Improved Tolerance of Artemisia ordosica to Drought Stress via Dark Septate Endophyte (DSE) Symbiosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Soil and Root Sampling
2.3. Isolation and Identification of DSE Strains
2.4. DSE Colonization and Diversity Analysis
2.5. Soil Physicochemical Properties
2.6. Drought Stress of the DSE Strains in Vitro
2.7. Inoculation Assay
2.7.1. DSE Inoculation and Plant Growth Conditions
2.7.2. Plant Growth Parameters
2.7.3. Plant Physiological Parameters
2.8. Statistical Analysis
3. Results
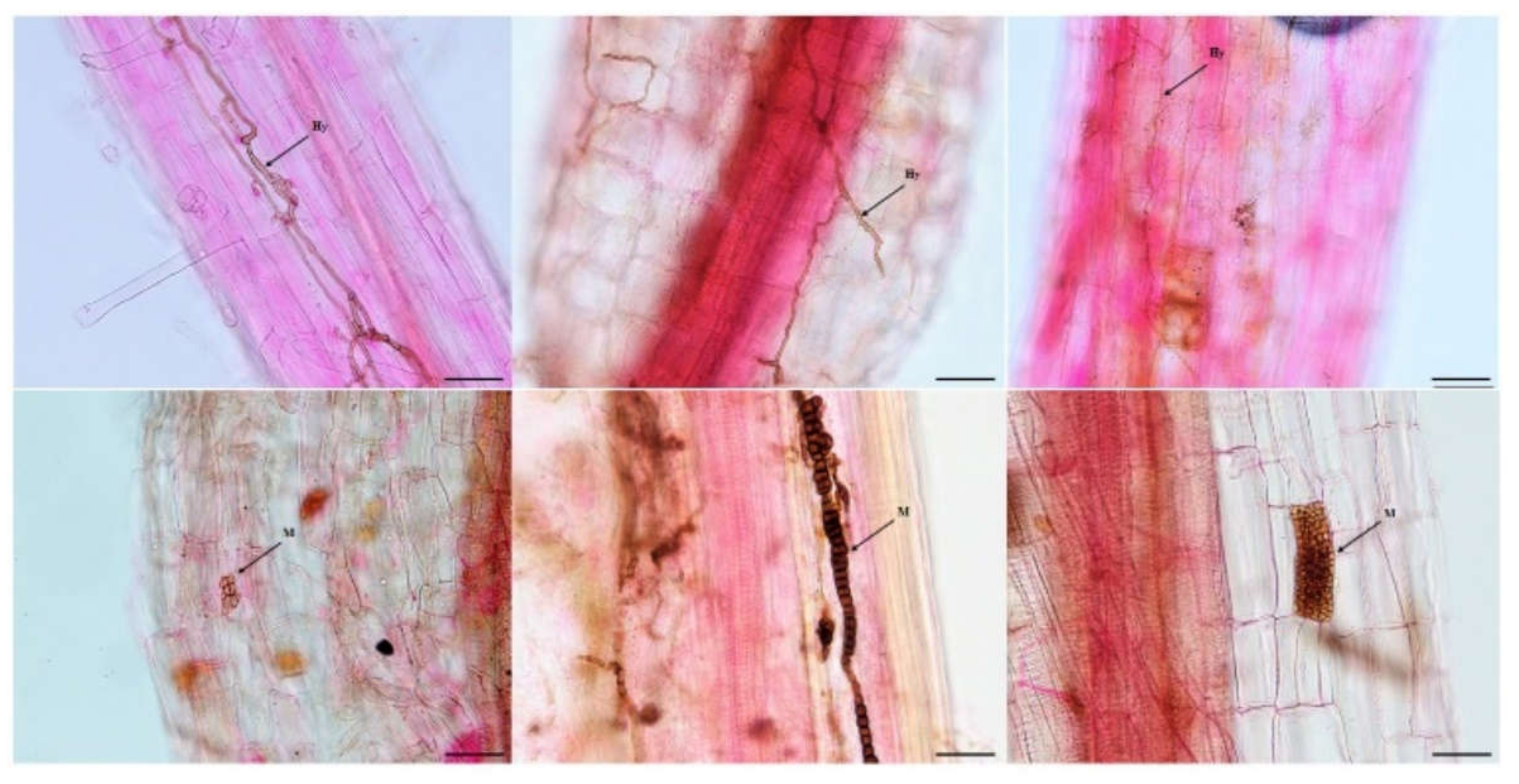
3.1. DSE Colonization in Artemisia ordosica
3.2. In Vitro Drought Stress Tolerance of the DSE Stains
3.3. Effect of DSEs on the Performance of Artemisia ordosica
3.3.1. Vegetative Growth of Artemisia ordosica
3.3.2. Biomass Production of Artemisia ordosica
3.3.3. Physiological Response Index of Artemisia ordosica
4. Discussion
4.1. Colonization and Species Diversity of DSEs in Artemisia ordosica
4.2. DSE Growth under Drought Stress
4.3. Effect of DSEs on the Performance of Artemisia ordosica
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Yu, H.; Dai, A.; Wei, Y.; Kang, L. Drylands face potential threat under 2 °C global warming target. Nat. Clim. Chang. 2017, 7, 417–422. [Google Scholar] [CrossRef]
- Sheffield, J.; Andreadis, K.M.; Wood, E.F.; Lettenmaier, D.P. Global and continental drought in the second half of the twentieth century, severity-area-duration analysis and temporal variability of large-scale events. J. Clim. 2009, 22, 1962–1981. [Google Scholar] [CrossRef]
- Peters, D.P.C.; Yao, J.; Sala, O.E.; Anderson, J.P. Directional climate change and potential reversal of desertification in arid and semiarid ecosystems. Glob. Chang. Biol. 2012, 18, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; An, H.; Deng, L.; Wang, Y.; Zhu, G.; Shangguan, Z. Effect of desertification on productivity in a desert steppe. Sci. Rep. 2016, 6, 27839. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Saleem, M.; Cheng, J.; Mi, J.; Chu, P.; Tuvshintogtokh, I.; Hu, S.; Bai, Y. Effects of aridity on soil microbial communities and functions across soil depths on the Mongolian Plateau. Funct. Ecol. 2019, 33, 1561–1571. [Google Scholar] [CrossRef]
- Na, X.; Yu, H.; Wang, P.; Zhu, W.; Niu, Y.; Huang, J. Vegetation biomass and soil moisture coregulate bacterial community succession under altered precipitation regimes in a desert steppe in northwestern China. Soil Biol. Biochem. 2019, 136, 107520. [Google Scholar] [CrossRef]
- Omer, A.; Zhu, G.; Zheng, Z.; Saleem, F. Natural and anthropogenic influences on the recent droughts in Yellow River Basin, China. Sci. Total Environ. 2020, 704, 135428. [Google Scholar] [CrossRef]
- Barker, J.D.; Kaspari, S.; Gabrielli, P.; Wegner, A.; Beaudon, E.; Sierra–Hernandez, M.R.; Thompson, L. Drought–induced biomass burning as a source of black carbon to the Central Himalaya since 1781 CE as reconstructed from the Dasuopu Ice Core. Atmos. Chem. Phys. 2021, 21, 5615–5633. [Google Scholar] [CrossRef]
- Meng, H.H.; Gao, X.Y.; Huang, J.F.; Zhang, M.L. Plant phylogeography in arid Northwest China: Retrospectives and perspectives. J. Syst. Evol. 2015, 53, 33–46. [Google Scholar] [CrossRef]
- Wei, W.; Guo, Z.; Shi, P.; Zhou, L.; Xie, B. Spatiotemporal changes of land desertification sensitivity in Northwest China from 2000 to 2017. J. Geogr. Sci. 2021, 31, 46–68. [Google Scholar] [CrossRef]
- Cui, Z.; Kang, H.; Wang, W.; Guo, W.; Guo, M.; Chen, Z. Vegetation restoration restricts rill development on dump slopes in coalfields. Sci. Total Environ. 2022, 820, 153203. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.J.; Ding, J.J.; Lin, W.; Li, Q.; Xu, C.; Zheng, Q.; Li, Y. Alleviation of the detrimental effect of water deficit on wheat (Triticum aestivum L.) growth by an indole acetic acid–producing endophytic fungus. Plant Soil 2019, 439, 373–391. [Google Scholar] [CrossRef]
- Gehring, C.; Sevanto, S.; Patterson, A.; Ulrich, D.; Kuske, C.R. Ectomycorrhizal and dark septate fungal associations of pinyon pine are differentially affected by experimental drought and warming. Front. Plant Sci. 2020, 11, 582574. [Google Scholar]
- Sebastiana, M.; Duarte, B.; Monteiro, F.; Malhóa, R.; Caçadorb, I.; Matosa, A.R. The leaf lipid composition of ectomycorrhizal oak plants shows a drought-tolerance signature. Plant Physiol. Bioch. 2019, 144, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes, a review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef]
- Santos, M.; Ignacio, C.; Fernando, D.; Brenda, S.; Alejandro, M. Advances in the role of dark septate endophytes in the plant resistance to abiotic and biotic stresses. J. Fungi 2021, 7, 939. [Google Scholar] [CrossRef]
- Yuan, Z.; Druzhinina, I.S.; Gibbons, J.G.; Zhong, Z.; Van de Peer, Y.; Rodriguez, R.J.; Liu, Z.; Wang, X.; Wei, H.; Wu, Q.; et al. Divergence of a genomic island leads to the evolution of melanization in a halophyte root fungus. ISME J. 2021, 15, 3468–3479. [Google Scholar] [CrossRef]
- Knapp, D.G.; Imrefi, I.; Boldpurev, E.; Csíkos, S.; Akhmetova, G.; Berek-Nagy, P.J.; Otgonsuren, B.; Kovács, G.M. Root-colonizing endophytic fungi of the dominant grass Stipa krylovii from a Mongolian steppe grassland. Front. Microbiol. 2019, 10, 2565. [Google Scholar] [CrossRef]
- Lugo, M.A.; Menoyo, E.; Allione, L.R.; Negritto, M.A.; Henning, J.A.; Anton, A.M. Arbuscular mycorrhizas and dark septate endophytes associated with grasses from the Argentine Puna. Mycologia 2018, 110, 654–665. [Google Scholar] [CrossRef]
- Li, X.; He, X.L.; Hou, L.F.; Ren, Y.; Wang, S.J.; Su, F. Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci. Rep. 2018, 8, 7896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Y.; Xia, L.; Yang, J.; Liu, J.; Zhao, L.; He, X. Fungal endophytic community and diversity associated with desert shrubs driven by plant identity and organ differentiation in extremely arid desert ecosystem. J. Fungi 2021, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Rayment, J.T.; Jones, S.; French, K. Seasonal patterns of fungal colonisation in Australian native plants of different ages. Symbiosis 2020, 80, 169–182. [Google Scholar] [CrossRef]
- Barrow, J.R. Atypical morphology of dark septate fungal root endophytes of Bouteloua in arid southwestern USA rangelands. Mycorrhiza 2003, 13, 239–247. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zeng, Q.; Chen, Y.; Chen, C.; Wang, W.; Hou, J.; Li, X. Colonization by dark septate endophytes improves the growth and rhizosphere soil microbiome of licorice plants under different water treatments. Appl. Soil Ecol. 2021, 166, 103993. [Google Scholar] [CrossRef]
- Li, Z.; Pan, J. Spatiotemporal changes in vegetation net primary productivity in the arid region of Northwest China, 2001 to 2012. Front. Earth Sci. 2018, 12, 108–124. [Google Scholar] [CrossRef]
- Gui, Z.; Li, L.; Qin, S.; Zhang, Y. Foliar water uptake of four shrub species in a semi–arid desert. J. Arid Environ. 2021, 195, 104629. [Google Scholar]
- Jiang, Y.; Tian, Y.; Zha, T.; Jia, X.; Bourque, C.P.A.; Liu, P.; Jin, C.; Jiang, X.; Li, X.; Wei, N.; et al. Dynamic changes in plant resource use efficiencies and their primary influence mechanisms in a typical desert shrub community. Forests 2021, 12, 1372. [Google Scholar] [CrossRef]
- Qin, J.; Si, J.; Jia, B.; Zhao, C.; Zhou, D.; He, X.; Wang, C.; Zhu, X. Water use characteristics of two dominant species in the mega–dunes of the Badain Jaran Desert. Water 2022, 14, 53. [Google Scholar] [CrossRef]
- Zha, T.; Qian, D.; Jia, X.; Bai, Y.; Tian, Y.; Bourque, C.P.A.; Ma, J.; Feng, W.; Wu, B.; Peltola, H. Soil moisture control of sap–flow response to biophysical factors in a desert–shrub species Artemisia Ordosica. Biogeosciences 2017, 14, 4533–4544. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; He, X.; Li, X.; Wang, S.; Zhao, L. Species composition and colonization of dark septate endophytes are affected by host plant species and soil depth in the Mu Us sandland, northwest China. Fungal Ecol. 2019, 39, 276–284. [Google Scholar]
- Ma, X.; Zhu, J.; Wang, Y.; Yan, W.; Zhao, C. Variations in water use strategies of sand-binding vegetation along a precipitation gradient in sandy regions, Northern China. J. Hydrol. 2021, 600, 126539. [Google Scholar] [CrossRef]
- Patterson, A.; Flores-Rentería, L.; Whipple, A.; Whitham, T.; Gehring, C. Common Garden experiments disentangle plant genetic and environmental contributions to ectomycorrhizal fungal community structure. New Phytol. 2019, 221, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest tree microbiomes and associated fungal endophytes, functional roles and impact on forest health. Forests 2019, 10, 42. [Google Scholar]
- Ruotsalainen, A.L.; Kauppinen, M.; Wli, P.R.; Saikkonen, K.; Helander, M.; Tuomi, J. Dark septate endophytes, mutualism from by–products? Trends Plant Sci. 2022, 27, 247–254. [Google Scholar] [CrossRef]
- Xie, L.L.; He, X.L.; Wang, K.; Hou, L.F.; Sun, Q. Spatial dynamics of dark septate endophytes in the roots and rhizospheres of Hedysarum scoparium in northwest China and the influence of edaphic variables. Fungal Ecol. 2017, 26, 135–143. [Google Scholar]
- Li, X.; He, C.; He, X.; Su, F.; Hou, L.; Ren, Y.; Hou, Y. Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development. Plant Soil 2019, 439, 259–272. [Google Scholar] [CrossRef]
- Han, L.; Shi, J.; He, C.; He, X. Temporal and spatial dynamics of dark septate endophytes in the roots of Lycium ruthenicum in the desert region of Northwest China. Agronomy 2021, 11, 648. [Google Scholar]
- Knapp, D.; Kovacs, G.M.; Zajta, E.; Groenewald, J.; Crous, P. Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia 2015, 35, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6, molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–163. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Samaga, P.V.; Rai, V.R. Diversity and bioactive potential of endophytic fungi from Nothapodytes foetida, Hypericum mysorense and Hypericum japonicum collected from Western Ghats of India. Ann. Microbiol. 2016, 66, 229–244. [Google Scholar] [CrossRef]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circular/United States Department of Agriculture: Washington, DC, USA, 1954.
- Bao, S.D. Agrochemical Analysis of Soil; Chinese Agricultural Press: Beijing, China, 2000; pp. 44–49. (In Chinese) [Google Scholar]
- Tarafdar, J.; Marschner, H. Phosphatase activity in the rhizosphere and hyphosphere of VA mycorrhizal wheat supplied with inorganic and organic phosphorus. Soil Biol. Biochem. 1994, 26, 387–395. [Google Scholar] [CrossRef]
- Hoffmann, G.; Teicher, K. A colorimetric technique for determining urease activity in soil. Dung Boden. 1961, 95, 55–63. (In German) [Google Scholar] [CrossRef]
- Chen, D.M.; Khalili, K.; Cairney, J.W.G. Influence of water stress on biomass production by isolates of an ericoid mycorrhizal endophyte of Woollsia pungens and Epacris microphylla (Ericaceae). Mycorrhiza 2003, 13, 173–176. [Google Scholar] [CrossRef]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. Methods Mol. Biol. 2010, 639, 273–280. [Google Scholar]
- Peever, T.L.; Higgins, V.J. Electrolyte leakage, lipoxygenase, and lipid peroxidation induced in tomato leaf tissue by specific and nonspecific elicitors from Cladosporium fulvum. Plant Physiol. 1989, 90, 867–875. [Google Scholar] [CrossRef] [Green Version]
- Christos, D.G.; Konstantinos, G.; George, Z. Mechanism of Coomassie brilliant blue G-250 binding to proteins: A hydrophobic assay for nanogram quantities of proteins. Anal Bioanal Chem. 2008, 3912, 391–403. [Google Scholar]
- Ellis, D.H.; Griffiths, D.A. The location and analysis of melanins in the cell walls of some soil fungi. Can. J. Microbiol. 1974, 20, 1379–1386. [Google Scholar] [CrossRef]
- Zhan, F.; He, Y.; Zu, Y.; Li, T.; Zhao, Z. Characterization of melanin isolated from a dark septate endophyte (DSE), Exophiala pisciphila. World J. Microbiol. Biotechnol. 2011, 27, 2483–2489. [Google Scholar] [CrossRef]
- Chen, J.H.; Jiang, H.W.; Hsieh, E.J.; Chen, H.; Chien, C.; Hsieh, H.; Lin, T. Drought and salt stress tolerance of an Arabidopsis glutathione S–transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol. 2012, 158, 340–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Wang, W.; Hou, J. Plant growth and soil microbial impacts of enhancing licorice with inoculating dark septate endophytes under drought stress. Front. Microbiol. 2019, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Method Enzymol. 1985, 113, 548–555. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hou, L.; Li, X.; He, X.; Zuo, Y.; Zhang, D.; Zhao, L. Effect of dark septate endophytes on plant performance of Artemisia ordosica and associated soil microbial functional group abundance under salt stress. Appl. Soil Ecol. 2021, 165, 103998. [Google Scholar] [CrossRef]
- Steven, B.; Hesse, C.; Gallegos-Graves, L.V.; Belnap, J.; Kuske, C.R. Fungal diversity in biological soil crusts of the Colorado plateau. In Proceedings of the 12th Biennial Conference of Research on the Colorado Plateau; U.S. Geological Survey: Reston, VA, USA, 2016. [Google Scholar]
- Zuo, Y.; Hu, Q.; Liu, J.; He, X.L. Relationship of root dark septate endophytes and soil factors to plant species and seasonal variation in extremely arid desert in Northwest China. Appl. Soil Ecol. 2022, 175, 104454. [Google Scholar] [CrossRef]
- Hou, L.; Yu, J.; Zhao, L.; He, X. Dark septate endophytes improve the growth and the tolerance of Medicago sativa and Ammopiptanthus mongolicus under cadmium stress. Front. Microbiol. 2020, 10, 3061. [Google Scholar] [CrossRef]
- He, C.; Liu, C.; Liu, H.; Wang, W.; Hou, J.; Li, X. Dual inoculation of dark septate endophytes and Trichoderma viride drives plant performance and rhizosphere microbiome adaptations of Astragalus mongholicus to drought. Environ. Microbiol. 2022, 24, 324–340. [Google Scholar] [CrossRef]
- Collins, S.L.; Sinsabaugh, R.L.; Crenshaw, C.; Green, L.; Porras–Alfaro, A.; Stursova, M.; Zeglin, L.H. Pulse dynamics and microbial processes in aridland ecosystems. J. Ecol. 2008, 96, 413–420. [Google Scholar] [CrossRef]
- Lagueux, D.; Jumpponen, A.; Porras-Alfaro, A.; Herrera, J.; Chung, Y.A.; Baur, L.E.; Smith, M.D.; Knapp, A.K.; Collins, S.L.; Rudgers, J.A. Experimental drought re-ordered assemblages of root-associated fungi across North American grasslands. J. Ecol. 2020, 2, 776–792. [Google Scholar] [CrossRef]
- Chu, H.; Wang, C.; Li, Z.; Wang, H.; Xiao, Y.; Chen, J.; Tang, M. The dark septate endophytes and ectomycorrhizal fungi effect on Pinus tabulaeformis Carr. seedling growth and their potential effects to pine wilt disease resistance. Forests 2019, 10, 140. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Zuo, Y.; He, X.; Hou, Y.; Li, M.; Li, B. Plant identity and soil variables shift the colonisation and species composition of dark septate endophytes associated with medicinal plants in a northern farmland in China. Appl. Soil Ecol. 2021, 167, 104042. [Google Scholar] [CrossRef]
- Upson, R.; Read, D.J.; Newsham, K.K. Nitrogen form influences the response of Deschampsia antarctica to dark septate root endophytes. Mycorrhiza 2009, 20, 1–11. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Belnap, J.; Rudgers, J.; Kuske, C.R.; Martinez, N.; Sandquist, D. Soil microbial responses to nitrogen addition in arid ecosystems. Front. Microbiol. 2015, 6, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.H.; Wang, C.K.; Luo, Y.Q. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Carrino-Kyker, S.R.; Kluber, L.A.; Petersen, S.M.; Coyle, K.P.; Hewins, C.R.; DeForest, J.L.; Smemo, K.A.; Burke, D.J. Mycorrhizal fungal communities respond to experimental elevation of soil pH and P availability in temperate hardwood forests. FEMS Microbiol. Ecol. 2016, 92, fiw024. [Google Scholar] [CrossRef]
- Fonseca, A.A.; Santos, D.A.; Passos, R.R.; Andrade, F.V.; Rangel, O.J.P. Phosphorus availability and grass growth in biochar–modified acid soil, a study excluding the effects of soil pH. Soil Use Manag. 2020, 36, 714–725. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberan, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Hao, B.; Jing, X.; He, J.; Ma, W.; Zhu, B. Minor responses of soil microbial biomass, community structure and enzyme activities to nitrogen and phosphorus addition in three grassland ecosystems. Plant Soil 2019, 444, 21–37. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Colpaert, J.V.; White, M.W.; Ouimette, A.P.; Macko, S.A. Nitrogen form, availability, and mycorrhizal colonization affect biomass and nitrogen isotope patterns in Pinus sylvestris. Plant Soil 2008, 310, 121–136. [Google Scholar] [CrossRef]
- Monica, I.F.D.; Saparrat, M.C.N.; Godeas, A.M.; Scervino, J.M. The co-existence between DSE and AMF symbionts affects plant P pools through P mineralization and solubilization processes. Fungal Ecol. 2015, 17, 10–17. [Google Scholar] [CrossRef]
- Renella, G.; Egamberdiyeva, D.; Landi, L.; Mench, M.; Nannipieri, P. Microbial activity and hydrolase activities during decomposition of root exudates released by an artificial root surface in Cd–contaminated soils. Soil Biol. Biochem. 2006, 38, 702–708. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B. A meta–analysis of soil extracellular enzyme activities in response to global change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Tabatabai, M.A. Enzyme activities in a limed agricultural soil. Biol. Fertil. Soils 2000, 31, 85–91. [Google Scholar] [CrossRef]
- Glassman, S.I.; Wang, I.J.; Bruns, T.D. Environmental filtering by pH and soil nutrients drives community assembly in fungi at fine spatial scales. Mol. Ecol. 2017, 26, 6960–6973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; He, X.L.; Zhou, Y.; Hou, Y.T.; Zuo, Y.L. Effects of dark septate endophytes on the performance of Hedysarum scoparium under water deficit stress. Front. Plant Sci. 2019, 10, 903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landolt, M.; Stroheker, S.; Queloz, V.; Gall, A.; Sieber, T.N. Does water availability influence the abundance of species of the Phialocephala fortinii s.l.—Acephala applanata complex (PAC) in roots of pubescent oak (Quercus pubescens) and Scots pine (Pinus sylvestris)? Fungal Ecol. 2020, 44, 100904. [Google Scholar] [CrossRef]
- He, C.; Wang, W.; Hou, J.; Li, X. Dark septate endophytes isolated from wild licorice roots grown in the desert regions of Northwest China enhance the growth of host plants under water deficit stress. Front. Microbiol. 2021, 12, 522449. [Google Scholar] [CrossRef]
- Baltruschat, H.; Fodor, J.; Harrach, B.D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.H.; Schafer, P.; Schwarczinger, I.; et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008, 180, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular mycorrhizal fungi alleviate drought stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) grasses via altering antioxidant enzyme activities and photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salducci, M.D.; Folzera, H.; Issartela, J.; Rabiera, J.; Masottia, V.; Prudentb, P.; Affrea, L.; Hardionc, L.; Tatonia, T.; Laffont-Schwobad, I. How can a rare protected plant cope with the metal and metalloid soil pollution resulting from past industrial activities? Phytometabolites, antioxidant activities and root symbiosis involved in the metal tolerance of Astragalus tragacantha. Chemosphere 2019, 217, 887–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collin–Hansen, C.; Andersen, R.A.; Steinnes, E. Molecular defense systems are expressed in the king bolete (Boletus edulis) growing near metal smelters. Mycologia 2005, 97, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hou, L.F.; Liu, J.Q.; Yang, J.Y.; Zou, Y.L.; Zhao, L.L.; He, X.L. Growth-promoting effects of dark septate endophytes on the non-mycorrhizal plant Isatis indigotica under diferent water conditions. Symbiosis 2021, 85, 291–303. [Google Scholar] [CrossRef]
- Petelenz-Kurdziel, E.; Eriksson, E.; Smedh, M.; Beck, C.; Hohmann, S.; Goksör, M. Quantification of cell volume changes upon hyperosmotic stress in Saccharomyces cerevisiae. Integr. Biol. 2011, 3, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, C.; Perrin, Y.; Leyval, C.; Blaudez, D. Melanization and ageing are not drawbacks for successful agrotransformation of dark septate endophytes. Fungal Biol. 2017, 121, 652–663. [Google Scholar] [CrossRef] [Green Version]
- Gaber, D.A.; Berthelot, C.; Camehl, I.; Kovács, G.M.; Blaudez, D.; Franken, P. Salt stress tolerance of dark septate endophytes is independent of melanin accumulation. Front. Microbiol. 2020, 11, 562931. [Google Scholar] [CrossRef]
- Addy, H.D.; Piercey, M.M.; Currah, R.S. Microfungal endophytes in roots. Can. J. Bot. 2005, 83, 1–13. [Google Scholar] [CrossRef]
- Mandyam, K.; Jumpponen, A. Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud. Mycol. 2005, 53, 173–189. [Google Scholar] [CrossRef] [Green Version]
- Peterson, R.L.; Wagg, C.; Pautler, M. Associations between microfungal endophytes and roots, do structural features indicate function. Botany 2008, 86, 445–456. [Google Scholar] [CrossRef]
- Wu, L.Q.; Lv, Y.L.; Meng, Z.X.; Chen, J.; Guo, S.X. The promoting role of an isolate of dark–septate fungus on its host plant Saussurea involucrate Kar. et Kir. Mycorrhiza 2010, 20, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, X.L. Dark septate endophyte improves drought tolerance of Ormosia hosiei Hemsley & E. H. Wilson by modulating root morphology, ultrastructure, and the ratio of root hormones. Forests 2019, 10, 830. [Google Scholar]
- Hosseini, F.; Mosaddeghi, M.R.; Dexter, A.R.; Sepehri, M. Maize water status and physiological traits as affected by root endophytic fungus Piriformospora indica under combined drought and mechanical stresses. Planta 2018, 247, 1229–1245. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hamayun, M.; Khan, S.A.; Kang, S.M.; Shinwari, Z.K.; Kamran, M.; Rehman, S.; Kim, J.G.; Lee, I.J. Pure culture of Metarhizium anisopliae LHL07 reprograms soybean to higher growth and mitigates salt stress. World J. Microbiol. Biotechnol. 2012, 28, 1483–1494. [Google Scholar] [CrossRef]
- Xu, L.; Wang, A.A.; Wang, J.; Wei, Q.; Zhang, W.Y. Piriformospora indica confers drought tolerance on Zea mays L. through enhancement of antioxidantactivity and expression of drought–related genes. Crop J. 2017, 5, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Qin, Y.; Yuan, Z.L. Potential of a halophyte–associated endophytic fungus for sustaining Chinese white poplar growth under salinity. Symbiosis 2018, 76, 109–116. [Google Scholar] [CrossRef]
- Deng, X.; Song, X.; Halifu, S.; Yu, W.; Song, R. Effects of dark septate endophytes strain A024 on damping–off biocontrol, plant growth, and the rhizosphere soil environment of Pinus sylvestris var. mongolica annual seedlings. Plants 2020, 9, 913. [Google Scholar] [CrossRef]
- Bouzouina, M.; Kouadria, R.; Lotmani, B. Fungal endophytes alleviate salt stress in wheat in terms of growth, ion homeostasis and osmoregulation. J. Appl. Microbiol. 2021, 130, 913–925. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Jung, H.Y.; Lee, J.H.; Lee, I.J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress, an example of Paecilomyces formosus LHL10. BMC Microbiol. 2012, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Priyadharsini, P.; Muthukumar, T. The root endophytic fungus Curvularia geniculata from Parthenium hysterophorus roots improves plant growth through phosphate solubilization and phytohormone production. Fungal Ecol. 2017, 27, 69–77. [Google Scholar] [CrossRef]
- Liu, H.G.; Wang, Y.J.; Hart, M.; Chen, H.; Tang, M. Arbuscular mycorrhizal symbiosis regulates hormone and osmotic equilibrium of Lycium barbarum L. under salt stress. Mycosphere 2016, 7, 828–843. [Google Scholar] [CrossRef]
- Lage–Pinto, F.; Oliveira, J.G.; Cunha, D.M.; Souza, C.M.M.; Rezende, C.E.; Azevedo, R.A.; Vitoria, A.P. Chlorophyll a fluorescence and ultrastructural changes in chloroplast of water hyacinth as indicators of environmental stress. Environ. Expl. Bot. 2008, 64, 307–313. [Google Scholar] [CrossRef]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.C.; Watt, M.; Roessner, U. Alleviation of salinity stress in plants by endophytic plant–fungal symbiosis, current knowledge, perspectives and future directions. Plant Soil 2020, 461, 219–244. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, X. Dark septate endophyte improves the drought–stress resistance of Ormosia hosiei seedlings by altering leaf morphology and photosynthetic characteristics. Plant Ecol. 2021, 222, 761–771. [Google Scholar] [CrossRef]











| Plant Height | Branching Number | Total Root Length | Root Surface Area | Root Volume | Root Diameter | Shoot Biomass | Root Biomass | Total Biomass | Root:Shoot Ratio | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | |
| drought | 17.57 | 0.001 | 365.16 | < 0.001 | 0.01 | 0.966 | 0.53 | 0.479 | 0.32 | 0.581 | 1.99 | 0.177 | 0.22 | 0.646 | 18.28 | 0.001 | 1.53 | 0.234 | 7.85 | 0.013 |
| DSE | 4.34 | 0.020 | 56.34 | < 0.001 | 11.79 | < 0.001 | 38.39 | < 0.001 | 8.15 | 0.002 | 1.76 | 0.195 | 65.80 | <0.001 | 127.37 | < 0.001 | 111.41 | < 0.001 | 11.08 | < 0.001 |
| drought × DSE | 3.51 | 0.040 | 65.86 | <0.001 | 15.74 | <0.001 | 34.11 | <0.001 | 4.96 | 0.013 | 1.07 | 0.391 | 82.25 | < 0.001 | 67.72 | < 0.001 | 119.85 | < 0.001 | 23.19 | < 0.001 |
| SOD | GSH | MDA | Auxin | Proline | Chlorophyll | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| drought | 3.08 | 0.098 | 193.48 | <0.001 | 0.01 | 0.997 | 27.19 | <0.001 | 32.16 | <0.001 | 1.18 | 0.293 |
| DSE | 7.50 | 0.002 | 30.96 | <0.001 | 10.99 | <0.001 | 6.64 | 0.004 | 15.35 | <0.001 | 49.69 | <0.001 |
| drought × DSE | 3.48 | 0.041 | 31.55 | <0.001 | 10.51 | <0.001 | 3.39 | 0.044 | 4.42 | 0.019 | 16.12 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, X.; Xu, M.; Ye, Q.; Gao, H.; He, X. Improved Tolerance of Artemisia ordosica to Drought Stress via Dark Septate Endophyte (DSE) Symbiosis. J. Fungi 2022, 8, 730. https://doi.org/10.3390/jof8070730
Li X, Zhang X, Xu M, Ye Q, Gao H, He X. Improved Tolerance of Artemisia ordosica to Drought Stress via Dark Septate Endophyte (DSE) Symbiosis. Journal of Fungi. 2022; 8(7):730. https://doi.org/10.3390/jof8070730
Chicago/Turabian StyleLi, Xia, Xue Zhang, Minghui Xu, Qiannan Ye, Huili Gao, and Xueli He. 2022. "Improved Tolerance of Artemisia ordosica to Drought Stress via Dark Septate Endophyte (DSE) Symbiosis" Journal of Fungi 8, no. 7: 730. https://doi.org/10.3390/jof8070730
APA StyleLi, X., Zhang, X., Xu, M., Ye, Q., Gao, H., & He, X. (2022). Improved Tolerance of Artemisia ordosica to Drought Stress via Dark Septate Endophyte (DSE) Symbiosis. Journal of Fungi, 8(7), 730. https://doi.org/10.3390/jof8070730






