. 2011, 43, 2176–2183. [Google Scholar] [CrossRef]
- Strickland, T.C.; Potter, T.L.; Joo, H. Tebuconazole Dissipation and Metabolism in Tifton Loamy Sand during Laboratory Incubation. Pest Manag. Sci. 2004, 60, 703–709. [Google Scholar] [CrossRef]
- Bending, G.D.; Rodríguez-Cruz, M.S.; Lincoln, S.D. Fungicide Impacts on Microbial Communities in Soils with Contrasting Management Histories. Chemosphere 2007, 69, 82–88. [Google Scholar] [CrossRef]
- Cycoń, M.; Piotrowska-Seget, Z.; Kaczyńska, A.; Kozdrój, J. Microbiological Characteristics of a Sandy Loam Soil Exposed to Tebuconazole and λ-Cyhalothrin under Laboratory Conditions. Ecotoxicology 2006, 15, 639–646. [Google Scholar] [CrossRef]
- Martínez-Toledo, M.V.; Salmerón, V.; Rodelas, B.; Pozo, C.; González-López, J. Effects of the Fungicide Captan on Some Functional Groups of Soil Microflora. Appl. Soil Ecol. 1998, 7, 245–255. [Google Scholar] [CrossRef]
- Monkiedje, A.; Ilori, M.O.; Spiteller, M. Soil Quality Changes Resulting from the Application of the Fungicides Mefenoxam and Metalaxyl to a Sandy Loam Soil. Soil Biol. Biochem. 2002, 34, 1939–1948. [Google Scholar] [CrossRef]
- Eberbach, P.L.; Douglas, L.A. Herbicide Effects on the Growth and Nodulation Potential of Rhizobium trifolii with Trifolium subterraneum L. Plant Soil 1989, 119, 15–23. [Google Scholar] [CrossRef]
- Lupi, L.; Miglioranza, K.S.B.; Aparicio, V.C.; Marino, D.; Bedmar, F.; Wunderlin, D.A. Occurrence of Glyphosate and AMPA in an Agricultural Watershed from the Southeastern Region of Argentina. Sci Total Environ. 2015, 536, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Newton, M.; Horner, L.M.; Cowell, J.E.; White, D.E.; Cole, E.C. Dissipation of Glyphosate and Aminomethylphosphonic Acid in North American Forests. J Agric Food Chem 1994, 42, 1795–1802. [Google Scholar] [CrossRef]
- Shushkova, T.; Ermakova, I.; Leontievsky, A. Glyphosate Bioavailability in Soil. Biodegradation 2010, 21, 403–410. [Google Scholar] [CrossRef]
- Ermakova, I.T.; Kiseleva, N.I.; Shushkova, T.; Zharikov, M.; Zharikov, G.A.; Leontievsky, A.A. Bioremediation of Glyphosate-Contaminated Soils. Appl Microbiol Biotechnol 2010, 88, 585–594. [Google Scholar] [CrossRef]
- Hadi, F.; Mousavi, A.; Noghabi, K.A.; Tabar, H.G.; Salmanian, A.H. New Bacterial Strain of the Genus Ochrobactrum with Glyphosate-Degrading Activity. J. Environ. Sci. Health B 2013, 48, 208–213. [Google Scholar] [CrossRef]
- Vázquez, M.B.; Moreno, M.V.; Amodeo, M.R.; Bianchinotti, M.V. Effects of Glyphosate on Soil Fungal Communities: A Field Study. Rev. Argent. Microbiol. 2021, 53, 349–358. [Google Scholar] [CrossRef]
- Ramanagouda, G.; Naik, M.K. Compatibility Studies of Indigenous Trichoderma Isolates with Pesticides. Indian Phytopathol. 2021, 74, 241–248. [Google Scholar] [CrossRef]




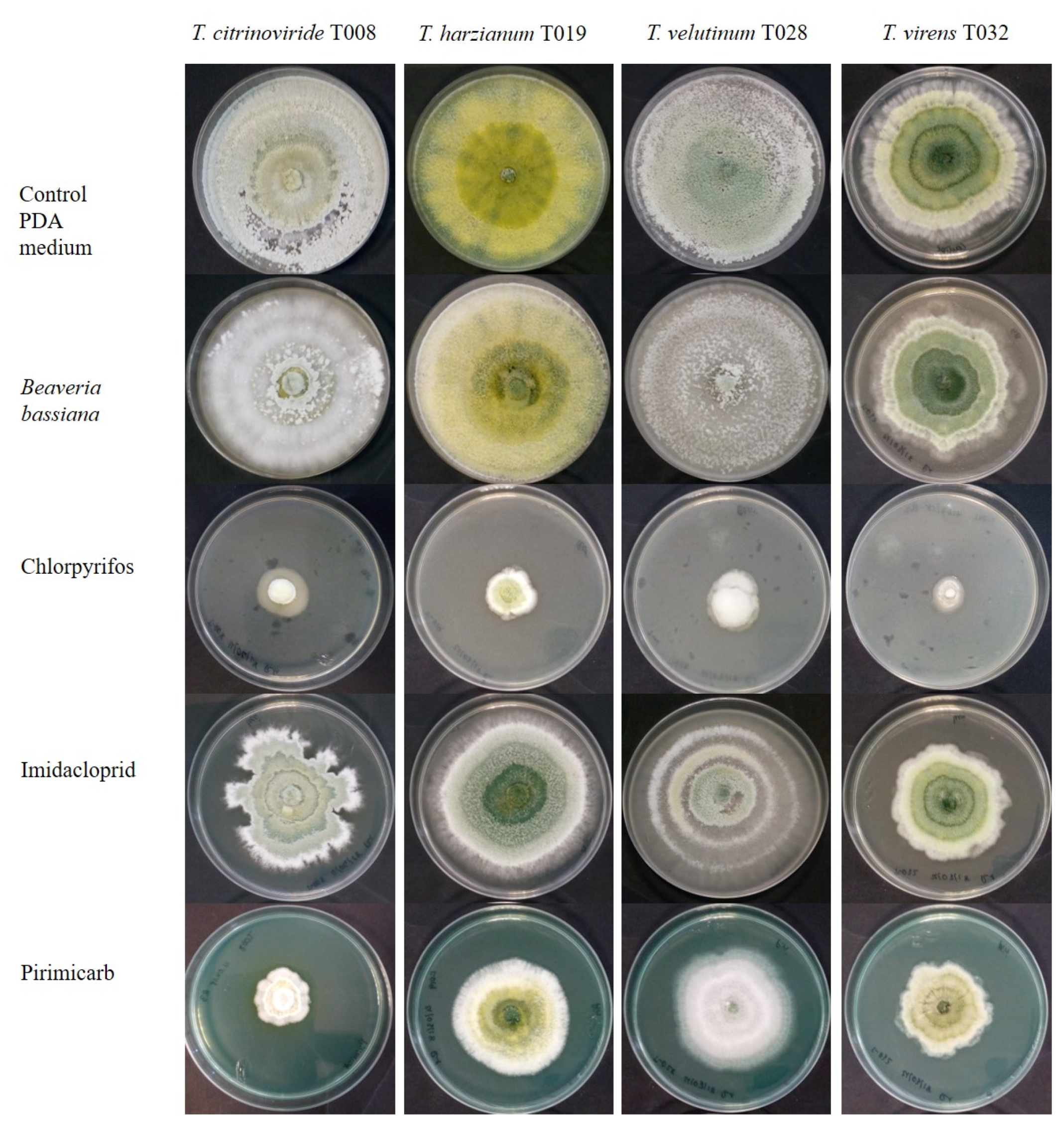

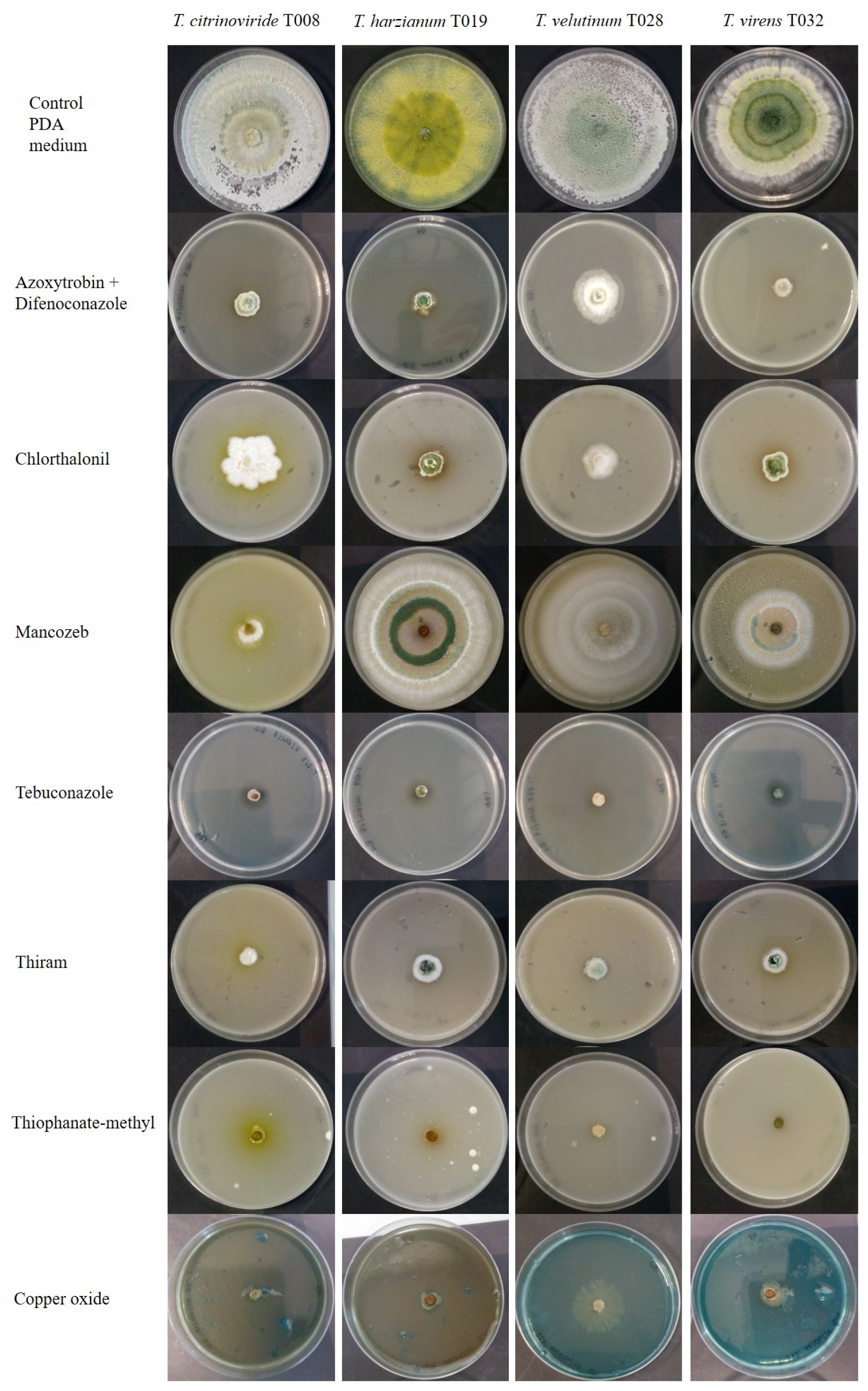

| Isolate (1) | Culture Collection (2) | Species | Crop | Type Sample | Localization |
|---|---|---|---|---|---|
| T008 | PAULET27 | T. citrinoviride | Bean | Selected seed | Fresno de la Vega (León) |
| T019 | PAULET38 | T. harzianum | Bean | Selected seed | Carrizo de la Ribera (León) |
| T028 | IASULE2 | T. velutinum | Bean | Soil | Villaobispo de Otero (León) |
| T032 | IASULE6 | T. virens | Wheat | Soil | Cebrones del Río (León) |
| Active Ingredient (%) | Mode of Action | Chemical Class | Recommended Field Dose | Observations |
|---|---|---|---|---|
| Acaricide | ||||
| Abamectin 1.8% weight/volume (w/v) | Contact and ingestion | Pentacyclone | 80–100 mL/hL | Permanence in soil between 2 weeks and 2 months. |
| It is fixed to the ground and is considered immobile on it. | ||||
| Deltamethrin 1.5% w/v | Contact and ingestion | Synthetic pyrethroid | 50–83 mL/hL | Its activity is reduced with temperatures above 35 °C. |
| Non-phytotoxic. | ||||
| Sulfur 80% w/v | Direct and remote contact by the gaseous compounds produced | - | 250 g/hL | Dose are reduced with high temperature and environmental dryness. Additionally, it had fungicide action. |
| Insecticide | ||||
| Beauveria bassiana 22% (4.4 × 1010 viable spores/g) | Parasitizing the host insect from egg to adult | Fungus: Phylum Deuteromycota | 62.5–125 g/hL | It is an entomopathogenic class of insects. |
| Chlorpyrifos 48 % w/v (1) | Ingestion, inhalation and contact | Organophosphate | 150–200 mL/hL * | It degrades slowly in the soil, with a half-life at 25 °C of 92 to 341 days in acid soils, and from 11 to 200 days in alkaline soils. |
| Imidacloprid 20% w/v | Contact and ingestion | Neonicotinoid | 50–75 mL/hL | Its residual effect varies between 15 and 21 days in the leaf and 45 and 65 days in the soil, increasing up to 165 and 247 days in very alkaline soils with low organic matter. |
| Pirimicarb 20% w/v | Contact, ingestion and inhalation | Carbamate | 100 g/hL | It remains in the soil between 7 and 234 days. |
| It is stable at pH 4. | ||||
| Fungicide | ||||
| Azoxistrobin 20% + Difenoconazole 12.5 % w/v | Preventive, curative and eradicator effect | Derived from ß-methoxyacrylic acid (Azoxistrobin) Triazole (Difenoconazole) | 100 mL/hL | Systemic, few residuals. |
| Chlorthalonil 50% w/v (2) | Contact activity Preventive and eradicating action | Polychlorinated aromatic derived from chlorisophthalic acid | 250–300 mL/hL | It has a persistence of 1.5–3 months depending on the moisture content and the soil temperature. |
| Copper 75% w/v (Copper oxide) | Preventive effect | - | 200 g/hL | It is strongly retained in the most superficial area of the soil, being practically immobile. |
| Mancozeb 80% w/v (3) | Preventive activity by contact | Diethyldithiocarbamate | 200 g/hL | It has a persistence of 6–15 days in the soil. |
| Thiophanate-methyl 45% w/v (4) | Preventive, curative effect | Thiocarbamate | 300 mL/hL | Secondary action on mite eggs and nematode. |
| It is converted to carbendazyme by photodegradation in the soil. Its persistence is approximately 1 month. | ||||
| Tebuconazole 25% w/v | Preventive, curative and eradicator effect | Triazole | 40–100 mL/hL | It degrades rapidly, and it does not accumulate in the soil. |
| Thiram 80% w/v (5) | Preventive activity by contact | Dimethyldithiocarbamate | 200 g/hL | Its persistence depends on the pH, concentration and type of soil, varying between 2 days and 32 weeks. |
| Herbicide | ||||
| Diquat 20% w/v (6) | Post-emergence, desiccant and defoliant, with contact activity and non-selective | Bipyridyl | 2 L/ha | Residual activity in the soil is of few days, inactivating quickly and completely. |
| Glyphosate 36% w/v | Post-emergence, foliar absorption, non-selective | Glycine | 3–6 L/ha | It quickly inactivates in the soil. Its persistence in silty-sandy soils is 19.2 days, being several years in clay soils. |
| Pendimethalin 33% w/v | Selective control Pre-emergence or early post-emergence | Dinitroaniline | 3–6 L/ha | Residual herbicide acting for 3–4 months. |
| Source of Variation | Df 1 | Day 2 | Day 5 | Day 7 |
|---|---|---|---|---|
| Autochthonous Trichoderma strains (ATs) | 3 | 691.937 ** | 3431.557 ** | 3095.672 ** |
| Group of pesticides (Gp) | 4 | 14,308.351 ** | 39,632.133 ** | 26,510.961 ** |
| ATs x Gp | 12 | 141.204 | 371.228 | 480.613 |
| Error | 296 | 84.303 | 470.840 | 651.822 |
| Total | 315 |
| Source of Variation | Df 1 | Day 2 | Day 5 | Day 7 |
|---|---|---|---|---|
| Autochthonous Trichoderma strains (ATs) | 3 | 369.093 ** | 3337.806 ** | 2357.338 ** |
| Pesticides (P) | 17 | 4594.332 ** | 15,945.741 ** | 16,312.180 ** |
| ATs x P | 50 | 71.787 ** | 476.623 ** | 461.477 ** |
| Error | 245 | 9.177 | 27.498 | 13.094 |
| Total | 315 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayo-Prieto, S.; Squarzoni, A.; Carro-Huerga, G.; Porteous-Álvarez, A.J.; Gutiérrez, S.; Casquero, P.A. Organic and Conventional Bean Pesticides in Development of Autochthonous Trichoderma Strains. J. Fungi 2022, 8, 603. https://doi.org/10.3390/jof8060603
Mayo-Prieto S, Squarzoni A, Carro-Huerga G, Porteous-Álvarez AJ, Gutiérrez S, Casquero PA. Organic and Conventional Bean Pesticides in Development of Autochthonous Trichoderma Strains. Journal of Fungi. 2022; 8(6):603. https://doi.org/10.3390/jof8060603
Chicago/Turabian StyleMayo-Prieto, Sara, Alessandra Squarzoni, Guzmán Carro-Huerga, Alejandra J. Porteous-Álvarez, Santiago Gutiérrez, and Pedro Antonio Casquero. 2022. "Organic and Conventional Bean Pesticides in Development of Autochthonous Trichoderma Strains" Journal of Fungi 8, no. 6: 603. https://doi.org/10.3390/jof8060603
APA StyleMayo-Prieto, S., Squarzoni, A., Carro-Huerga, G., Porteous-Álvarez, A. J., Gutiérrez, S., & Casquero, P. A. (2022). Organic and Conventional Bean Pesticides in Development of Autochthonous Trichoderma Strains. Journal of Fungi, 8(6), 603. https://doi.org/10.3390/jof8060603









