Effects of Volatile Organic Compounds Produced by Pseudomonas aurantiaca ST-TJ4 against Verticillium dahliae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Growth Conditions
2.2. Effects of ST-TJ4 VOCs on V. dahliae Radial Growth
2.3. Effects of ST-TJ4 VOCs on V. dahliae Mycelial Biomass
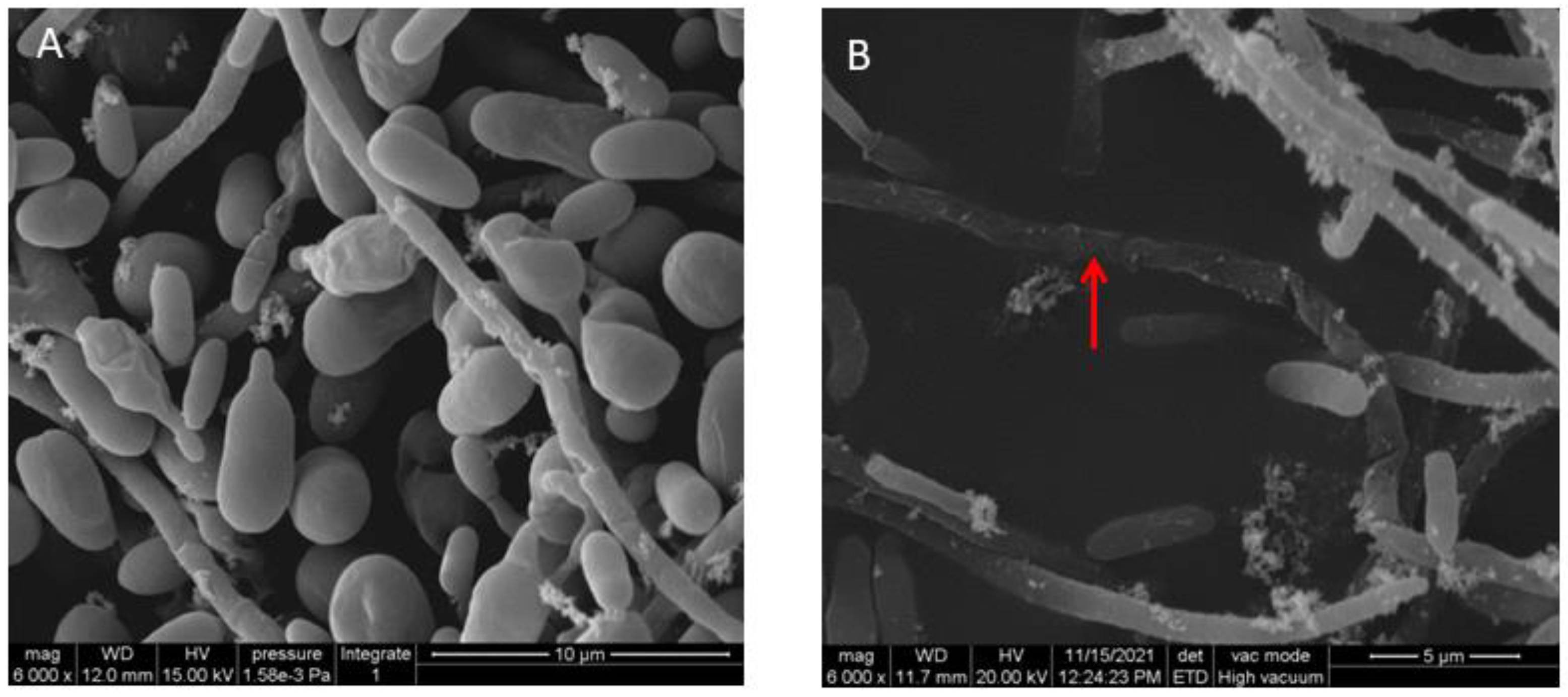
2.4. Scanning Electron Microscopy (SEM)
2.5. Effects of ST-TJ4 VOCs on V. dahliae Conidia Germination
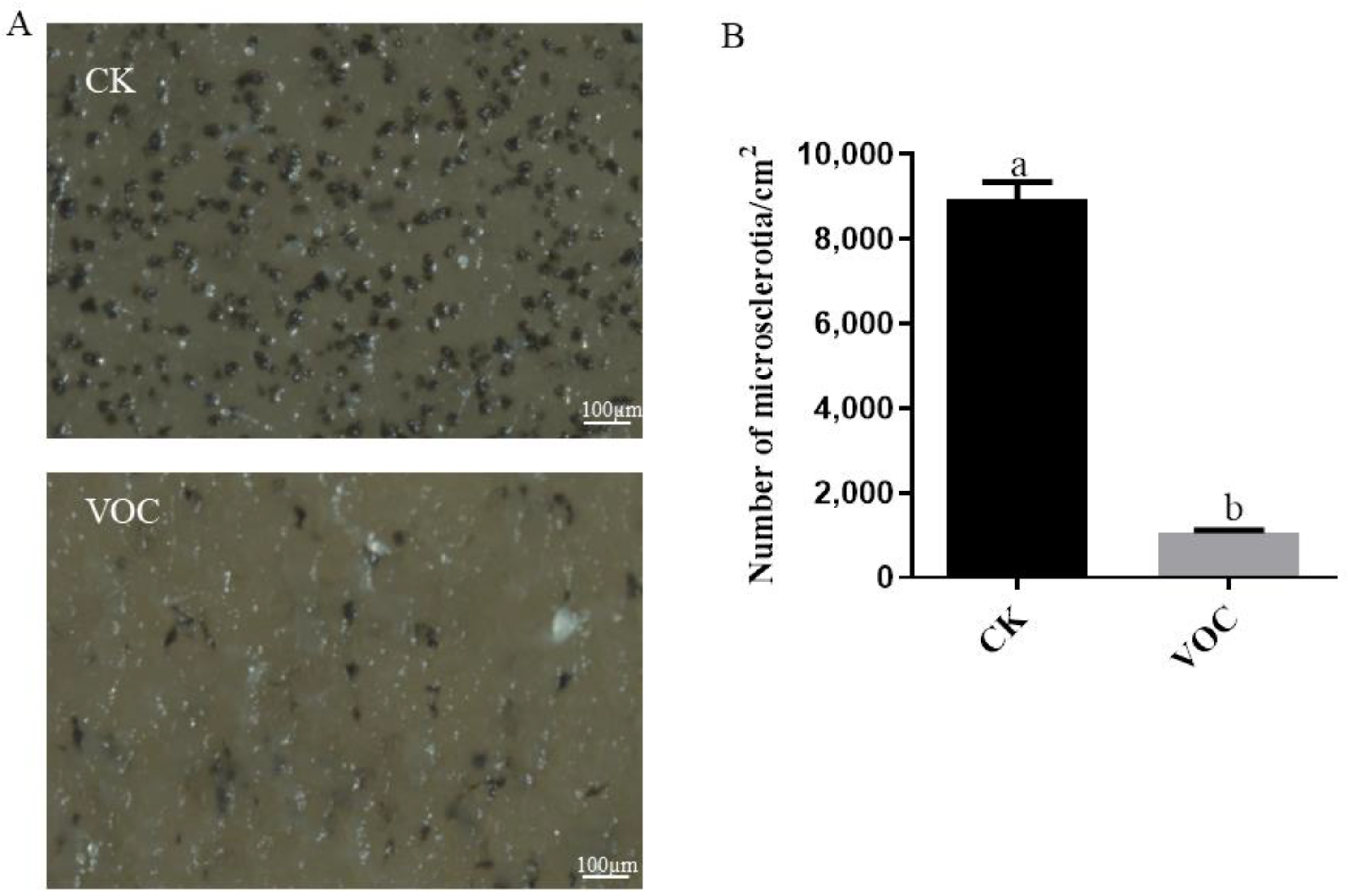
2.6. Effects of ST-TJ4 VOCs on V. dahliae Microsclerotia Formation
2.7. Effects of ST-TJ4 VOCs on V. dahliae Melanin Genes Expression
2.8. Effects of ST-TJ4 VOCs on V. dahliae Melanin Production
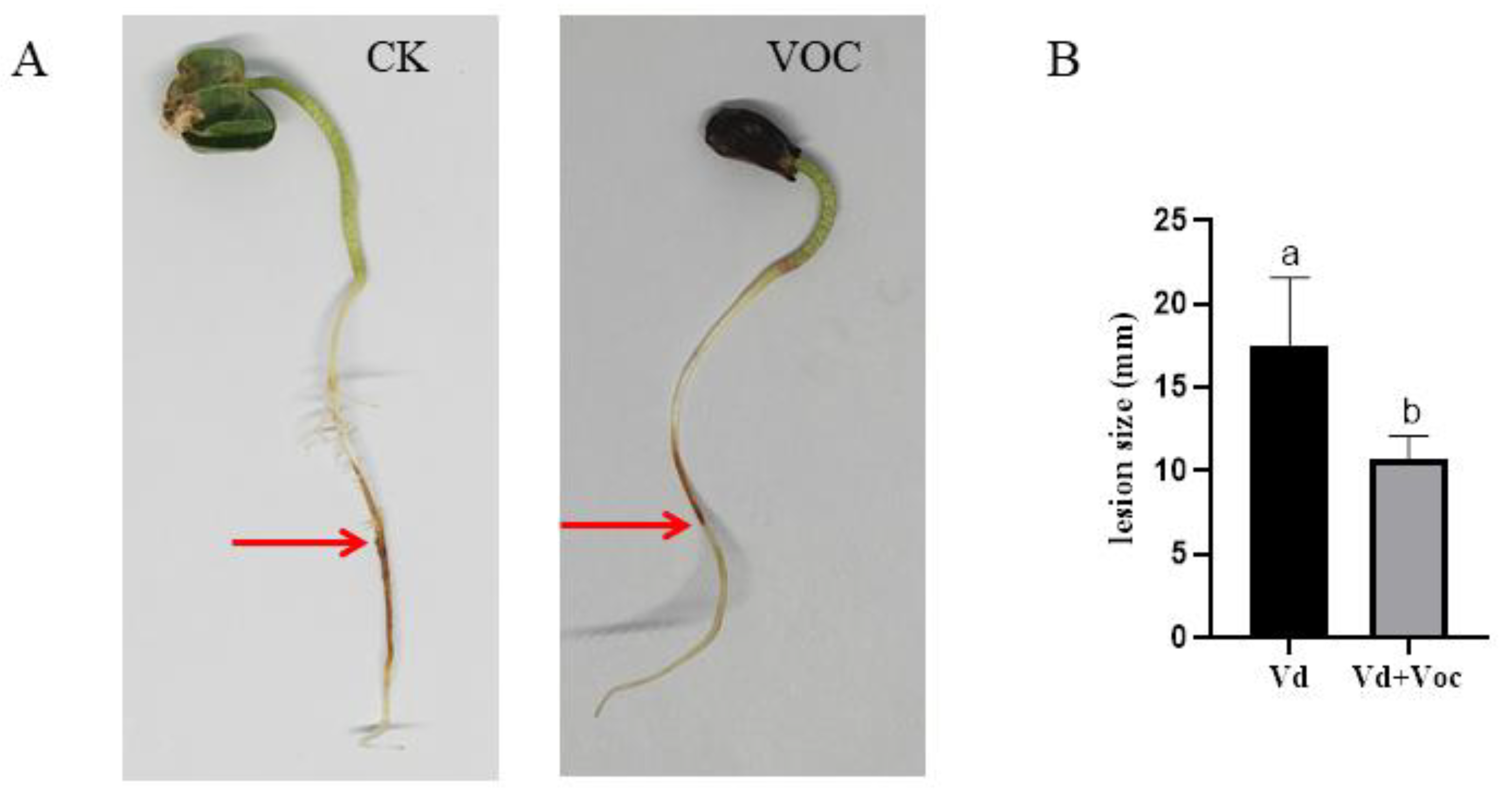
2.9. Effects of ST-TJ4 VOCs on V. dahliae Inoculated Cotton Seedlings
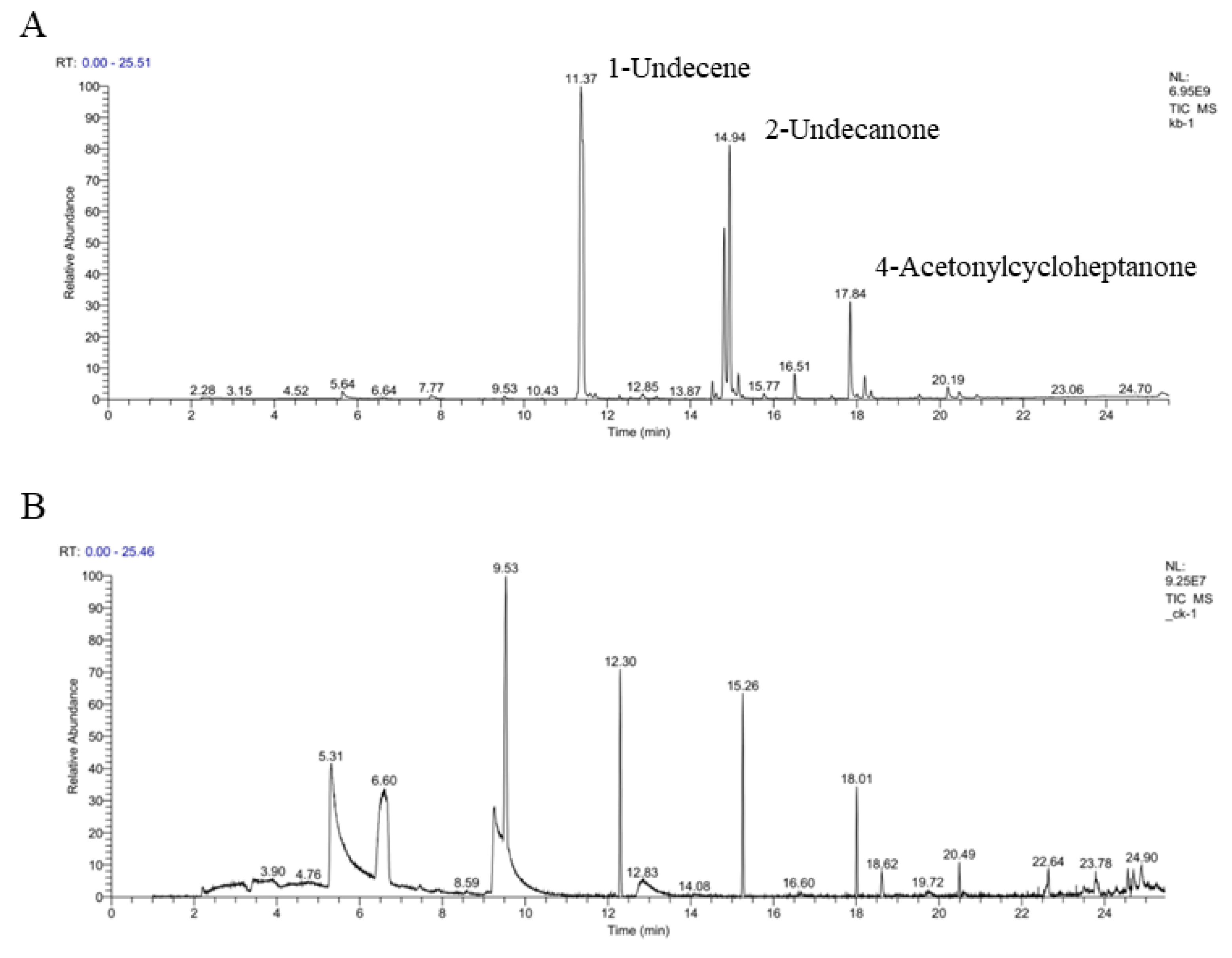
2.10. Identification of VOCs by GC/MS Analysis
2.11. Effects of Synthetic VOCs on V. dahliae Radial Growth
2.12. Statistical Analysis
3. Results
3.1. Effect of VOCs Produced by P. aurantiaca ST-TJ4 on V. dahliae Growth
3.2. Effect of VOCs Produced by P. aurantiaca ST-TJ4 on V. dahliae Hyphae Morphology
3.3. Effect of VOCs Produced by P. aurantiaca ST-TJ4 on V. dahliae Spore Germination
3.4. Effect of VOCs Produced by P. aurantiaca ST-TJ4 on V. dahliae Microsclerotia Formation
3.5. Effects of ST-TJ4 VOCs on V. dahliae Melanin Formation
3.6. Inhibition by the VOCs Produced by ST-TJ4 Strain of Infection by V. dahliae
3.7. Identification of VOCs by GC/MS Analysis
3.8. Effects of Synthetic VOCs on V. dahliae Radial Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, Pathogenicity, and Management of Verticillium Species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawagishi, H.; Finkel, T. Unraveling the Truth about Antioxidants: ROS and disease: Finding the right balance. Nat. Med. 2014, 20, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Li, P.T.; Wang, M.; Lu, Q.W.; Ge, Q.; Rashid, M.; Liu, A.Y.; Gong, J.W.; Shang, H.H.; Gong, W.K.; Li, J.W.; et al. Comparative transcriptome analysis of cotton fiber development of Upland cotton (Gossypium hirsutum) and Chromosome Segment Sub-stitution Lines from G. hirsutum × G. barbadense. Bmc Genom. 2017, 18, 705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Tao, Y.; Zhao, S.; Yin, X.; Chen, J.; Wang, M.; Cai, Y.; Niu, Q. A novel peroxiredoxin from the antagonistic endophytic bacterium Enterobacter sp. V1 contributes to cotton resistance against Verticillium dahliae. Plant Soil 2020, 454, 395–409. [Google Scholar] [CrossRef]
- Zhang, T.J.; Yu, L.X.; McCord, P.; Miller, D.; Bhamidimarri, S.; Johnson, D.; Monteros, M.J.; Ho, J.; Reisen, P.; Samac, D.A. Iden-tification of Molecular Markers Associated with Verticillium Wilt Resistance in Alfalfa (Medicago sativa L.) Using High-Resolution Melting. PLoS ONE 2014, 9, e115953. [Google Scholar] [CrossRef]
- Long, L.; Zhao, J.-R.; Xu, F.-C.; Yang, W.-W.; Liao, P.; Gao, Y.; Gao, W.; Song, C.-P. Silencing of GbANS reduces cotton resistance to Verticillium dahliae through decreased ROS scavenging during the pathogen invasion process. Plant Cell Tissue Organ Cult (PCTOC) 2018, 135, 213–221. [Google Scholar] [CrossRef]
- Li, C.-H.; Shi, L.; Han, Q.; Hu, H.-L.; Zhao, M.-W.; Tang, C.-M.; Li, S.-P. Biocontrol of verticillium wilt and colonization of cotton plants by an endophytic bacterial isolate. J. Appl. Microbiol. 2012, 113, 641–651. [Google Scholar] [CrossRef]
- Ji, S.H.; Paul, N.C.; Deng, J.X.; Kim, Y.S.; Yun, B.S.; Yu, S.H. Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant diseases. Mycobiology 2013, 41, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Schöller, C.E.; Gürtler, H.; Pedersen, R.; Molin, S.; Wilkins, K. Volatile metabolites from Actinomycetes. J. Agric. Food Chem. 2002, 50, 2615. [Google Scholar] [CrossRef]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef]
- Splivallo, R.; Bossi, S.; Maffei, M.; Bonfante, P. Discrimination of truffle fruiting body versus mycelial aromas by stir bar sorptive extraction. Phytochemistry 2007, 68, 2584–2598. [Google Scholar] [CrossRef]
- Zhou, J.; Dai, C.; Chen, Y.; Yang, T. Ecological functions of volatiles produced by plant growth-promoting micro-organisms. Acta Autom. Sin. 2013, 21, 141. [Google Scholar] [CrossRef]
- Xie, S.; Liu, J.; Gu, S.; Chen, X.; Jiang, H.; Ding, T. Antifungal activity of volatile compounds produced by endophytic Bacillus subtilis DZSY21 against Curvularia lunata. Ann. Microbiol. 2020, 70, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Liu, Y.; Li, X.; Zhang, C.; Feng, Z.; Peng, X.; Li, Z.; Qin, S.; Xing, K. Volatile Organic Compounds Produced by Pseudomonas chlororaphis subsp. aureofaciens SPS-41 as Biological Fumigants To Control Ceratocystis fimbriata in Postharvest Sweet Potatoes. J. Agric. Food. Chem. 2019, 67, 3702–3710. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Qiao, X.; Li, Z.; Li, F.; Chen, M.; Wang, Y.; Huang, Y.; Cui, H. Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiol. Lett. 2013, 341, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.-L.; Li, P.-S.; Wu, X.-Q.; Wu, T.-Y.; Sun, X.-R. Forest Tree Associated Bacterial Diffusible and Volatile Organic Compounds against Various Phytopathogenic Fungi. Microorganisms 2020, 8, 590. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, K.; Nielsen, P. Diversity of Pseudomonas strains isolated with King’s B and Gould’s S1 agar determined by repetitive extragenic palindromic-polymerase chain reaction, 16S rDNA sequencing and Fourier transform infrared spectroscopy char-acterisation. FEMS Microbiol. Lett. 1999, 173, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhou, L.; Wang, L.; Zhao, X.; Liang, L.; Chen, F. Wilt of Shantung Maple Caused by Verticillium dahliae in China. Plant Dis. 2018, 102, 249. [Google Scholar] [CrossRef]
- Kong, W.-L.; Rui, L.; Ni, H.; Wu, X.-Q. Antifungal Effects of Volatile Organic Compounds Produced by Rahnella aquatilis JZ-GX1 against Colletotrichum gloeosporioides in Liriodendron chinense × tulipifera. Front. Microbiol. 2020, 11, 1114. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, S.; Yang, Y.; Zhang, J.; Zhao, D.; Pan, Y.; Fan, S.; Yang, Z.; Zhu, J. Antifungal Effects of Volatiles Produced by Bacillus subtilis against Alternaria solani in Potato. Front. Microbiol. 2020, 11, 1196. [Google Scholar] [CrossRef]
- Fang, Y.L.; Xia, L.M.; Wang, P.; Zhu, L.H.; Ye, J.R.; Huang, L. The MAPKKK CgMck1 Is Required for Cell Wall Integrity, Appressorium Development, and Pathogenicity in Colletotrichum gloeosporioides. Genes 2018, 9, 543. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Guo, Q.G.; Su, Z.H.; Dong, L.H.; Wang, P.P.; Zhang, X.Y.; Lu, X.Y.; Zhao, W.S.; Qu, Y.H.; Li, S.Z.; et al. Effect of fengycin produced by Bacillus subtilis NCD-2 on the conidial germinationand microsclerotia formation of Verticillium dahliae. Acta. Phytopathol. Sin. 2020, 50, 739–747. [Google Scholar]
- Li, Z.; Ding, B.; Zhou, X.; Wang, G.-L. The Rice Dynamin-Related Protein OsDRP1E Negatively Regulates Programmed Cell Death by Controlling the Release of Cytochrome c from Mitochondria. PLoS Pathog. 2017, 13, e1006157. [Google Scholar] [CrossRef] [Green Version]
- Su, C.L.; Kang, H.; Mei, Z.D.; Yang, S.Y.; Liu, X.M.; Pu, J.J.; Zhang, H. Expression analysis of pathogenic genes during the infection of Colletotrichum gloeosporioides to mango, (in Chinese). Biotechnol. Bull. 2018, 34, 182–186. [Google Scholar]
- Bashyal, B.M.; Chand, R.; Kushwaha, C.; Sen, D.; Prasad, L.C.; Joshi, A.K. Association of melanin content with conidiogenesis in Bipolaris sorokiniana of barley (Hordeum vulgare L.). World J. Microbiol. Biotechnol. 2009, 26, 309–316. [Google Scholar] [CrossRef]
- Cao, Z.Y.; Dong, J.G.; Yang, S.Y.; Yao, X.X. Characteristics and spectral analysis of melanin in Setosphaeria turcica. Acta Phyto-Pathol. Sin. 2007, 37, 410–417. [Google Scholar]
- Zhou, J.L.; Feng, Z.L.; Feng, H.J.; Li, Y.Q.; Yuan, Y.; Li, Z.F.; Wei, F.; Shi, Y.Q.; Zhao, L.H.; Sun, Z.X.; et al. Biocontrol effect and mechanism of cotton endophytic bacterium Bacillus cereus YUPP-10 against verticillium wilt in Gossypium hirsutum. Sci. Agric. Sin. 2017, 50, 2717–2727. [Google Scholar]
- Han, X.; Shen, D.X.; Xiong, Q.; Bao, B.H.; Zhang, W.B.; Dai, T.T.; Zhao, Y.J.; Borriss, R.; Fan, B. The Plant-Beneficial Rhizo-bacterium Bacillus velezensis FZB42 Controls the Soybean Pathogen Phytophthora sojae Due to Bacilysin Production. Appl. Environ. Microb. 2021, 87, e01601-21. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef]
- McCormick, A.C.; Unsicker, S.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef]
- Mari, M.; Bautista-Baños, S.; Sivakumar, D. Decay control in the postharvest system: Role of microbial and plant volatile or-ganic compounds. Postharvest Biol. Technol. 2016, 122, 70–81. [Google Scholar] [CrossRef]
- Zhong, T.; Wang, Z.; Zhang, M.; Wei, X.; Kan, J.; Zalán, Z.; Wang, K.; Du, M. Volatile organic compounds produced by Pseudomonas fluorescens ZX as potential biological fumigants against gray mold on postharvest grapes. Biol. Control 2021, 163, 104754. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef] [Green Version]
- Gu, Q.; Yang, Y.; Yuan, Q.; Shi, G.; Wu, L.; Lou, Z.; Huo, R.; Wu, H.; Borriss, R.; Gao, X. Bacillomycin D Produced by Bacillus amyloliquefaciens Is Involved in the Antagonistic Interaction with the Plant-Pathogenic Fungus Fusarium graminearum. Appl. Environ. Microbiol. 2017, 83, e01075-17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, T.; Xu, M.; Guo, J.; Zhang, C.; Feng, Z.; Peng, X.; Li, Z.; Xing, K.; Qin, S. Antifungal effect of volatile organic compounds produced by Pseudomonas chlororaphis subsp. aureofaciens SPS-41 on oxidative stress and mitochondrial dysfunction of Ceratocystis fimbriata. Pestic. Biochem. Physiol. 2021, 173, 104777. [Google Scholar] [CrossRef]
- Duressa, D.; Anchieta, A.; Chen, D.; Klimes, A.; Garcia-Pedrajas, M.D.; Dobinson, K.F.; Klosterman, S.J. RNA-seq analyses of gene expression in the microsclerotia of Verticillium dahliae. BMC Genom. 2013, 14, 607. [Google Scholar] [CrossRef] [Green Version]
- Henson, J.M.; Butler, M.J.; Day, A.W. THE DARK SIDE OF THE MYCELIUM: Melanins of Phytopathogenic Fungi. Annu. Rev. Phytopathol. 1999, 37, 447–471. [Google Scholar] [CrossRef]
- Nosanchuk, J.D.; Casadevall, A. The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 2003, 5, 203–223. [Google Scholar] [CrossRef]
- Howard, R.J.; Valent, B. BREAKING AND ENTERING: Host Penetration by the Fungal Rice Blast Pathogen Magnaporthe grisea. Annu. Rev. Microbiol. 1996, 50, 491–512. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Fang, Y.; Anchieta, A.; Goldman, P.H.; Hernandez, G.; Klosterman, S.J. Transcription factor VdCmr1 is required for pigment production, protection from UV irradiation, and regulates expression of melanin biosynthetic genes in Verticillium dahliae. Microbiology 2018, 164, 685. [Google Scholar] [CrossRef]
- Antonopoulos, D.F.; Tjamos, S.E.; Antoniou, P.P.; Rafeletos, P.; Tjamos, E.C. Effect of Paenibacillus alvei, strain K165, on the germination of Verticillium dahliae microsclerotia in planta. Biol. Control 2008, 46, 166–170. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.Q.; Ye, Y.H.; Tang, C.M. Antifungal activity of chaetoviridin A from Chaetomium globosum CEF-082 me-tabolites against Verticillium dahliae in cotton. Mol. Plant-Microbe Interact. MPMI 2021, 34, 758–769. [Google Scholar] [CrossRef]
- Yu, D.; Fang, Y.; Tang, C.; Klosterman, S.J.; Tian, C.; Wang, Y. Genomewide Transcriptome Profiles Reveal How Bacillus subtilis Lipopeptides Inhibit Microsclerotia Formation in Verticillium dahliae. Mol. Plant Microbe Interact. 2019, 32, 622–634. [Google Scholar] [CrossRef]
- Chang, P.-K.; Cary, J.W.; Lebar, M.D. Biosynthesis of conidial and sclerotial pigments in Aspergillus species. Appl. Microbiol. Biotechnol. 2020, 104, 2277–2286. [Google Scholar] [CrossRef]
- A Farag, M.; Song, G.C.; Park, Y.-S.; Audrain, B.; Lee, S.; Ghigo, J.-M.; Kloepper, J.W.; Ryu, C.-M. Biological and chemical strategies for exploring inter- and intra-kingdom communication mediated via bacterial volatile signals. Nat. Protoc. 2017, 12, 1359–1377. [Google Scholar] [CrossRef]
- Romoli, R.; Papaleo, M.C.; de Pascale, D.; Tutino, M.L.; Michaud, L.; LoGiudice, A.; Fani, R.; Bartolucci, G. Characterization of the volatile profile of Antarctic bacteria by using solid-phase microextraction-gas chromatography-mass spectrometry. Biol. Mass Spectrom. 2011, 46, 1051–1059. [Google Scholar] [CrossRef]
- Ghader, M.; Shokoufi, N.; Es-Haghi, A.; Kargosha, K. Headspace solid-phase microextraction (HS-SPME) combined with GC–MS as a process analytical technology (PAT) tool for monitoring the cultivation of C. tetani. J. Chromatogr. B 2018, 1083, 222–232. [Google Scholar] [CrossRef]
- Castillo, M.; Silva, E.D.; Cmara, J.S.; Khadem, M. Molecular Identification and VOMs Characterization of Saccharomyces cere-visiae Strains Isolated from Madeira Region Winery Environments. Processes 2020, 8, 1058. [Google Scholar] [CrossRef]
- Báez-Vallejo, N.; Camarena-Pozos, D.A.; Monribot-Villanueva, J.L.; Ramírez-Vázquez, M.; Carrión-Villarnovo, G.L.; Guerrero-Analco, J.A.; Partida-Martínez, L.P.; Reverchon, F. Forest tree associated bacteria for potential biological control of Fusarium solani and of Fusarium kuroshium, causal agent of Fusarium dieback. Microbiol. Res. 2020, 235, 126440. [Google Scholar] [CrossRef]




| Primer | Sequence (5′–3′) | Gene Name |
|---|---|---|
| VDAG-00183-F | GAAGGGTGTGACTGTCAATG | VdT3HR |
| VDAG-00183-R | TTGATCCACTCGCAGTCTTC | |
| VDAG-00190-F | CTTGACGACGTGAACGTTAC | VdPKS |
| VDAG-00190-R | GCTGTCTCAACACAGAGTTC | |
| VDAG-02273-F | CTATTGCGACGATTGCTCTG | VdHYD |
| VDAG-02273-R | AACGGCCAGACCAAGAATAC | |
| VDAG-03393-F | GAGTTCCTCGCCATGATCTC | VdSCD |
| VDAG-03393-R | TACTCGCTCCAACGAATCTC | |
| VDAG-03665-F | AGGTCGTACAAGCCATCAAG | VdT4HR |
| VDAG-03665-R | CTCGCGTGTTGATGTTGAAG | |
| β-tubulin-F | TTTCCAGATCACCCACTCC | Reference gene |
| β-tubulin-R | ACGACCGAGAAGGTAGCC |
| Retention Time (min) | Relative Peak Area (%) | CAS# | Compound |
|---|---|---|---|
| 7.77 | 0.56 ± 0.02 | 5874-90-8 | 2-Heptanone |
| 5.64 | 0.7 ± 0.04 | 1066-42-8 | Silanediol, dimethyl- |
| 7.77 | 0.56 ± 0.02 | 110-43-0 | 2-Heptanone |
| 11.73 | 46.26 ± 2.19 | 821-95-4 | 1-Undecene |
| 12.85 | 0.47 ± 0.15 | 143-08-8 | 1-Nonanol |
| 14.53 | 1.1 ± 0.06 | NA | 5-Isopropenyl-2-methyl-7-oxabicyclo [4.1.0]heptan-2-ol |
| 14.62 | 0.35 | NA | E-1,6-Undecadiene |
| 14.81 | 13.76 ± 0.65 | 86428-60-6 | 4-Acetonylcycloheptanone |
| 14.94 | 20.82 ± 0.62 | 112-12-9 | 2-Undecanone |
| 15.16 | 1.32 ± 0.12 | 143-13-5 | Acetic acid, nonyl ester |
| 15.77 | 0.41 ± 0.03 | 53447-47-5 | Lilac aldehyde D |
| 16.51 | 1.74 ± 0.38 | 830-13-7 | Cyclododecanone |
| 17.84 | 7.27 ± 0.4 | 81634-99-3 | 3-Decen-1-ol, acetate, (Z)- |
| 18.19 | 1.61 ± 0.15 | 593-08-8 | 2-Tridecanone |
| 18.34 | 0.46 ± 0.03 | 4704-31-8 | Vinyl decanoate |
| 19.5 | 0.31 ± 0.12 | 16778-27-1 | 2(3H)-Benzofuranone, hexahydro-4,4,7a-trimethyl- |
| 20.19 | 0.98 ± 0.07 | 56600-19-2 | 6-Octadecynenitrile |
| 20.46 | 0.55 ± 0.04 | 88592-11-4 | Hexadecenenitrile |
| 20.89 | 0.33 ± 0.02 | 502-72-7 | Cyclopentadecanone |
| 25.33 | 0.81 ± 0.21 | 630-01-3 | Hexacosane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, H.; Kong, W.-L.; Zhang, Y.; Wu, X.-Q. Effects of Volatile Organic Compounds Produced by Pseudomonas aurantiaca ST-TJ4 against Verticillium dahliae. J. Fungi 2022, 8, 697. https://doi.org/10.3390/jof8070697
Ni H, Kong W-L, Zhang Y, Wu X-Q. Effects of Volatile Organic Compounds Produced by Pseudomonas aurantiaca ST-TJ4 against Verticillium dahliae. Journal of Fungi. 2022; 8(7):697. https://doi.org/10.3390/jof8070697
Chicago/Turabian StyleNi, Hang, Wei-Liang Kong, Yu Zhang, and Xiao-Qin Wu. 2022. "Effects of Volatile Organic Compounds Produced by Pseudomonas aurantiaca ST-TJ4 against Verticillium dahliae" Journal of Fungi 8, no. 7: 697. https://doi.org/10.3390/jof8070697
APA StyleNi, H., Kong, W.-L., Zhang, Y., & Wu, X.-Q. (2022). Effects of Volatile Organic Compounds Produced by Pseudomonas aurantiaca ST-TJ4 against Verticillium dahliae. Journal of Fungi, 8(7), 697. https://doi.org/10.3390/jof8070697






