Effect of Oligogalacturonides on Seed Germination and Disease Resistance of Sugar Beet Seedling and Root
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of OGs
2.2. Plant Materials
2.3. Fungal Pathogens
2.4. The Effect of OGs on Mycelial Growth of R. solani AG-4HGI
2.5. Assessment of OGs Effect on the Germination of Sugar Beet Seeds
2.6. Ability of OGs to Prevent Sugar Beet Seedling Damping-Off Caused by R. solani AG-4HGI
2.7. Ability of OGs to Prevent Sugar Beet Root Rot Caused by R. solani AG-4HGI
2.8. Effect of OGs on the Expression of Defense-Related Genes in Sugar Beet Roots
2.9. Data Analysis
3. Results and Discussions
3.1. Preparation and Characterization of OGs
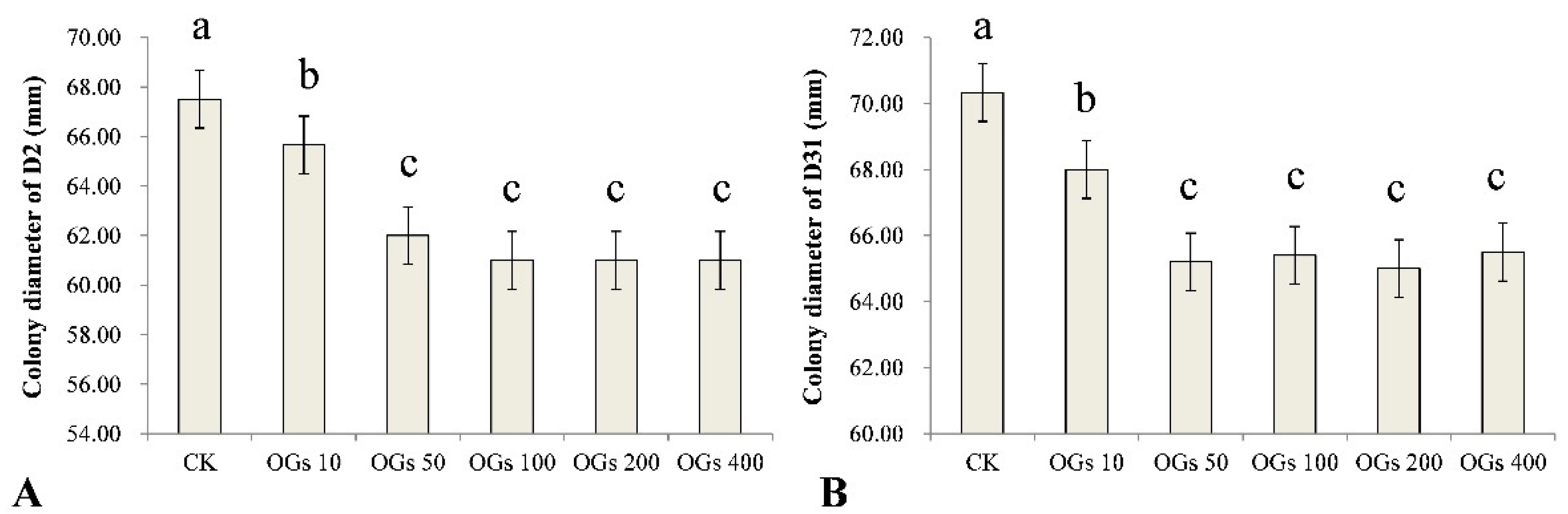
3.2. Inhibitory Effect of OGs on Mycelial Growth of R. solani AG-4HGI
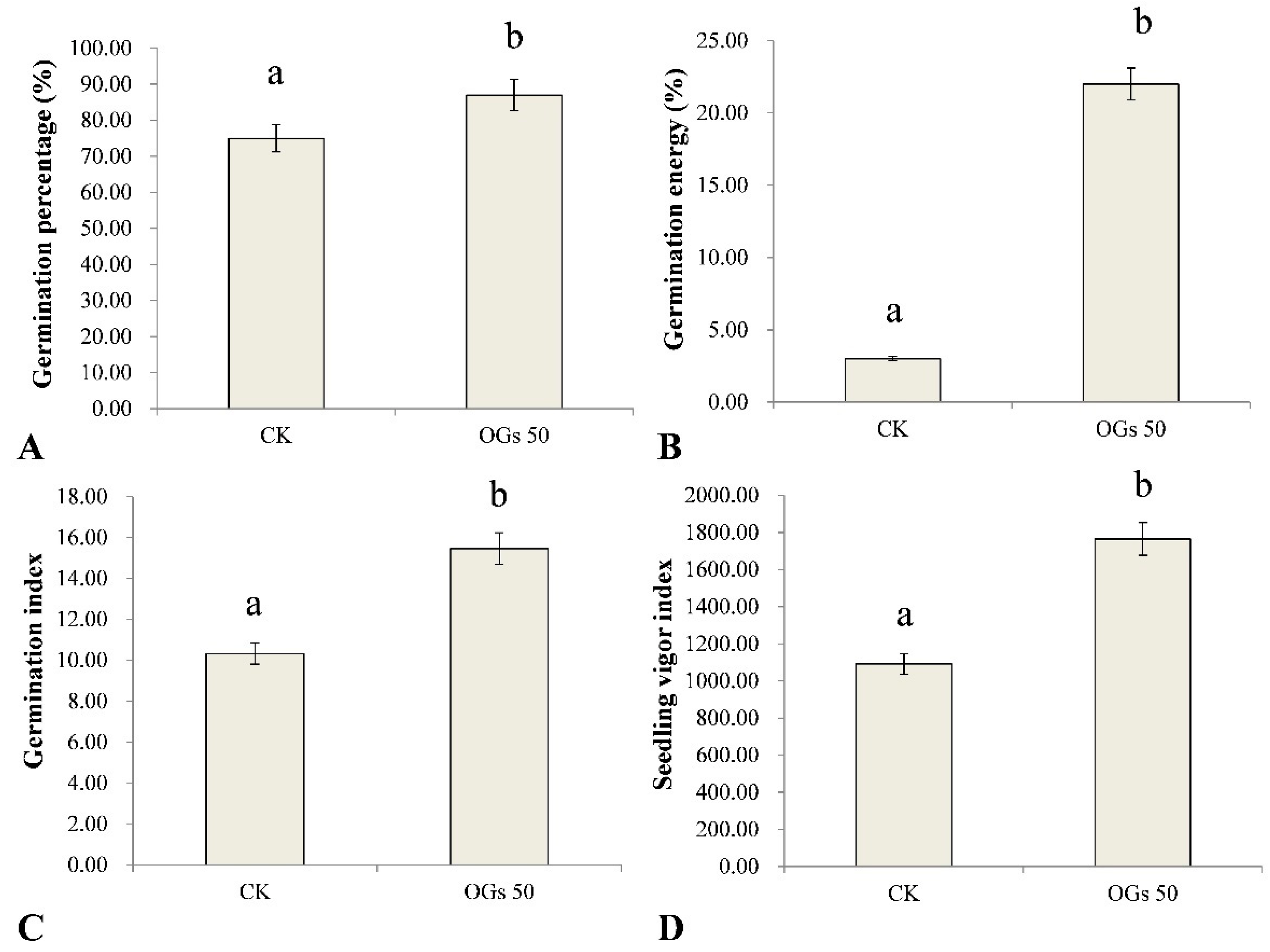
3.3. Effect of OGs on Sugar Beet Seed Germination
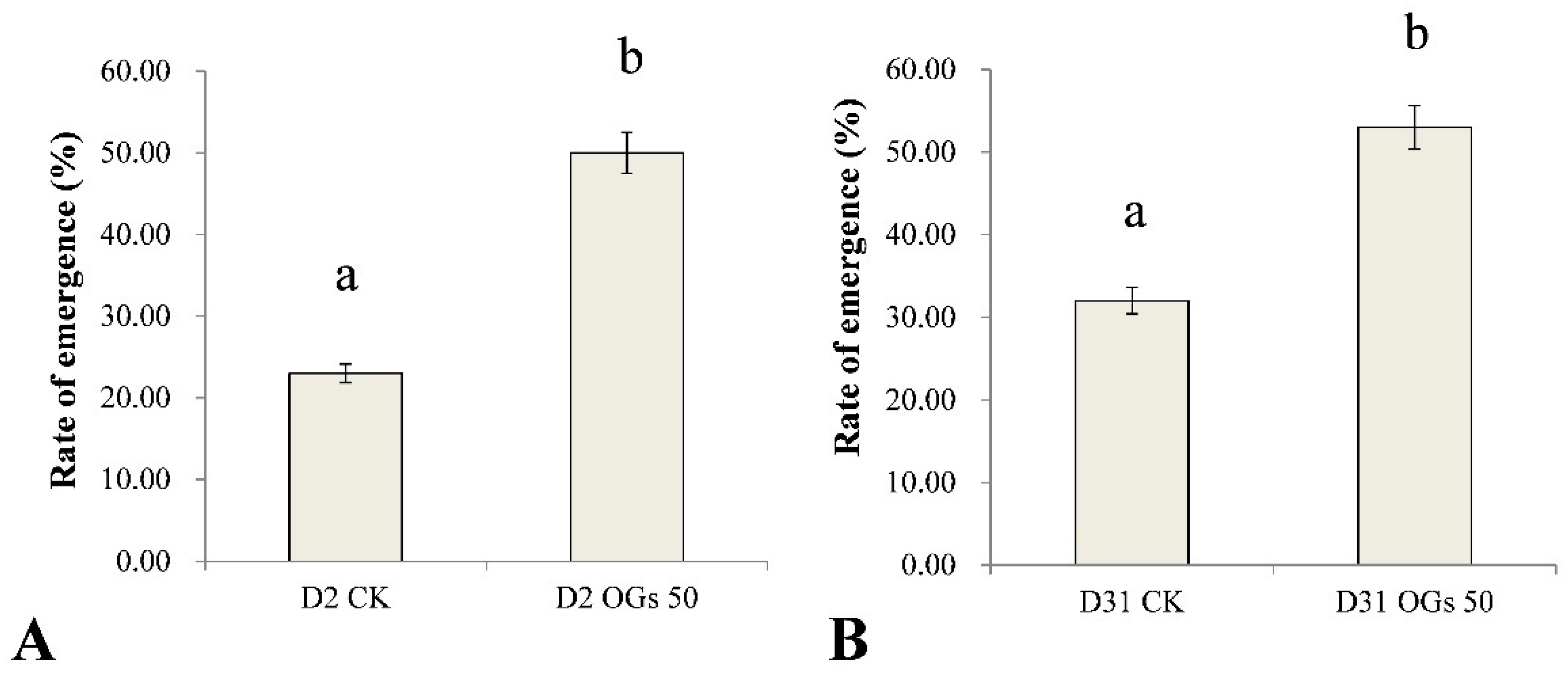
3.4. Ability of OGs to Prevent Sugar Beet Damping-Off and Root Rot Caused by R. solani AG-4HGI
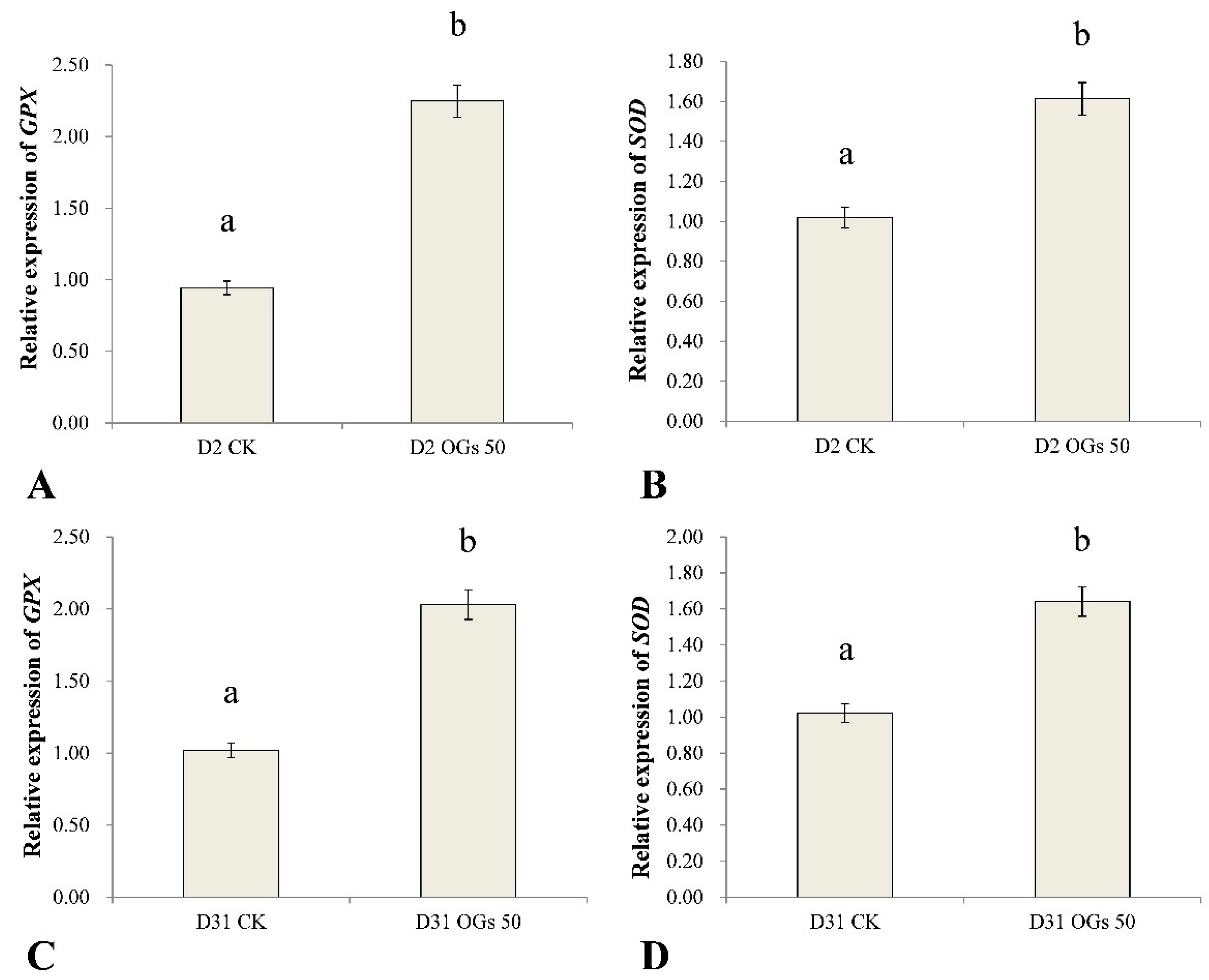
3.5. The Effect of OGs on Defense-Related Gene Expression in Sugar Beet Roots
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Combo, A.M.M.; Aguedo, M.; Quievy, N.; Danthine, S.; Goffin, D.; Jacquet, N.; Blecker, C.; Devaux, J.; Paquot, M. Characterization of sugar beet pectic-derived oligosaccharides obtained by enzymatic hydrolysis. Int. J. Biol. Macromol. 2013, 52, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Elboutachfaiti, R.; Delattre, C.; Michaud, P.; Courtois, B.; Courtois, J. Oligogalacturonans production by free radical depolymerization of polygalacturonan. Int. J. Biol. Macromol. 2008, 43, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Savatin, D.V.; Sicilia, F.; Gramegna, G.; Cervone, F.; De Lorenzo, G. Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 2013, 4, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, S.; Galletti, R.; Denoux, C.; De Lorenzo, G.; Ausubel, F.M.; Dewdney, J. Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol. 2007, 144, 367–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallarino, J.G.; Osorio, S. Signaling role of oligogalacturonides derived during cell wall degradation. Plant Signal. Behav. 2012, 7, 1447–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiro, M.D.; Bowers, J.F.; Cosgrove, D.J. A comparison of oligogalacturonide- and auxin-induced extracellular alkalinization and growth responses in roots of intact cucumber seedlings. Plant Physiol. 2002, 130, 895–903. [Google Scholar] [CrossRef] [Green Version]
- Camejo, D.; Martí, M.C.; Jiménez, A.; Cabrera, J.C.; Olmos, E.; Sevilla, F. Effect of oligogalacturonides on root length, extracellular alkalinization and O2-accumulation in alfalfa. J. Plant Physiol. 2011, 168, 566–575. [Google Scholar] [CrossRef]
- Moscatiello, R.; Baldan, B.; Squartini, A.; Mariani, P.; Navazio, L. Oligogalacturonides: Novel signaling molecules in rhizobium-legume communications. Mol. Plant-Microbe Interact. 2012, 25, 1387–1395. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.R.; Darvill, A.G.; Dell, A.A. Host-pathogen interactions: XXIX. oligogalacturonides released from sodium polypectate by endopolygalacturonic acid lyase are elicitors of phytoalexins in soybean. Plant Physiol. 1986, 80, 568–577. [Google Scholar] [CrossRef] [Green Version]
- Selim, S.; Sanssené, J.; Rossard, S.; Courtois, J. Systemic induction of the defensin and phytoalexin pisatin pathways in pea (Pisum sativum) against Aphanomyces euteiches by acetylated and nonacetylated oligogalacturonides. Molecules 2017, 22, 1017. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.R.; Hahlbrock, K. Induction of defense responses in cultured parsley cells by plant cell wall fragments. Plant Physiol. 1987, 84, 1286–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howlader, P.; Bose, S.K.; Jia, X.; Zhang, C.; Yin, H. Oligogalacturonides induce resistance in Arabidopsis thaliana by triggering salicylic acid and jasmonic acid pathways against Pst DC3000. Int. J. Biol. Macromol. 2020, 164, 4054–4064. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Pneumas, W.J. Pectic polysaccharides elicit chitinase accumulation in tobacco. Physiol. Plant. 1988, 74, 740–744. [Google Scholar] [CrossRef]
- Bellincampi, D.; Dipierro, N.; Salvi, G.; Cervone, F.; De Lorenzo, G. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolb gene expression in tobacco leaf explants. Plant Physiol. 2000, 122, 1379–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galletti, R.; Denoux, C.; Gambetta, S.; Dewdney, J.; Ausubel, F.M.; De Lorenzo, G.; Ferrari, S. The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Physiol. 2008, 148, 1695–1706. [Google Scholar] [CrossRef] [Green Version]
- Randoux, B.; Renard-Merlier, D.; Mulard, G.; Rossard, S.; Duyme, F.; Sanssene, J.; Courtois, J.; Durand, R.; Reignault, P. Distinct defenses induced in wheat against powdery mildew by acetylated and nonacetylated oligogalacturonides. Phytopathology 2010, 100, 1352–1363. [Google Scholar] [CrossRef] [Green Version]
- Rasul, S.; Dubreuil-Maurizi, C.; Lamotte, O.; Koen, E.; Jeandroz, S. Nitric oxide production mediates oligogalacturonide-triggered immunity and resistance to Botrytis cinerea in Arabidopsis thaliana. Plant Signal. Behav. 2014, 7, 1483–1499. [Google Scholar] [CrossRef]
- Rasul, S.; Wendehenne, D.; Jeandroz, S. Study of oligogalacturonides-triggered nitric oxide (NO) production provokes new questioning about the origin of NO biosynthesis in plants. Plant Signal. Behav. 2012, 7, 1031–1033. [Google Scholar] [CrossRef] [Green Version]
- Côté, F.; Hahn, M.G. Oligosaccharins: Structures and signal transduction. Plant Mol. Biol. 1994, 26, 1379–1411. [Google Scholar] [CrossRef]
- Davidsson, P.; Broberg, M.; Kariola, T.; Sipari, N.; Pirhonen, M.; Palva, E.T. Short oligogalacturonides induce pathogen resistance-associated gene expression in Arabidopsis thaliana. BMC Plant Biol. 2017, 17, 19. [Google Scholar] [CrossRef] [Green Version]
- Harveson, R.M.; Hanson, L.E.; Hein, G.L. Root diseases caused by fungi and oomycetes. In Compendium of Beet Diseases and Pests, 2nd ed.; American Phytopathological Society: St. Paul, MN, USA, 2009; pp. 21–24. [Google Scholar]
- O’Sullivan, E.; Kavanagn, J.A. Characteristics and pathogenicity of isolates of Rhizoctonia spp. associated with damping-off of sugar beet. Plant Pathol. 1991, 40, 128–135. [Google Scholar] [CrossRef]
- Rush, C.M.; Carling, D.E.; Harveson, R.M.; Mathieson, J.T. Prevalence and pathogenicity of anastomosis groups of Rhizoctonia solani from wheat and sugar beet in Texas. Plant Dis. 1994, 78, 349–352. [Google Scholar] [CrossRef]
- Strausbaugh, C.A.; Eujayl, I.A.; Panella, L.W.; Hanson, L.E. Virulence, distribution, and diversity of Rhizoctonia solani from sugar beet in Idaho and Oregon. Can. J. Plant Pathol. 2011, 33, 210–226. [Google Scholar] [CrossRef]
- Windels, C.E.; Nabben, D.J. Characterization and pathogenicity of anastomosis groups of Rhizoctonia solani isolated from Beta vulgaris. Phytopathology 1989, 79, 83–88. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.T.; Wu, S.Y.; Wang, P.P.; Han, C.G.; Wu, X.H. Anastomosis group and pathogenicity of Rhizoctonia spp. associated with seedling damping-off of sugar beet in China. Eur. J. Plant Pathol. 2019, 153, 869–878. [Google Scholar] [CrossRef]
- Scortica, A.; Capone, M.; Narzi, D.; Frezzini, M.; Scafati, V.; Giovannoni, M.; Angelucci, F.; Guidoni, L.; Mattei, B.; Benedetti, M. A molecular dynamics-guided mutagenesis identifies two aspartic acid residues involved in the pH-dependent activity of OG-OXIDASE 1. Plant Physiol. Biochem. 2021, 169, 171–182. [Google Scholar] [CrossRef]
- Ochoa-Meza, L.C.; Quintana-Obregón, E.A.; Vargas-Arispuro, I.; Falcón-Rodríguez, A.B.; Aispuro-Hernández, E.; Virgen-Ortiz, J.J.; Martínez-Téllez, M.Á. Oligosaccharins as elicitors of defense responses in wheat. Polymers 2021, 13, 3105. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Li, S.; Tan, H.D.; Li, K.K.; Chen, W.; Yin, H. A highly efficient biocatalytic conversion of renewable pectic polysaccharide biomass into bioactive oligogalacturonides. ACS Food Sci. Technol. 2021, 1, 338–346. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2003. [Google Scholar]
- Puglisi, I.; Barone, V.; Fragala, F.; Stevanato, P.; Baglieri, A.; Vitale, A. Effect of microalgal extracts from Chlorella vulgaris and Scenedesmus quadricauda on germination of Beta vulgaris seeds. Plants 2020, 9, 675. [Google Scholar] [CrossRef]
- Bolton, M.D.; Panella, L.; Campbell, L.; Khan, M.F.R. Temperature, moisture, and fungicide effects in managing Rhizoctonia root and crown rot of sugar beet. Phytopathology 2010, 100, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, X.F.; Kennedy, J.F.; Jiang, M.G.; Cai, Q.N.; Wu, X.H. Chitosan induces resistance to tuber rot in stored potato caused by Alternaria tenuissima. Int. J. Biol. Macromol. 2019, 140, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zuo, J.H.; Wang, Q.; Na, Y.; Gao, L.P. Inhibitory effect of chitosan on growth of the fungal phytopathogen, Sclerotinia sclerotiorum, and Sclerotinia rot of carrot. J. Integr. Agric. 2015, 14, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, S.; Zhao, X.; Du, Y.; Lin, B. Oligochitosan induces cell death and hydrogen peroxide accumulation in tobacco suspension cells. Pesticide Biochem. Physiol. 2008, 90, 106–113. [Google Scholar] [CrossRef]
- Hu, X.K.; Jiang, X.L.; Hwang, H.M.; Liu, S.L.; Guan, H.S. Promotive effects of alginate-derived oligosaccharide on maize seed germination. J. Appl. Phycol. 2004, 16, 73–76. [Google Scholar] [CrossRef]
- Udchumpisai, W.; Uttapap, D.; Wandee, Y.; Kotatha, D.; Rungsardthong, V. Promoting effect of pectic-oligosaccharides produced from pomelo peel on rice seed germination and early seedling growth. J. Plant Growth Regul. 2022. [CrossRef]
- Podlaski, S.; Chomontowski, C. Various methods of assessing sugar beet seed vigour and its impact on the germination process, field emergence and sugar yield. Sugar Tech. 2020, 22, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Ugena, L.; Hylova, A.; Podlesakova, K.; Humplik, J.F.; Dolezal, K.; De Diego, N.; Spichal, L. Characterization of biostimulant mode of action using novel multi-trait high-throughput screening of Arabidopsis germination and rosette growth. Front. Plant Sci. 2018, 9, 1327. [Google Scholar] [CrossRef] [Green Version]
- Osorio, S.; Castillejo, C.; Quesada, M.A.; Medina-Escobar, N.; Brownsey, G.J.; Suau, R.; Heredia, A.; Botella, M.A.; Valpuesta, V. Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca). Plant J. 2008, 54, 43–55. [Google Scholar] [CrossRef]
- Aziz, A.; Heyraud, A.; Lambert, B. Oligogalacturonide signal transduction, induction of defense-related responses and protection of grapevine against Botrytis cinerea. Planta 2004, 218, 767–774. [Google Scholar] [CrossRef]
- Camejo, D.; Marti, M.C.; Olmos, E.; Torres, W.; Sevilla, F.; Jimenez, A. Oligogalacturonides stimulate antioxidant system in alfalfa roots. Biol. Plantarum 2012, 56, 537–544. [Google Scholar] [CrossRef]


| Gene | GenBank Accession Number | Primer Sequence | Product Size (bp) |
|---|---|---|---|
| GPX | XM_019250362.1 | F: AGCAGGCCGTGTCACTTTR: TATGCCCGACGACCAATC | 193 |
| SOD | XM_010672327 | F: TGATTTGGCGTGGTTTCGR: ATCTTCACTGGGTTGGGCTAT | 153 |
| GAPDH | XM_010679634 | F: GCTCAACATCGTCCCTCTR: TCTCAGCCTCACATTTCTC | 118 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Wu, C.; Li, K.; Kennedy, J.F.; Wisniewski, M.; Gao, L.; Han, C.; Liu, J.; Yin, H.; Wu, X. Effect of Oligogalacturonides on Seed Germination and Disease Resistance of Sugar Beet Seedling and Root. J. Fungi 2022, 8, 716. https://doi.org/10.3390/jof8070716
Zhao C, Wu C, Li K, Kennedy JF, Wisniewski M, Gao L, Han C, Liu J, Yin H, Wu X. Effect of Oligogalacturonides on Seed Germination and Disease Resistance of Sugar Beet Seedling and Root. Journal of Fungi. 2022; 8(7):716. https://doi.org/10.3390/jof8070716
Chicago/Turabian StyleZhao, Can, Chunyan Wu, Kuikui Li, John F. Kennedy, Michael Wisniewski, Lihong Gao, Chenggui Han, Jia Liu, Heng Yin, and Xuehong Wu. 2022. "Effect of Oligogalacturonides on Seed Germination and Disease Resistance of Sugar Beet Seedling and Root" Journal of Fungi 8, no. 7: 716. https://doi.org/10.3390/jof8070716
APA StyleZhao, C., Wu, C., Li, K., Kennedy, J. F., Wisniewski, M., Gao, L., Han, C., Liu, J., Yin, H., & Wu, X. (2022). Effect of Oligogalacturonides on Seed Germination and Disease Resistance of Sugar Beet Seedling and Root. Journal of Fungi, 8(7), 716. https://doi.org/10.3390/jof8070716







