Comparative Genomics of Three Aspergillus Strains Reveals Insights into Endophytic Lifestyle and Endophyte-Induced Plant Growth Promotion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Endophytic Fungi
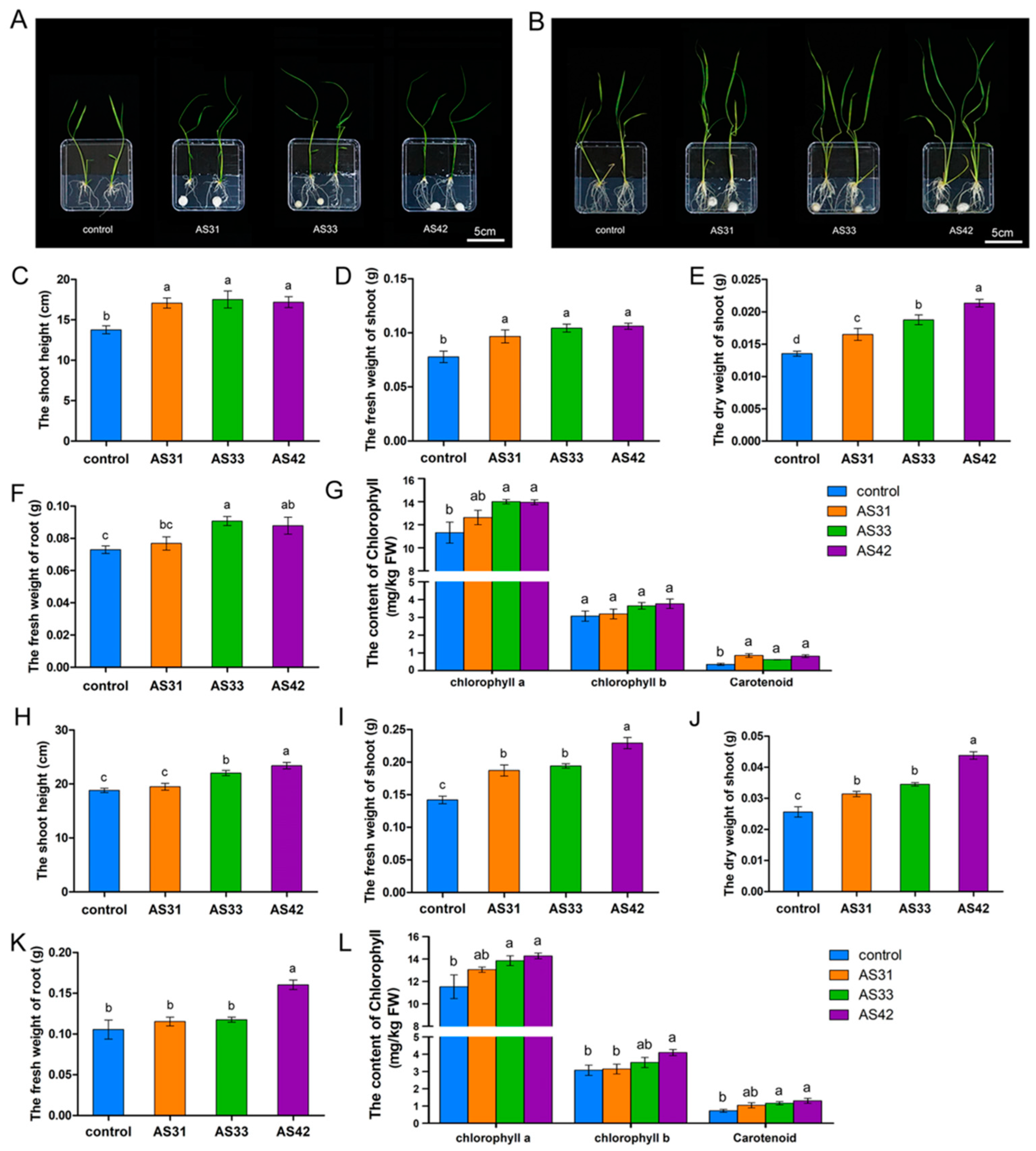
2.2. Plant Recolonization Experiments Assessing the Effect of Each Fungal Strain on Plant Growth
2.3. Determination of Pi Concentrations in Rice Shoots and Roots
2.4. Fungal Colonization
2.5. RNA Extraction and Gene Expression Analysis
2.6. Whole Genome Sequencing, De Novo Assembly, and Functional Annotation
2.7. Statistical Analysis
3. Results
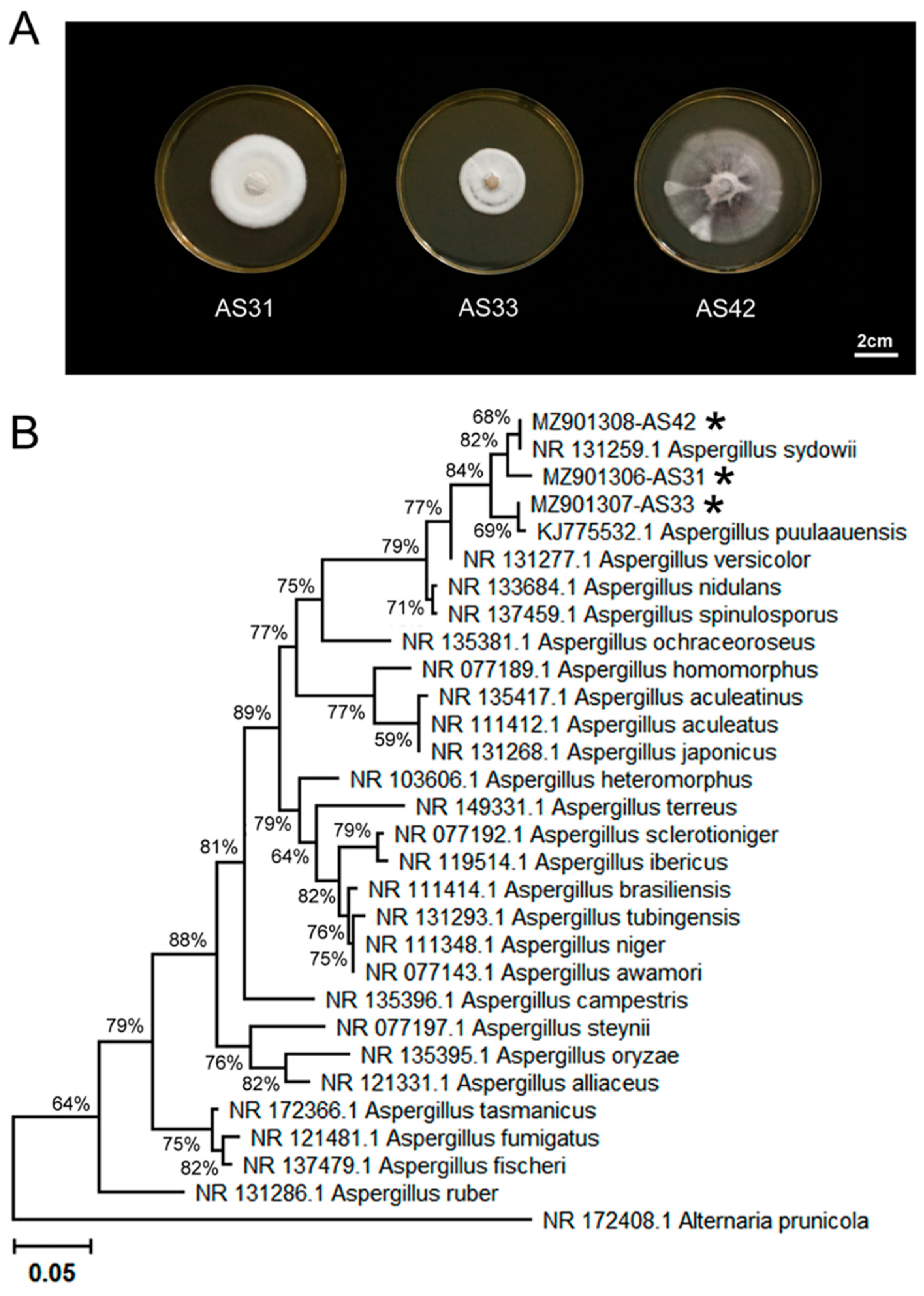
3.1. Characterization of Three Aspergillus Isolates and Plant Growth-Promoting Properties
3.2. Genome Features of Strains AS31, AS33, and AS42
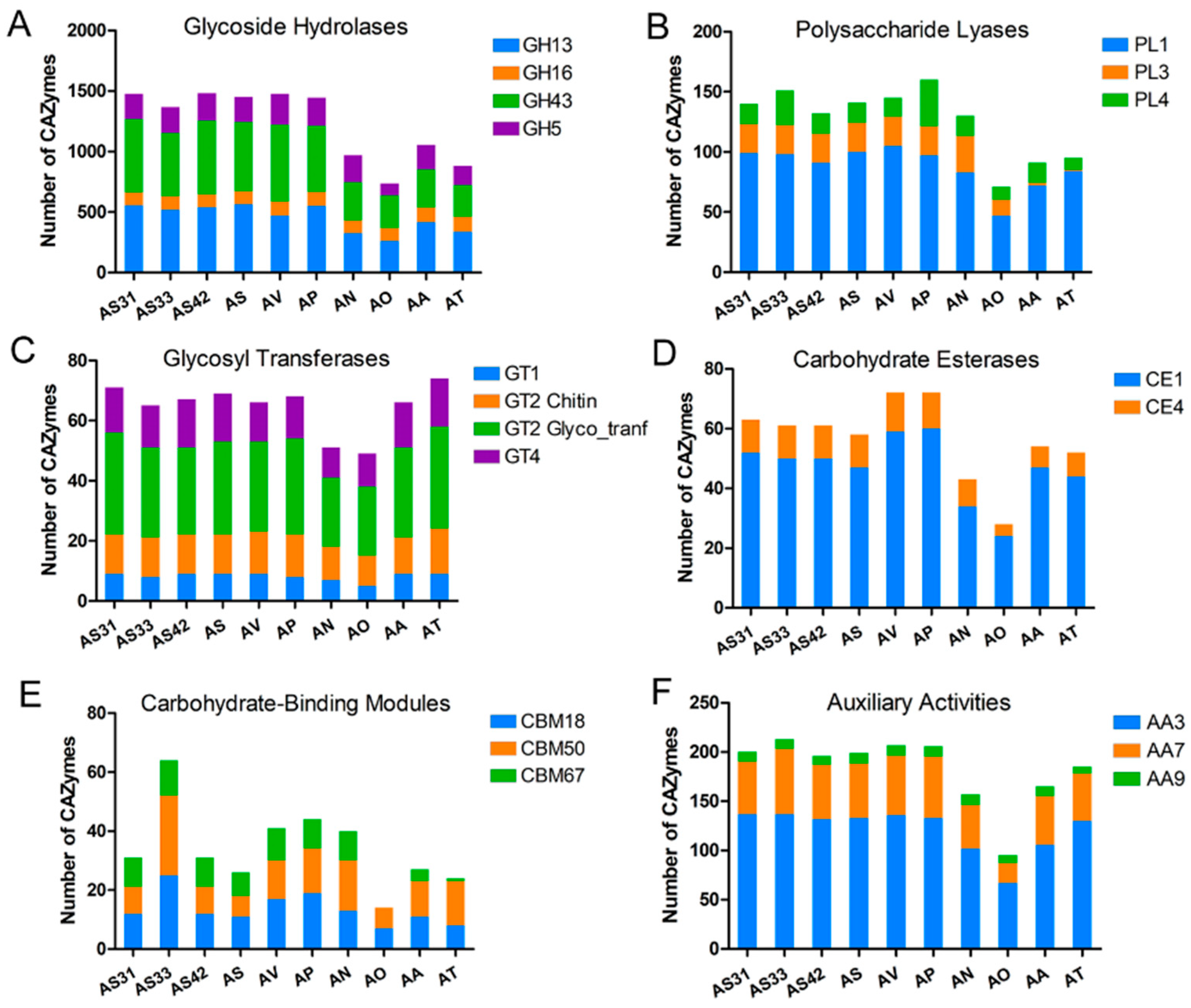
3.3. Comparative Analysis of CAZymes and SSPs
3.4. Identification of Secondary Metabolism Gene Clusters
3.5. Endophytes Altered Pi transportation and Distribution in Rice by Regulating the Expression of Pi Transport Genes in the Host Plant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Elsas, J.D.; Chiurazzi, M.; Mallon, C.A.; Elhottovā, D.; Krištůfek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2012, 109, 1159–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagg, C.; Bender, S.F.; Widmer, F.; Van Der Heijden, M.G. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Mishra, J.; Prakash, J.; Arora, N.K. Role of beneficial soil microbes in sustainable agriculture and environmental management. Clim. Chang. Environ. Sustain. 2016, 4, 137–149. [Google Scholar] [CrossRef]
- Smith, S.E.; Gianinazzi-Pearson, V.; Koide, R.; Cairney, J.W.G. Nutrient transport in mycorrhizas: Structure, physiology and consequences for efficiency of the symbiosis. Plant Soil 1994, 159, 103–113. [Google Scholar] [CrossRef]
- Nehls, U.; Grunze, N.; Willmann, M.; Reich, M.; Küster, H. Sugar for my honey: Carbohydrate partitioning in ectomycorrhizal symbiosis. Phytochemistry 2007, 68, 82–91. [Google Scholar] [CrossRef]
- Schardl, C.L.; Craven, K.D.; Speakman, S.; Stromberg, A.; Lindstrom, A.; Yoshida, R. A Novel Test for Host-Symbiont Codivergence Indicates Ancient Origin of Fungal Endophytes in Grasses. Syst. Biol. 2008, 57, 483–498. [Google Scholar] [CrossRef] [Green Version]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011, 90, 1829–1845. [Google Scholar] [CrossRef]
- Pozo, M.J.; Zabalgogeazcoa, I.; de Aldana, B.R.V.; Martinez-Medina, A. Untapping the potential of plant mycobiomes for applications in agriculture. Curr. Opin. Plant Biol. 2021, 60, 102034. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2019, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, F.; Mosaddeghi, M.R.; Hajabbasi, M.A.; Sabzalian, M.R. Role of fungal endophyte of tall fescue (Epichloë coenophiala) on water availability, wilting point and integral energy in texturally-different soils. Agric. Water Manag. 2016, 163, 197–211. [Google Scholar] [CrossRef]
- Poveda Arias, J.; Eugui Arrizabalaga, D.; Abril Urías, P.; Velasco, P. Endophytic fungi as direct plant growth promoters for sustainable agricultural production. Symbiosis 2021, 85, 1–19. [Google Scholar] [CrossRef]
- Carswell, C.; Grant, B.R.; Theodorou, M.E.; Harris, J.; Niere, J.O.; Plaxton, W.C. The fungicide phosphonate disrupts the phosphate-starvation response in Brassica nigra seedlings. Plant Physiol. 1996, 110, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghothama, K.G. Phosphate transport and signaling. Curr. Opin. Plant Biol. 2000, 3, 182–187. [Google Scholar] [CrossRef]
- Goff, S.A. A Draft Sequence of the Rice Genome (Oryza sativa L. ssp. japonica). Science 2002, 296, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Paszkowski, U.; Kroken, S.; Roux, C.; Briggs, S.P. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 13324–13329. [Google Scholar] [CrossRef] [Green Version]
- Hiruma, K.; Gerlach, N.; Sacristan, S.; Nakano, R.T.; Hacquard, S.; Kracher, B.; Neumann, U.; Ramirez, D.; Bucher, M.; O’Connell, R.J.; et al. Root Endophyte Colletotrichum tofieldiae Confers Plant Fitness Benefits that Are Phosphate Status Dependent. Cell 2016, 165, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Sawers, R.J.H.; Svane, S.F.; Quan, C.; Gronlund, M.; Wozniak, B.; Gebreselassie, M.N.; Gonzalez-Munoz, E.; Montes, R.A.C.; Baxter, I.; Goudet, J.; et al. Phosphorus acquisition efficiency in arbuscular mycorrhizal maize is correlated with the abundance of root-external hyphae and the accumulation of transcripts encoding PHT1 phosphate transporters. New Phytol. 2017, 214, 632–643. [Google Scholar] [CrossRef] [Green Version]
- Opitz, M.W.; Daneshkhah, R.; Lorenz, C.; Ludwig, R.; Steinkellner, S.; Wieczorek, K. Serendipita indica changes host sugar and defense status in Arabidopsis thaliana: Cooperation or exploitation? Planta 2021, 253, 74. [Google Scholar] [CrossRef]
- Sista Kameshwar, A.K.; Qin, W. Comparative study of genome-wide plant biomass-degrading CAZymes in white rot, brown rot and soft rot fungi. Mycology 2018, 9, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Su, Z.; Wang, C.; Kubicek, C.P.; Feng, X.; Mao, L.; Wang, J.; Chen, C.; Lin, F.; Zhang, C. The rice endophyte Harpophora oryzae genome reveals evolution from a pathogen to a mutualistic endophyte. Sci. Rep. 2014, 4, 5783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Presti, L.; Lanver, D.; Schweizer, G.; Tanaka, S.; Liang, L.; Tollot, M.; Zuccaro, A.; Reissmann, S.; Kahmann, R. Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 2015, 66, 513–545. [Google Scholar] [CrossRef]
- Sánchez-Vallet, A.; Fouché, S.; Fudal, I.; Hartmann, F.E.; Soyer, J.L.; Tellier, A.; Croll, D. The genome biology of effector gene evolution in filamentous plant pathogens. Annu. Rev. Phytopathol. 2018, 56, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Win, J.; Krasileva, K.V.; Kamoun, S.; Shirasu, K.; Staskawicz, B.J.; Banfield, M.J. Sequence divergent RXLR effectors share a structural fold conserved across plant pathogenic oomycete species. PLoS Pathog. 2012, 8, e1002400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancheeva, E.; Daletos, G.; Proksch, P. Bioactive secondary metabolites from endophytic fungi. Curr. Med. Chem. 2020, 27, 1836–1854. [Google Scholar] [CrossRef]
- Alam, B.; Li, J.; Ge, Q.; Khan, M.A.; Gong, J.; Mehmood, S.; Yuan, Y.; Gong, W. Endophytic Fungi: From Symbiosis to Secondary Metabolite Communications or Vice Versa? Front. Plant Sci. 2021, 12, 791033. [Google Scholar] [CrossRef]
- Wiemann, P.; Sieber, C.M.K.; Von Bargen, K.W.; Studt, L.; Niehaus, E.M.; Espino, J.J.; Huss, K.; Michielse, C.B.; Albermann, S.; Wagner, D.; et al. Deciphering the Cryptic Genome: Genome-wide Analyses of the Rice Pathogen Fusarium fujikuroi Reveal Complex Regulation of Secondary Metabolism and Novel Metabolites. PLoS Pathog. 2013, 9, e1003475. [Google Scholar] [CrossRef] [Green Version]
- De Maayer, P.; Chan, W.Y.; Venter, S.N.; Toth, I.K.; Birch, P.R.J.; Joubert, F.; Coutinho, T.A. Genome sequence of Pantoea ananatis LMG20103, the causative agent of Eucalyptus blight and dieback. J. Bacteriol. 2010, 192, 2936–2937. [Google Scholar] [CrossRef] [Green Version]
- Kahlke, T.; Goesmann, A.; Hjerde, E.; Willassen, N.P.; Haugen, P. Unique core genomes of the bacterial family vibrionaceae: Insights into niche adaptation and speciation. BMC Genom. 2012, 13, 179. [Google Scholar]
- Lòpez-Fernàndez, S.; Sonego, P.; Moretto, M.; Pancher, M.; Engelen, K.; Pertot, I.; Campisano, A. Whole-genome comparative analysis of virulence genes unveils similarities and differences between endophytes and other symbiotic bacteria. Front. Microbiol. 2015, 6, 419. [Google Scholar] [PubMed] [Green Version]
- Knapp, D.G.; Nemeth, J.B.; Barry, K.; Hainaut, M.; Henrissat, B.; Johnson, J.; Kuo, A.; Lim, J.H.P.; Lipzen, A.; Nolan, M.; et al. Comparative genomics provides insights into the lifestyle and reveals functional heterogeneity of dark septate endophytic fungi. Sci. Rep. 2018, 8, 6321. [Google Scholar]
- Parkhill, J.; Sebaihia, M.; Preston, A.; Murphy, L.D.; Thomson, N.; Harris, D.E.; Holden, M.T.G.; Churcher, C.M.; Bentley, S.D.; Mungall, K.L.; et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 2003, 35, 32–40. [Google Scholar] [CrossRef] [PubMed]
- De Vries, R.P.; Riley, R.; Wiebenga, A.; Aguilar-Osorio, G.; Amillis, S.; Uchima, C.A.; Anderluh, G.; Asadollahi, M.; Askin, M.; Barry, K.; et al. Comparative genomics reveals high biological diversity and specific adaptations in the industrially and medically important fungal genus Aspergillus. Genome Biol. 2017, 18, 28. [Google Scholar] [CrossRef] [Green Version]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.B.; Hubka, V.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [Green Version]
- Bastos, R.W.; Valero, C.; Silva, L.P.; Schoen, T.; Drott, M.; Brauer, V.; Silva-Rocha, R.; Lind, A.; Steenwyk, J.L.; Rokas, A. Functional Characterization of Clinical Isolates of the Opportunistic Fungal Pathogen Aspergillus nidulans. mSphere 2020, 5, 2. [Google Scholar]
- Klich, M.A.; Cary, J.W.; Beltz, S.B.; Bennett, C.A. Phylogenetic and Morphological Analysis of Aspergillus ochraceoroseus. Mycologia 2003, 95, 1252. [Google Scholar] [CrossRef]
- Cary, J.W.; Ehrlich, K.C.; Beltz, S.B.; Harris-Coward, P.; Klich, M.A. Characterization of the Aspergillus ochraceoroseus aflatoxin/sterigmatocystin biosynthetic gene cluster. Mycologia 2009, 101, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.J.; Wang, Q.T.; Cheng, Y.H.; Hou, C.L. Identification of Aspergillus tubingensis causing pomegranate fruit rot in China. Australas. Plant Pathol. 2021, 50, 233240. [Google Scholar]
- Khizar, M.; Haroon, U.; Kamal, A.; Inam, W.; Chaudhary, H.J.; Munis, M.F.H. Evaluation of virulence potential of Aspergillus tubingensis and subsequent biochemical and enzymatic defense response of cotton. Microsc. Res. Tech. 2021, 84, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Escobar Diaz, P.A.; Santos, R.M.D.; Baron Cozentino, N.C.; Oniel, J.A.; Rigobelo, E.C. Effect of Aspergillus and Bacillus concentration on cotton growth promotion. Front. Microbiol. 2021, 12, 737385. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Saxena, J.; Sharma, V. Effect of phosphate-solubilizing fungi Aspergillus awamori S29 on mungbean (Vigna radiata cv. RMG 492) growth. Folia Microbiol. 2012, 57, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V.; Singh, O.; Nayyar, H.; Kaur, J.; Tewari, R. Stimulatory effect of phosphate-solubilizing fungal strains (Aspergillus awamori and Penicillium citrinum) on the yield of chickpea (Cicer arietinum L. cv. GPF2). Soil Biol. Biochem. 2008, 40, 718–727. [Google Scholar] [CrossRef]
- Ali, R.; Gul, H.; Hamayun, M.; Rauf, M.; Iqbal, A.; Shah, M.; Hussain, A.; Bibi, H.; Lee, I.J. Aspergillus awamori ameliorates the physicochemical characteristics and mineral profile of mung bean under salt stress. Chem. Biol. Technol. Agric. 2021, 8, 9. [Google Scholar] [CrossRef]
- Letsiou, S.; Bakea, A.; Le Goff, G.; Lopes, P.; Gardikis, K.; Alonso, C.; Alvarez, P.A.; Ouazzani, J. In vitro protective effects of marine-derived Aspergillus puulaauensis TM124-S4 extract on H2O2-stressed primary human fibroblasts. Toxicol. In Vitro 2020, 66, 104869. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, Y.; Li, N.; Ni, D.; Yang, Y.; Wang, X. Differences in the Characteristics and Pathogenicity of Colletotrichum camelliae and C. fructicola Isolated From the Tea Plant [Camellia sinensis (L.) O. Kuntze]. Front. Microbiol. 2018, 9, 3060. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Lee, Y.C.; Johnson, J.M.; Chien, C.T.; Sun, C.; Cai, D.; Lou, B.; Oelmüller, R.; Yeh, K.W. Growth promotion of Chinese cabbage and Arabidopsis by Piriformospora indica is not stimulated by mycelium-synthesized auxin. Mol. Plant-Microbe Interact. 2010, 24, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhou, J.; Xu, R.; Meng, M.; Yu, X.; Dai, C. Auxin, Cytokinin, and Ethylene Involved in Rice N Availability Improvement Caused by Endophyte Phomopsis liquidambari. J. Plant Growth Regul. 2018, 37, 128–143. [Google Scholar] [CrossRef]
- Nanamori, M.; Shinano, T.; Wasaki, J.; Yamamura, T.; Rao, I.M.; Osaki, M. Low Phosphorus Tolerance Mechanisms: Phosphorus Recycling and Photosynthate Partitioning in the Tropical Forage Grass, Brachiaria Hybrid Cultivar Mulato Compared with Rice. Plant Cell Physiol. 2004, 45, 460–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Z.; Zhang, C.; Lin, F.; Kubicek, C.P. Identity, diversity, and molecular phylogeny of the endophytic mycobiota in the roots of rare wild rice (Oryza granulate) from a nature reserve in Yunnan, China. Appl. Environ. Microbiol. 2010, 76, 1642–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesny, F.; Miyauchi, S.; Thiergart, T.; Pickel, B.; Atanasova, L.; Karlsson, M.; Huttel, B.; Barry, K.W.; Haridas, S.; Chen, C.; et al. Genetic determinants of endophytism in the Arabidopsis root mycobiome. Nat. Commun. 2021, 12, 7227. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Jackman, S.D.; Vandervalk, B.P.; Mohamadi, H.; Chu, J.; Yeo, S.; Hammond, S.A.; Jahesh, G.; Khan, H.; Coombe, L.; Warren, R.L.; et al. Abyss 2.0: Resource-efficient assembly of large genomes using a bloom filter. Genome Res. 2017, 27, 768–777. [Google Scholar] [CrossRef] [Green Version]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience 2012, 1, 18. [Google Scholar] [CrossRef]
- Hoff, K.J.; Lange, S.; Lomsadze, A.; Borodovsky, M.; Stanke, M. BRAKER1: Unsupervised RNA-Seq-Based Genome Annotation with GeneMark-ET and AUGUSTUS. Bioinformatics 2015, 32, 767–769. [Google Scholar] [CrossRef]
- Zhang, H.; Yohe, T.; Huang, L.; Entwistle, S.; Wu, P.; Yang, Z.; Busk, P.K.; Xu, Y.; Yin, Y. dbCAN2: A meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2018, 46, W95–W101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortázar, A.R.; Aransay, A.M.; Alfaro, M.; Oguiza, J.A.; Lavín, J.L. SECRETOOL: Integrated secretome analysis tool for fungi. Amino Acids 2014, 46, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Gramaje, D.; Berlanas, C.; Martinez-Diz, M.D.; Diaz-Losada, E.; Antonielli, L.; Beier, S.; Gorfer, M.; Schmoll, M.; Compant, S. Comparative genomic of Dactylonectria torresensis strains from grapevine, soil and weed highlights potential mechanisms in pathogenicity and endophytic lifestyle. J. Fungi 2020, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [Green Version]
- Hage, H.; Rosso, M.N. Evolution of Fungal Carbohydrate-Active Enzyme Portfolios and Adaptation to Plant Cell-Wall Polymers. J. Fungi 2021, 7, 185. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef] [Green Version]
- Kuchkarova, N.N.; Toshmatov, Z.O.; Zhou, S.; Han, C.; Shao, H. Secondary metabolites with plant growth regulator activity produced by an endophytic fungus Purpureocillium sp. from Solanum rostratum. Chem. Nat. Compd. 2020, 56, 775–776. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, M.K.; Pham, H.Q.; Gu, M.J.; Zhu, B.H.; Son, S.H.; Hahn, D.; Shin, J.H.; Yu, J.H.; Park, H.S.; et al. The velvet Regulator VosA Governs Survival and Secondary Metabolism of Sexual Spores in Aspergillus nidulans. Genes 2020, 11, 103. [Google Scholar] [CrossRef] [Green Version]
- Caceres, I.; Al Khoury, A.; El Khoury, R.; Lorber, S.; Oswald, I.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.D. Aflatoxin Biosynthesis and Genetic Regulation: A Review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef] [Green Version]
- Almario, J.; Jeena, G.; Wunder, J.; Langen, G.; Zuccaro, A.; Coupland, G.; Bucher, M. Root-associated fungal microbiota of nonmycorrhizal Arabis alpina and its contribution to plant phosphorus nutrition. Proc. Natl. Acad. Sci. USA 2017, 114, E9403–E9412. [Google Scholar] [CrossRef] [Green Version]
- Adnan, M.; Alshammari, E.; Ashraf, S.A.; Patel, K.; Lad, K.; Patel, M. Physiological and molecular characterization of biosurfactant producing endophytic fungi Xylaria regalis from the cones of Thuja plicata as a potent plant growth promoter with its potential application. BioMed Res. Int. 2018, 2018, 7362148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.; Zhu, Q.; Zhang, F.; Zhang, W.; Yuan, J.; Sun, K.; Xu, F.; Dai, C. Enhanced nitrogen and phosphorus activation with an optimized bacterial community by endophytic fungus Phomopsis liquidambari in paddy soil. Microbiol. Res. 2019, 221, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Gu, M.; Xia, Y.; Dai, X.; Dai, C.; Zhang, J.; Wang, S.; Qu, H.; Yamaji, N.; Ma, J.; et al. OsPHT1;3 Mediates Uptake, Translocation and Remobilization of Phosphate under Extremely Low Phosphate Regimes. Plant Physiol. 2018, 179, 656–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, Y.; Pineros, M.A.; Wang, Z.; Wang, W.; Li, C.; Wu, Z.; Kochian, L.V.; Wu, P. Phosphate transporters OsPHT1; 9 and OsPHT1; 10 are involved in phosphate uptake in rice. Plant Cell Environ. 2014, 37, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ying, S.; Huang, H.; Li, K.; Wu, P.; Shou, H. Involvement of OsSPX1 in phosphate homeostasis in rice. Plant J. 2009, 57, 895–904. [Google Scholar] [CrossRef]
- Sun, S.; Gu, M.; Cao, Y.; Huang, X.; Zhang, X.; Ai, P.; Zhao, J.; Fan, X.; Xu, G. A constitutive expressed phosphate transporter, OsPht1; 1, modulates phosphate uptake and translocation in phosphate-replete rice. Plant Physiol. 2012, 159, 1571–1581. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Ren, H.; Gu, M.; Zhao, J.; Sun, S.; Zhang, X.; Chen, J.; Wu, P.; Xu, G. The phosphate transporter gene OsPht1; 8 is involved in phosphate homeostasis in rice. Plant Physiol. 2011, 156, 1164–1175. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Anasontzis, G.E.; Labourel, A.; Champion, C.; Haon, M.; Kemppainen, M.; Commun, C.; Deveau, A.; Pardo, A.; Veneault-Fourrey, C.; et al. The ectomycorrhizal basidiomycete Laccaria bicolor releases a secreted beta-1,4 endoglucanase that plays a key role in symbiosis development. New Phytol. 2018, 220, 1309–1321. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Vienken, K.; Weber, R.; Bunting, S.; Requena, N.; Fischer, R. A putative high affinity hexose transporter, hxtA, of Aspergillus nidulans is induced in vegetative hyphae upon starvation and in ascogenous hyphae during cleistothecium formation. Fungal Genet. Biol. 2004, 41, 148–156. [Google Scholar] [CrossRef]
- Helber, N.; Wippel, K.; Sauer, N.; Schaarschmidt, S.; Hause, B.; Requena, N. A Versatile Monosaccharide Transporter That Operates in the Arbuscular Mycorrhizal Fungus Glomus sp Is Crucial for the Symbiotic Relationship with Plants. Plant Cell 2011, 23, 3812–3823. [Google Scholar] [CrossRef] [Green Version]
- Newis, K.; Lenfant, N.; Lombard, V.; Henrissat, B. Dividing the large glycoside hydrolase family 43 into subfamilies: A motivation for detailed enzyme characterization. Appl. Environ. Microbiol. 2016, 82, 16862. [Google Scholar]
- Scheiblbrandner, S.; Ludwig, R. Cellobiose dehydrogenase: Bioelectrochemical insights and applications. Bioelectrochemistry 2020, 131, 107345. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, M.C.; Valent, B. Filamentous plant pathogen effectors in action. Nat. Rev. Microbiol. 2013, 11, 800–814. [Google Scholar] [CrossRef]
- Akum, F.N.; Steinbrenner, J.; Biedenkopf, D.; Imani, J.; Kogel, K.H. The Piriformospora indica effector PIIN_08944 promotes the mutualistic Sebacinalean symbiosis. Front. Plant Sci. 2015, 6, 906. [Google Scholar] [CrossRef] [Green Version]
- Queiroz, C.B.D.; Santana, M.F. Prediction of the secretomes of endophytic and nonendophytic fungi reveals similarities in host plant infection and colonization strategies. Mycologia 2020, 112, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Gudiño, M.E.; Blanco-Touriñán, N.; Arbona, V.; Gómez-Cadenas, A.; Blázquez, M.A.; Navarro-García, F. β-Lactam antibiotics modify root architecture and indole glucosinolate metabolism in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 2086–2098. [Google Scholar] [PubMed]
- Palmer, J.M.; Wiemann, P.; Greco, C.; Chiang, Y.M.; Wang, C.C.; Lindner, D.L.; Keller, N.P. The sexual spore pigment asperthecin is required for normal ascospore production and protection from UV light in Aspergillus nidulans. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab055. [Google Scholar] [CrossRef]




| AS31 | AS33 | AS42 | |
|---|---|---|---|
| Accession number | JAIOTV000000000 | JAIOTX000000000 | JAIOTW000000000 |
| Number of scaffolds | 538 | 812 | 836 |
| Genome size (Mb) | 36.8 | 34.8 | 35.3 |
| N50 length (bp) | 246,959 | 284,941 | 76,567 |
| GC content (%) | 49.94 | 49.57 | 50.47 |
| N rate (%) | 0 | 0 | 0.0001 |
| Gene number | 12,933 | 12,364 | 13,211 |
| Gene average length (bp) | 1474 | 1514 | 1453 |
| Gene length/Genome (%) | 53.85 | 47.48 | 54.87 |
| Repeat (%) | 2.28 | 1.62 | 6.43 |
| NR | 12,759 | 12,685 | 12,949 |
| GO | 4093 | 4117 | 4128 |
| eggNOG | 7732 | 7864 | 7852 |
| KEGG | 4644 | 4684 | 4612 |
| Swiss | 7667 | 7829 | 7745 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, M.; Xu, X.; Peng, J.; Li, C.; Zhang, H.; Lian, C.; Chen, Y.; Shen, Z.; Chen, C. Comparative Genomics of Three Aspergillus Strains Reveals Insights into Endophytic Lifestyle and Endophyte-Induced Plant Growth Promotion. J. Fungi 2022, 8, 690. https://doi.org/10.3390/jof8070690
Jing M, Xu X, Peng J, Li C, Zhang H, Lian C, Chen Y, Shen Z, Chen C. Comparative Genomics of Three Aspergillus Strains Reveals Insights into Endophytic Lifestyle and Endophyte-Induced Plant Growth Promotion. Journal of Fungi. 2022; 8(7):690. https://doi.org/10.3390/jof8070690
Chicago/Turabian StyleJing, Minyu, Xihui Xu, Jing Peng, Can Li, Hanchao Zhang, Chunlan Lian, Yahua Chen, Zhenguo Shen, and Chen Chen. 2022. "Comparative Genomics of Three Aspergillus Strains Reveals Insights into Endophytic Lifestyle and Endophyte-Induced Plant Growth Promotion" Journal of Fungi 8, no. 7: 690. https://doi.org/10.3390/jof8070690
APA StyleJing, M., Xu, X., Peng, J., Li, C., Zhang, H., Lian, C., Chen, Y., Shen, Z., & Chen, C. (2022). Comparative Genomics of Three Aspergillus Strains Reveals Insights into Endophytic Lifestyle and Endophyte-Induced Plant Growth Promotion. Journal of Fungi, 8(7), 690. https://doi.org/10.3390/jof8070690









