Cuticular Wax Modification by Epichloë Endophyte in Achnatherum inebrians under Different Soil Moisture Availability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Treatments
2.2. Extraction of Cuticular Wax
2.3. Chemical Analysis
2.4. RNA Preparation and Transcriptome Sequencing
2.5. Differentially Expressed Gene (DEG) Analysis
2.6. Statistical Analysis
3. Results
3.1. Composition and Proportion of Leaf Cuticular Wax
3.2. Carbon Chain Length of Wax Components
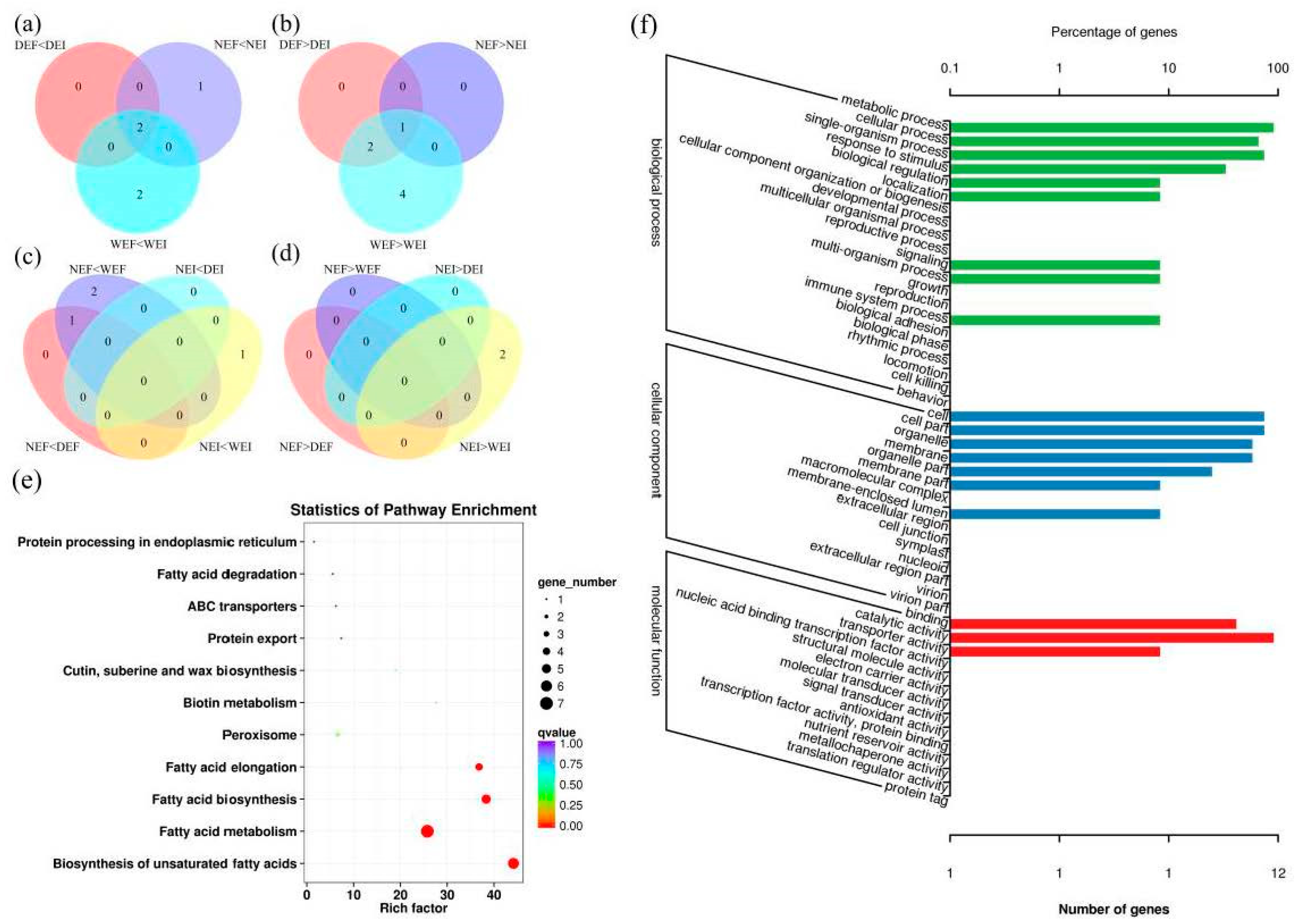
3.3. Differentially Expressed Gene (DEG) Analysis
3.4. KEGG Pathway Enrichment Analysis of the DEGs
3.5. GO Functional-Enrichment Analysis of the DEGs
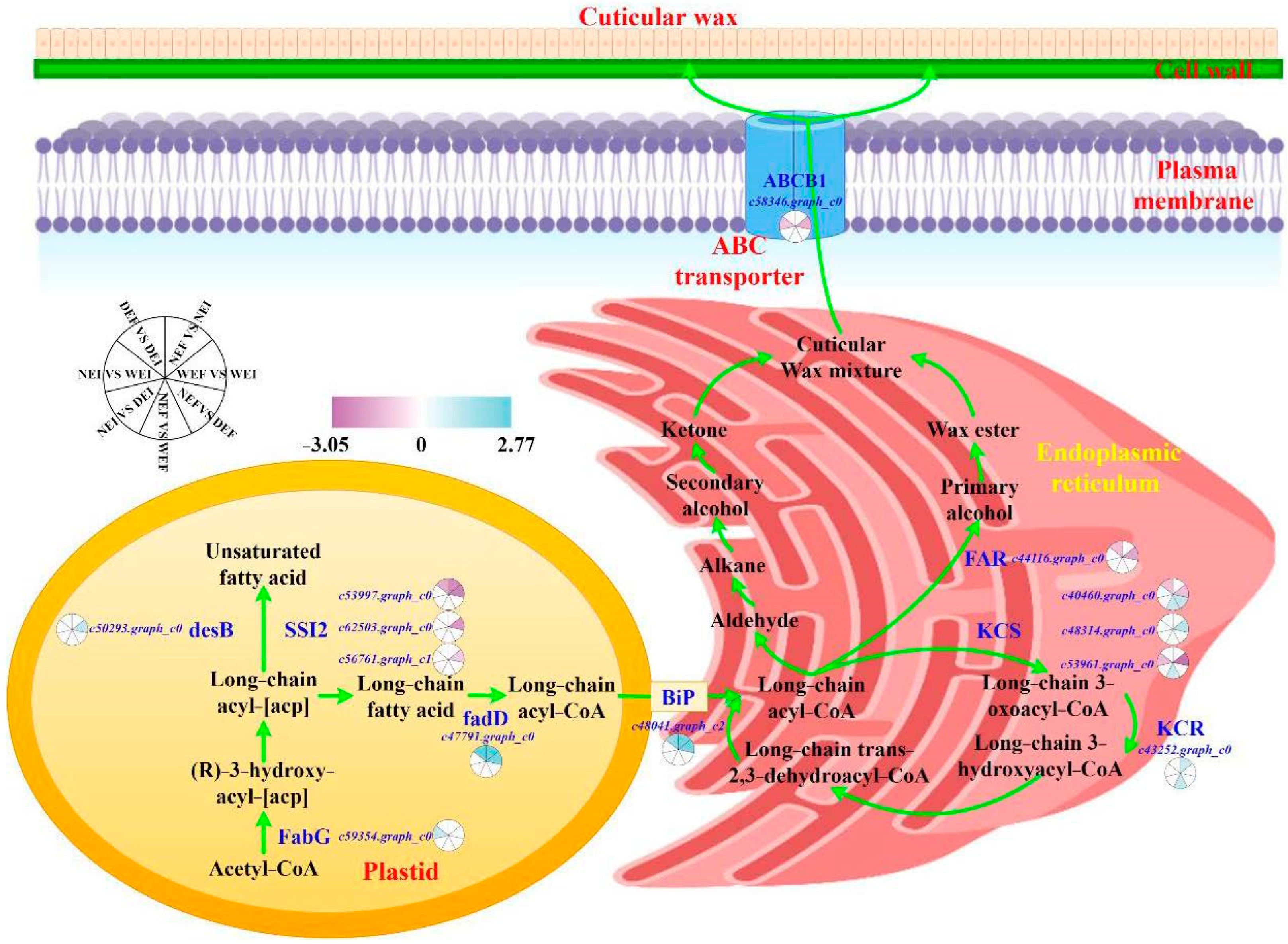
3.6. Biosynthesis Pathway of Cuticular Wax
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Ault, T.R. On the essentials of drought in a changing climate. Science 2020, 368, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Bastías, D.A.; Martinez-Ghersa, M.A.; Ballaré, C.L.; Gundel, P.E. Epichloë Fungal Endophytes and Plant Defenses: Not Just Alkaloids. Trends Plant Sci. 2017, 22, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef]
- Lowman, S.; Dura, S.; Mei, C.S.; Nowak, J. Strategies for enhancement of switchgrass (Panicum virgatum L.) performance under limited nitrogen supply based on utilization of N-fixing bacterial endophytes. Plant Soil 2016, 405, 47–63. [Google Scholar] [CrossRef]
- Johnson, L.J.; de Bonth, A.C.M.; Briggs, L.R.; Caradus, J.R.; Finch, S.C.; Fleetwood, D.J.; Fletcher, L.R.; Hume, D.E.; Johnson, R.D.; Popay, A.J.; et al. The exploitation of epichloae endophytes for agricultural benefit. Fungal Divers. 2013, 60, 171–188. [Google Scholar] [CrossRef]
- Kuldau, G.; Bacon, C. Clavicipitaceous endophytes: Their ability to enhance resistance of grasses to multiple stresses. Biol. Control 2008, 46, 57–71. [Google Scholar] [CrossRef]
- Leuchtmann, A.; Bacon, C.W.; Schardl, C.L.; White, J.F.; Tadych, M. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia 2014, 106, 202–215. [Google Scholar] [CrossRef]
- Li, C.J.; Nan, Z.B.; Paul, V.H.; Dapprich, P.D.; Liu, Y. A new Neotyphodium species symbiotic with drunken horse grass (Achnatherum inebrians) in China. Mycotaxon 2004, 90, 141–147. [Google Scholar]
- Chen, L.; Li, X.Z.; Li, C.J.; Swoboda, G.A.; Young, C.A.; Sugawara, K.; Leuchtmann, A.; Schardl, C.L. Two distinct Epichloë species symbiotic with Achnatherum inebrians, drunken horse grass. Mycologia 2015, 107, 863–873. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Zhang, X.X.; Christensen, M.J.; Nan, Z.B.; Li, C.J. Epichloë endophyte affects the ability of powdery mildew (Blumeria graminis) to colonise drunken horse grass (Achnatherum inebrians). Fungal Ecol. 2015, 16, 26–33. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.J.; Nan, Z.B.; Matthew, C. Neotyphodium endophyte increases Achnatherum inebrians (drunken horse grass) resistance to herbivores and seed predators. Weed Res. 2012, 52, 70–78. [Google Scholar] [CrossRef]
- Chen, N.; He, R.L.; Chai, Q.; Li, C.J.; Nan, Z.B. Transcriptomic analyses giving insights into molecular regulation mechanisms involved in cold tolerance by Epichloë endophyte in seed germination of Achnatherum inebrians. Plant Growth Regul. 2016, 80, 367–375. [Google Scholar] [CrossRef]
- Zhang, X.X.; Fan, X.M.; Li, C.J.; Nan, Z.B. Effects of cadmium stress on seed germination, seedling growth and antioxidative enzymes in Achnatherum inebrians plants infected with a Neotyphodium endophyte. Plant Growth Regul. 2010, 60, 91–97. [Google Scholar] [CrossRef]
- Xia, C.; Christensen, M.J.; Zhang, X.X.; Nan, Z.B. Effect of Epichloë gansuensis endophyte and transgenerational effects on the water use efficiency, nutrient and biomass accumulation of Achnatherum inebrians under soil water deficit. Plant Soil 2018, 424, 555–571. [Google Scholar] [CrossRef]
- Cameron, K.D.; Teece, M.A.; Smart, L.B. Increased accumulation of cuticular wax and expression of lipid transfer protein in response to periodic drying events in leaves of tree tobacco. Plant Physiol. 2006, 140, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Post-Beittenmiller, D. Biochemistry and molecular biology of wax production in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 405–430. [Google Scholar] [CrossRef] [Green Version]
- Kunst, L.; Samuels, A.L. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 2003, 42, 51–80. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, L. Plant cuticles shine: Advances in wax biosynthesis and export. Curr. Opin. Plant Biol. 2009, 12, 721–727. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W.; Koch, S.; Hommes, A.; Wandelt, K.; Mamdouh, W.; De-Feyter, S.; Broekmann, P. Structural analysis of wheat wax (Triticum aestivum, c.v. ‘Naturastar’ L.): From the molecular level to three dimensional crystals. Planta 2006, 223, 258–270. [Google Scholar] [CrossRef]
- Javelle, M.; Vernoud, V.; Depège-Fargeix, N.; Arnould, C.; Oursel, D.; Domergue, F.; Sarda, X.; Rogowsky, P.M. Overexpression of the epidermis-specific homeodomain-leucine zipper IV transcription factor OUTER CELL LAYER1 in maize identifies target genes involved in lipid metabolism and cuticle biosynthesis. Plant Physiol. 2010, 154, 273–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avato, P.; Mikkelsen, J.D.; Wettstein-knowles, P.V. Synthesis of epicuticular primary alcohols and intracellular fatty acids by tissue slices from cer-j59 barley leaves. Carlsberg Res. Commun. 1982, 47, 377–390. [Google Scholar] [CrossRef]
- Wang, Y.T.; Sun, Y.L.; Wang, M.L.; Wang, Y.; Shi, X.; Li, C.L.; Quan, L.; Wang, Z.H.; Chen, Y.F. Composition and ultrastructure variation of leaf culticular wax at different developing stages of Aegilops Tauschii. J. Triticeae Crops 2014, 34, 1516–1521. [Google Scholar]
- Li, T.T.; Zhang, Y.Y.; Sun, Y.L.; Wang, Y.T.; Wang, M.L.; Hu, S.W.; Wang, Y.; Shi, X.; Quan, L.; Wang, Z.H. Cuticular Wax Composition and Microscopic Structure of Different Organs of Brachypodium distachyon. J. Triticeae Crops 2014, 34, 969–975. [Google Scholar]
- Yao, L.H.; Ni, Y.; Guo, N.; He, Y.J.; Gao, J.H.; Guo, Y.J. Leaf cuticular waxes in Poa pratensis and their responses to altitudes. Acta Prataculturae Sin. 2018, 27, 97–105. [Google Scholar]
- Zhang, D.; Yang, H.F.; Wang, X.C.; Qiu, Y.J.; Tian, L.H.; Qi, X.Q.; Qu, L.Q. Cytochrome P450 family member CYP96B5 hydroxylates alkanes to primary alcohols and is involved in rice leaf cuticular wax synthesis. New Phytol. 2020, 225, 2094–2107. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Rao, L.Q.; Xiang, Z.X.; Hu, X.M.; Wang, X.J. Epidermis wax content and drought resistance among different tall fescue (Festuca Arundinacea schreb.) varieties. Acta Bot. Boreali-Occident. Sin. 2007, 27, 1417–1421. [Google Scholar]
- Saneoka, H.; Ogata, S. Relationship between water use efficiency and cuticular wax deposition in warm season forage crops grown under water deficit conditions. Soil Sci. Plant Nutr. 1987, 33, 439–448. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Wan, L.Y.; Zhang, L.X.; Zhang, Z.J.; Zhang, H.W.; Quan, R.D.; Zhou, S.R.; Huang, R.F. An ethylene response factor OsWR1 responsive to drought stress transcriptionally activates wax synthesis related genes and increases wax production in rice. Plant Mol. Biol. 2012, 78, 275–288. [Google Scholar] [CrossRef]
- Zhao, D.B.; Zhang, H.L.; Wang, X.; Qi, J.C.; Hui, H.S.; Lin, L.H.; Wang, F.; Zheng, X.G. Relationship between epicuticular wax components and its content and drought resistance in barley leaf. Xinjiang Agric. Sci. 2017, 54, 43–50. [Google Scholar]
- Li, L.; Du, Y.C.; He, C.; Dietrich, C.R.; Li, J.K.; Ma, X.L.; Wang, R.; Liu, Q.; Liu, S.Z.; Wang, G.Y.; et al. Maize glossy6 is involved in cuticular wax deposition and drought tolerance. J. Exp. Bot. 2019, 70, 3089–3099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuffer, M.G.; Coe, E.H.; Wessler, S.R. Mutants of maize. Q. Rev. Biol. 1998, 73, 207. [Google Scholar]
- Yu, D.; Ranathunge, K.; Huang, H.; Pei, Z.; Franke, R.; Schreiber, L.; He, C. Wax Crystal-Sparse Leaf1 encodes a beta-ketoacyl CoA synthase involved in biosynthesis of cuticular waxes on rice leaf. Planta 2008, 228, 675–685. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Xiong, L.Z. Putative megaenzyme DWA1 plays essential roles in drought resistance by regulating stress-induced wax deposition in rice. Proc. Natl. Acad. Sci. USA 2013, 110, 17790–17795. [Google Scholar] [CrossRef] [Green Version]
- Kou, M.Z. The Responses of Achnatherum inebrians-Epichloë Endophyte Symbiont to Blumeria graminis. Master’s Thesis, Lanzhou University, Lanzhou, China, 2021. [Google Scholar]
- Alexa, A.; Rahnenfuhrer, J. topGO: Enrichment Analysis for Gene Ontology; R Package Version 2.18.0; Cranio: Saarbrücken, Germany, 2010. [Google Scholar]
- Xie, C.; Mao, X.Z.; Huang, J.K.; Ding, Y.; Wu, J.M.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L.P. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res 2011, 39, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Lewandowska, M.; Keyl, A.; Feussner, I. Wax biosynthesis in response to danger: Its regulation upon abiotic and biotic stress. New Phytol. 2020, 227, 698–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.B.; Suh, M.C. Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species. Plant Cell Rep. 2015, 34, 557–572. [Google Scholar] [CrossRef]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lü, S.; Joubès, J.; Jenks, M.A. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef] [Green Version]
- Bernard, A.; Domergue, F.; Pascal, S.; Jetter, R.; Renne, C.; Faure, J.D.; Haslam, R.P.; Napier, J.A.; Lessire, R.; Joubes, J. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. Plant Cell 2012, 24, 3106–3118. [Google Scholar] [CrossRef] [Green Version]
- Pascal, S.; Bernard, A.; Deslous, P.; Gronnier, J.; Fournier-Goss, A.; Domergue, F.; Rowland, O.; Joubes, J. Arabidopsis CER1-LIKE1 functions in a cuticular very-long-chain alkane-forming complex. Plant Physiol. 2019, 179, 415–432. [Google Scholar] [CrossRef] [Green Version]
- Pighin, J.A.; Zheng, H.Q.; Balakshin, L.J.; Goodman, I.P.; Western, T.L.; Jetter, R.; Kunst, L.; Samuels, A.L. Plant cuticular lipid export requires an ABC transporter. Science 2004, 306, 702–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente, M.A.; Faria, J.A.; Soares-Ramos, J.R.; Reis, P.A.; Pinheiro, G.L.; Piovesan, N.D.; Morais, A.T.; Menezes, C.C.; Cano, M.A.; Fietto, L.G.; et al. The ER luminal binding protein (BiP) mediates an increase in drought tolerance in soybean and delays drought-induced leaf senescence in soybean and tobacco. J. Exp. Bot. 2009, 60, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.T.; Hao, P.C.; Chen, G.X.; Han, C.X.; Li, X.H. Molecular cloning, phylogenetic analysis, and expression profiling of endoplasmic reticulum molecular chaperone BiP genes from bread wheat (Triticum aestivum L.). BMC Plant Biol. 2014, 14, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
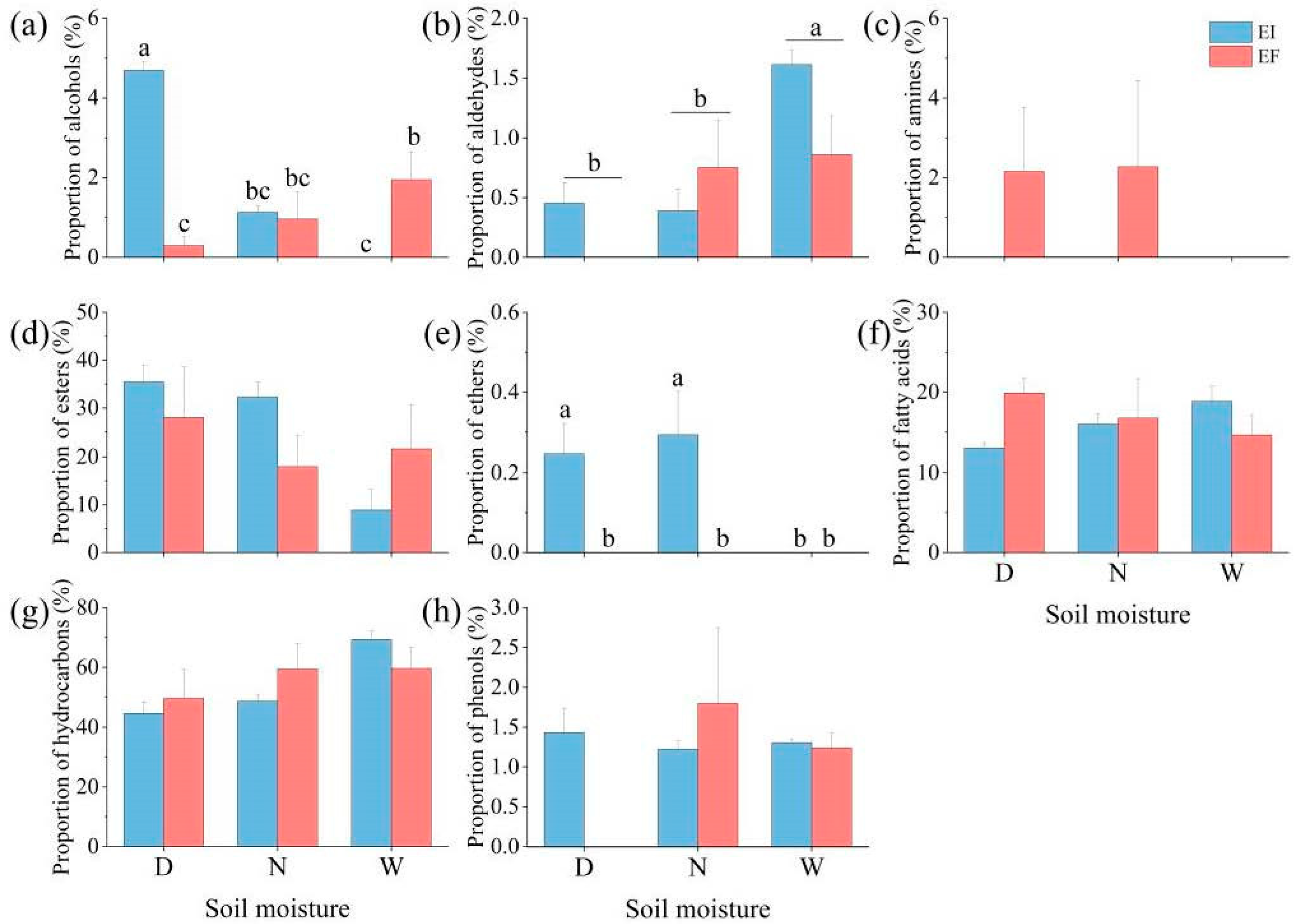
| Alcohols | Aldehydes | Amines | Esters | Ethers | Fatty Acids | Hydrocarbons | Phenols | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | df | F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P |
| E | 1 | 6.52 | 0.025 | 2.104 | 0.173 | 2.691 | 0.127 | 0.291 | 0.599 | 17.171 | 0.001 | 0.272 | 0.612 | 0.154 | 0.701 | 0.809 | 0.386 |
| W | 2 | 8.442 | 0.005 | 9.309 | 0.004 | 0.674 | 0.528 | 3.025 | 0.086 | 4.381 | 0.037 | 0.01 | 0.99 | 3.776 | 0.053 | 1.893 | 0.193 |
| E × W | 2 | 29.873 | 0 | 2.961 | 0.09 | 0.674 | 0.528 | 2.185 | 0.155 | 4.381 | 0.037 | 2.31 | 0.142 | 1.379 | 0.289 | 2.998 | 0.088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Ju, Y.; Kou, M.; Tian, M.; Christensen, M.J.; Zhang, X.; Nan, Z. Cuticular Wax Modification by Epichloë Endophyte in Achnatherum inebrians under Different Soil Moisture Availability. J. Fungi 2022, 8, 725. https://doi.org/10.3390/jof8070725
Zhao Z, Ju Y, Kou M, Tian M, Christensen MJ, Zhang X, Nan Z. Cuticular Wax Modification by Epichloë Endophyte in Achnatherum inebrians under Different Soil Moisture Availability. Journal of Fungi. 2022; 8(7):725. https://doi.org/10.3390/jof8070725
Chicago/Turabian StyleZhao, Zhenrui, Yawen Ju, Mingzhu Kou, Mei Tian, Michael John Christensen, Xingxu Zhang, and Zhibiao Nan. 2022. "Cuticular Wax Modification by Epichloë Endophyte in Achnatherum inebrians under Different Soil Moisture Availability" Journal of Fungi 8, no. 7: 725. https://doi.org/10.3390/jof8070725
APA StyleZhao, Z., Ju, Y., Kou, M., Tian, M., Christensen, M. J., Zhang, X., & Nan, Z. (2022). Cuticular Wax Modification by Epichloë Endophyte in Achnatherum inebrians under Different Soil Moisture Availability. Journal of Fungi, 8(7), 725. https://doi.org/10.3390/jof8070725









