The Fungal Protein Mes1 Is Required for Morphogenesis and Virulence in the Dimorphic Phytopathogen Ustilago maydis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Plasmid and Strain Construction
2.3. Light Microscopy and Image Analysis
2.4. Transmission Electron Microscopy (TEM)
2.5. Mating and Plant Infections
2.6. Bioinformatic Analyses
3. Results
3.1. The MesA/Mes1 Homologue in U. maydis
3.2. Mes1 Is Required for Full Filamentous Growth in U. maydis
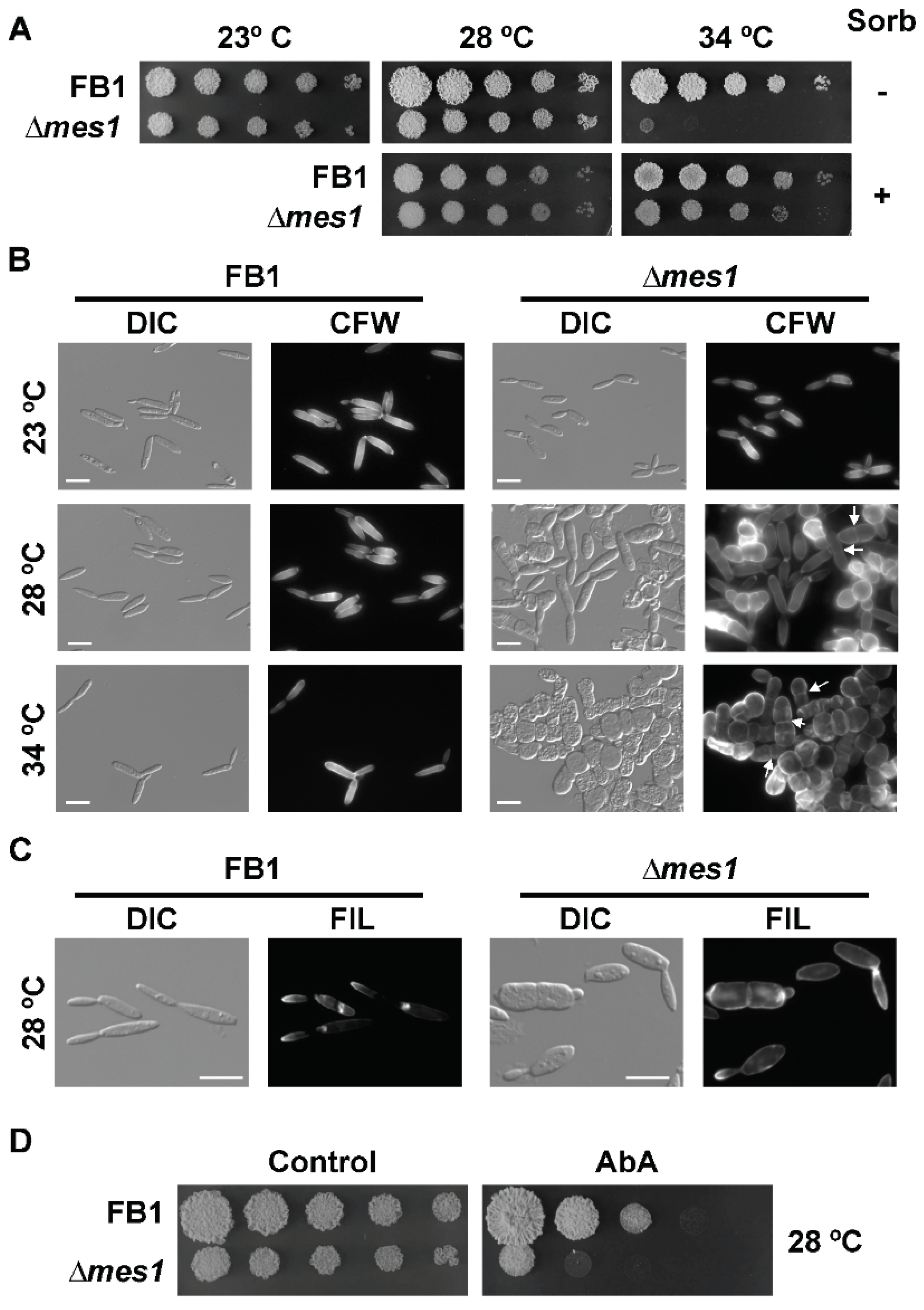
3.3. Mes1 Is Required for Yeast Growth in U. maydis
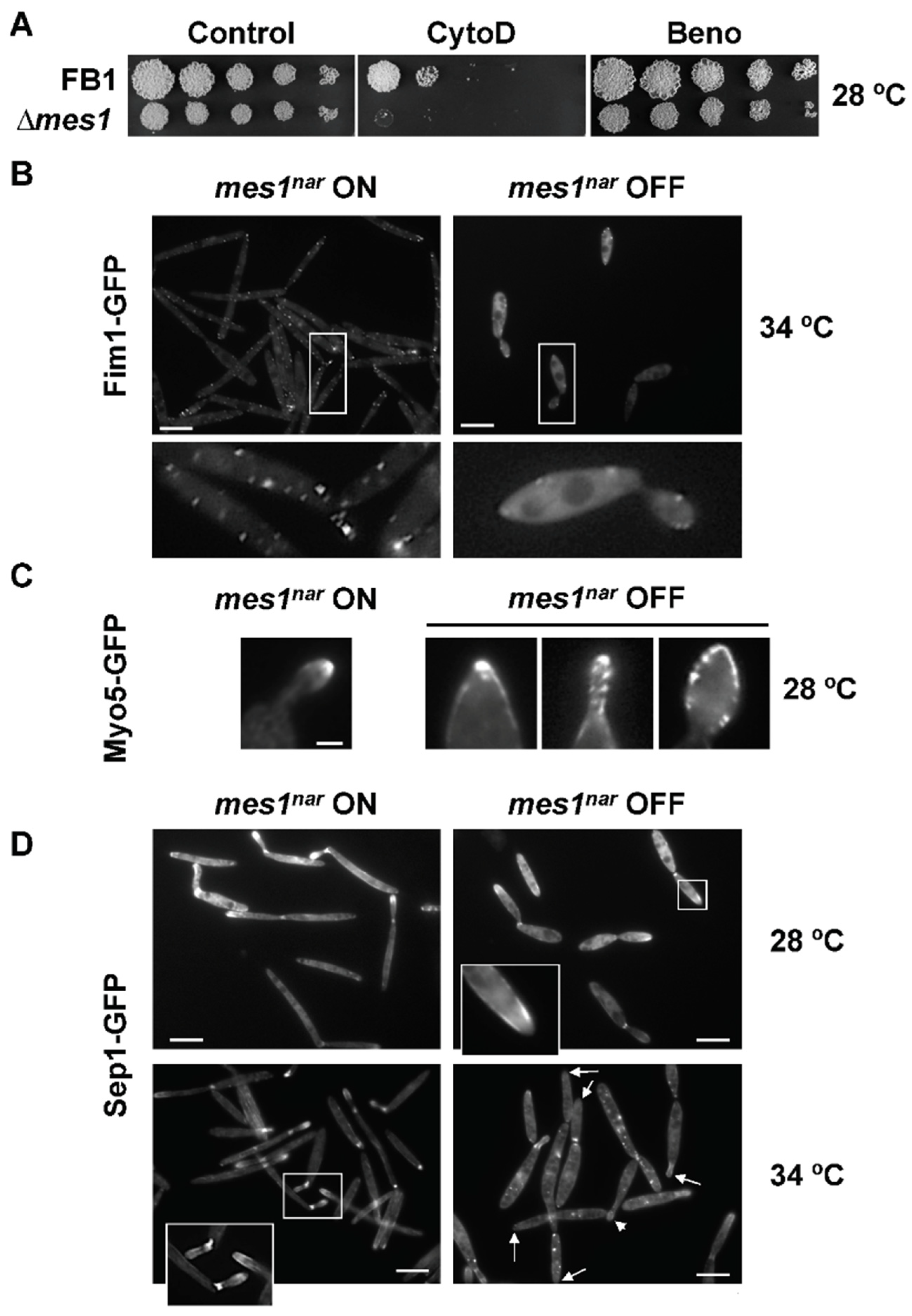
3.4. Deletion of mes1 Affects the Actin Cytoskeleton
3.5. The ∆mes1 Strain Is More Sensitive to Membrane and Cell Wall Disruptants
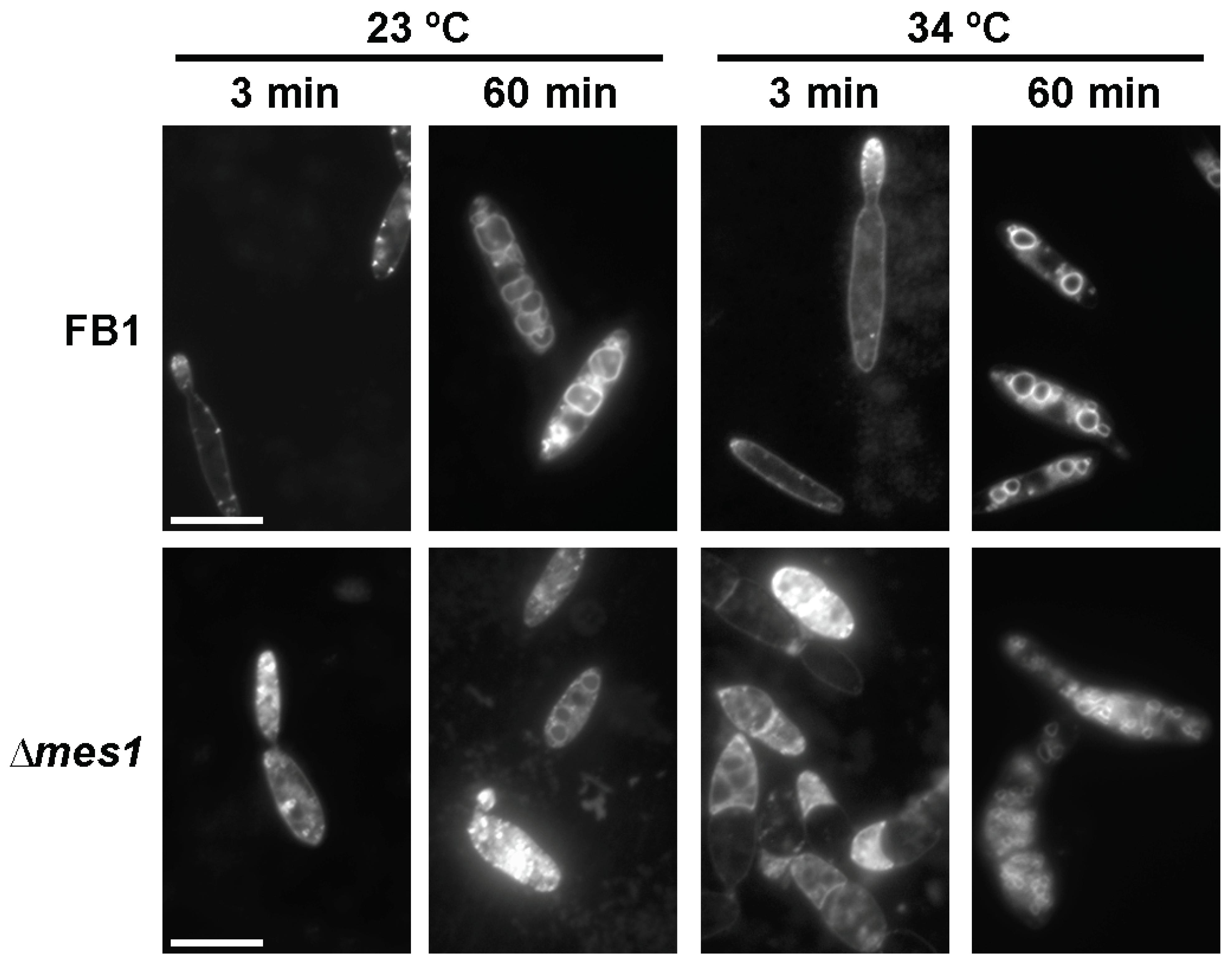
3.6. Endocytosis in the ∆mes1 Mutant
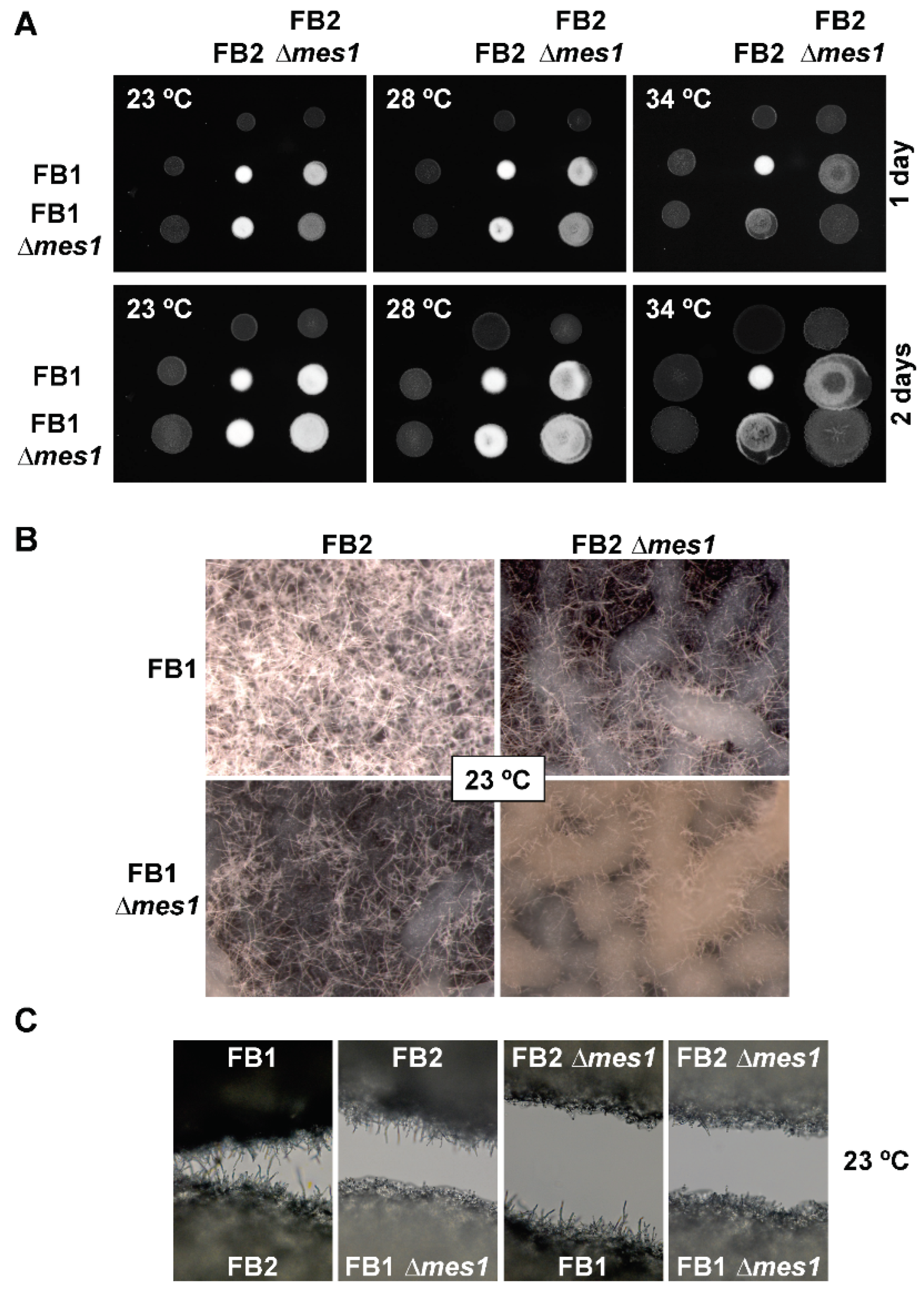
3.7. Role of Mes1 in Mating and Plant Infection
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gow, N.R. Yeast-Hyphal Dimorphism. The Growing Fungus; Chapman and Hall: London, UK, 1995; pp. 403–422. [Google Scholar]
- Boyce, K.J.; Andrianopoulos, A. Fungal dimorphism: The switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol. Rev. 2015, 39, 797–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, G.M. Dimorphism in fungal pathogens of mammals, plants, and insects. PLoS Pathog. 2015, 11, e1004608. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.; van de Veerdonk, F.L.; Brown, A.J.; Netea, M.G. Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nat. Rev. Microbiol. 2011, 10, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Kadosh, D. Regulatory mechanisms controlling morphology and pathogenesis in Candida albicans. Curr. Opin. Microbiol. 2019, 52, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martin, J.; Bardetti, P.; Castanheira, S.; de la Torre, A.; Tenorio-Gomez, M. Virulence-specific cell cycle and morphogenesis connections in pathogenic fungi. Semin. Cell Dev. Biol. 2016, 57, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Medoff, G.; Sacco, M.; Maresca, B.; Schlessinger, D.; Painter, A.; Kobayashi, G.S.; Carratu, L. Irreversible block of the mycelial-to-yeast phase transition of Histoplasma capsulatum. Science 1986, 231, 476–479. [Google Scholar] [CrossRef]
- Boyce, K.J.; Hynes, M.J.; Andrianopoulos, A. Control of morphogenesis and actin localization by the Penicillium marneffei RAC homolog. J. Cell Sci. 2003, 116, 1249–1260. [Google Scholar] [CrossRef] [Green Version]
- Mahlert, M.; Leveleki, L.; Hlubek, A.; Sandrock, B.; Bolker, M. Rac1 and Cdc42 regulate hyphal growth and cytokinesis in the dimorphic fungus Ustilago maydis. Mol. Microbiol. 2006, 59, 567–578. [Google Scholar] [CrossRef]
- Pearson, C.L.; Xu, K.; Sharpless, K.E.; Harris, S.D. MesA, a novel fungal protein required for the stabilization of polarity axes in Aspergillus nidulans. Mol. Biol. Cell 2004, 15, 3658–3672. [Google Scholar] [CrossRef] [Green Version]
- Rittenour, W.R.; Harris, S.D. Characterization of Fusarium graminearum Mes1 reveals roles in cell-surface organization and virulence. Fungal Genet. Biol. 2008, 45, 933–946. [Google Scholar] [CrossRef]
- Harsay, E.; Schekman, R. Avl9p, a member of a novel protein superfamily, functions in the late secretory pathway. Mol. Biol. Cell 2007, 18, 1203–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolker, M. Ustilago maydis—A valuable model system for the study of fungal dimorphism and virulence. Microbiology 2001, 147, 1395–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Herrera, J.; Perez-Rodriguez, F.; Velez-Haro, J. The signaling mechanisms involved in the dimorphic phenomenon of the Basidiomycota fungus Ustilago maydis. Int. Microbiol. 2020, 23, 121–126. [Google Scholar] [CrossRef]
- Agrios, G. Plant Pathology; Academic Press: London, UK, 1997. [Google Scholar]
- Bolker, M.; Urban, M.; Kahmann, R. The a mating type locus of U. maydis specifies cell signaling components. Cell 1992, 68, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Feldbrugge, M.; Kamper, J.; Steinberg, G.; Kahmann, R. Regulation of mating and pathogenic development in Ustilago maydis. Curr. Opin. Microbiol. 2004, 7, 666–672. [Google Scholar] [CrossRef]
- Steinberg, G.; Schliwa, M.; Lehmler, C.; Bolker, M.; Kahmann, R.; McIntosh, J.R. Kinesin from the plant pathogenic fungus Ustilago maydis is involved in vacuole formation and cytoplasmic migration. J. Cell Sci. 1998, 111, 2235–2246. [Google Scholar] [CrossRef]
- Snetselaar, K.; Mims, C. Sporidial fusion and infection of maize seedlings by the smut fungus Ustilago maydis. Mycologia 1992, 84, 193–203. [Google Scholar] [CrossRef]
- Snetselaar, K.; Mims, C. Infection of maize stigmas by Ustilago maydis: Light and electron microscopy. Phytopathology 1993, 83, 843–850. [Google Scholar] [CrossRef]
- Banuett, F.; Herskowitz, I. Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc. Natl. Acad. Sci. USA 1989, 86, 5878–5882. [Google Scholar] [CrossRef] [Green Version]
- Holliday, R. Ustilago maydis. In Handbook of Genetics; King, R.C., Ed.; Plenum Press: New York, NY, USA, 1974; pp. 575–595. [Google Scholar]
- Brachmann, A.; Weinzierl, G.; Kamper, J.; Kahmann, R. Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol. Microbiol. 2001, 42, 1047–1063. [Google Scholar] [CrossRef]
- Garcia-Muse, T.; Steinberg, G.; Perez-Martin, J. Characterization of B-type cyclins in the smut fungus Ustilago maydis: Roles in morphogenesis and pathogenicity. J. Cell. Sci. 2004, 117, 487–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canovas, D.; Studt, L.; Marcos, A.T.; Strauss, J. High-throughput format for the phenotyping of fungi on solid substrates. Sci. Rep. 2017, 7, 4289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamper, J. A PCR-based system for highly efficient generation of gene replacement mutants in Ustilago maydis. Mol. Genet. Genomics 2004, 271, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Brachmann, A.; Konig, J.; Julius, C.; Feldbrugge, M. A reverse genetic approach for generating gene replacement mutants in Ustilago maydis. Mol. Genet. Genom. 2004, 272, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Tabares, I.; Perez-Martin, J. Septins from the phytopathogenic fungus Ustilago maydis are required for proper morphogenesis but dispensable for virulence. PLoS ONE 2010, 5, e12933. [Google Scholar] [CrossRef]
- Castillo-Lluva, S.; Alvarez-Tabares, I.; Weber, I.; Steinberg, G.; Perez-Martin, J. Sustained cell polarity and virulence in the phytopathogenic fungus Ustilago maydis depends on an essential cyclin-dependent kinase from the Cdk5/Pho85 family. J. Cell Sci. 2007, 120, 1584–1595. [Google Scholar] [CrossRef] [Green Version]
- Snetselaar, K.M.; Bolker, M.; Kahmann, R. Ustilago maydis mating hyphae orient their growth toward pheromone sources. Fungal Genet. Biol. 1996, 20, 299–312. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015, 16, 157. [Google Scholar] [CrossRef] [Green Version]
- Ojeda-Lopez, M.; Chen, W.; Eagle, C.E.; Gutierrez, G.; Jia, W.L.; Swilaiman, S.S.; Huang, Z.; Park, H.S.; Yu, J.H.; Canovas, D.; et al. Evolution of asexual and sexual reproduction in the aspergilli. Stud. Mycol. 2018, 91, 37–59. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bryant, S.H. CD-Search: Protein domain annotations on the fly. Nucleic Acids Res. 2004, 32, W327–W331. [Google Scholar] [CrossRef] [PubMed]
- Amos, B.; Aurrecoechea, C.; Barba, M.; Barreto, A.; Basenko, E.Y.; Bazant, W.; Belnap, R.; Blevins, A.S.; Bohme, U.; Brestelli, J.; et al. VEuPathDB: The eukaryotic pathogen, vector and host bioinformatics resource center. Nucleic Acids Res. 2022, 50, D898–D911. [Google Scholar] [CrossRef] [PubMed]
- Cserzo, M.; Wallin, E.; Simon, I.; von Heijne, G.; Elofsson, A. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: The dense alignment surface method. Protein Eng. 1997, 10, 673–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamper, J.; Kahmann, R.; Bolker, M.; Ma, L.J.; Brefort, T.; Saville, B.J.; Banuett, F.; Kronstad, J.W.; Gold, S.E.; Muller, O.; et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 2006, 444, 97–101. [Google Scholar] [CrossRef]
- Canovas, D.; Perez-Martin, J. Sphingolipid biosynthesis is required for polar growth in the dimorphic phytopathogen Ustilago maydis. Fungal Genet. Biol. 2009, 46, 190–200. [Google Scholar] [CrossRef]
- Li, S.; Du, L.; Yuen, G.; Harris, S.D. Distinct ceramide synthases regulate polarized growth in the filamentous fungus Aspergillus nidulans. Mol. Biol. Cell 2006, 17, 1218–1227. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.W.; Konopka, J.B. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryot. Cell 2004, 3, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Nichols, C.B.; Fraser, J.A.; Heitman, J. PAK kinases Ste20 and Pak1 govern cell polarity at different stages of mating in Cryptococcus neoformans. Mol. Biol. Cell 2004, 15, 4476–4489. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, F.J.; Douglas, L.M.; Konopka, J.B. Sterol-rich plasma membrane domains in fungi. Eukaryot. Cell 2007, 6, 755–763. [Google Scholar] [CrossRef] [Green Version]
- Nagiec, M.M.; Nagiec, E.E.; Baltisberger, J.A.; Wells, G.B.; Lester, R.L.; Dickson, R.C. Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. J. Biol. Chem. 1997, 272, 9809–9817. [Google Scholar] [CrossRef] [Green Version]
- Weber, I.; Gruber, C.; Steinberg, G. A class-V myosin required for mating, hyphal growth, and pathogenicity in the dimorphic plant pathogen Ustilago maydis. Plant Cell 2003, 15, 2826–2842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delley, P.A.; Hall, M.N. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 1999, 147, 163–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momany, M.; Talbot, N.J. Septins Focus Cellular Growth for Host Infection by Pathogenic Fungi. Front. Cell Dev. Biol. 2017, 5, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuranda, K.; Leberre, V.; Sokol, S.; Palamarczyk, G.; Francois, J. Investigating the caffeine effects in the yeast Saccharomyces cerevisiae brings new insights into the connection between TOR, PKC and Ras/cAMP signalling pathways. Mol. Microbiol. 2006, 61, 1147–1166. [Google Scholar] [CrossRef]
- Pringle, J.R.; Preston, R.A.; Adams, A.E.; Stearns, T.; Drubin, D.G.; Haarer, B.K.; Jones, E.W. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989, 31, 357–435. [Google Scholar]
- Ram, A.F.; Wolters, A.; Ten Hoopen, R.; Klis, F.M. A new approach for isolating cell wall mutants in Saccharomyces cerevisiae by screening for hypersensitivity to calcofluor white. Yeast 1994, 10, 1019–1030. [Google Scholar] [CrossRef] [Green Version]
- Gaughran, J.P.; Lai, M.H.; Kirsch, D.R.; Silverman, S.J. Nikkomycin Z is a specific inhibitor of Saccharomyces cerevisiae chitin synthase isozyme Chs3 in vitro and in vivo. J. Bacteriol. 1994, 176, 5857–5860. [Google Scholar] [CrossRef] [Green Version]
- Weber, I.; Assmann, D.; Thines, E.; Steinberg, G. Polar localizing class V myosin chitin synthases are essential during early plant infection in the plant pathogenic fungus Ustilago maydis. Plant Cell 2006, 18, 225–242. [Google Scholar] [CrossRef] [Green Version]
- Fischer-Parton, S.; Parton, R.M.; Hickey, P.C.; Dijksterhuis, J.; Atkinson, H.A.; Read, N.D. Confocal microscopy of FM4-64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J. Microsc. 2000, 198, 246–259. [Google Scholar] [CrossRef] [Green Version]
- Wedlich-Soldner, R.; Bolker, M.; Kahmann, R.; Steinberg, G. A putative endosomal t-SNARE links exo- and endocytosis in the phytopathogenic fungus Ustilago maydis. EMBO J. 2000, 19, 1974–1986. [Google Scholar] [CrossRef]
- Day, P.R.; Anagnostakis, S.L. Corn smut dikaryon in culture. Nat. New Biol. 1971, 231, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Heitman, J. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 2006, 60, 69–105. [Google Scholar] [CrossRef] [PubMed]


| Tumour Formation a | |||
|---|---|---|---|
| Inoculum | Genotype | Total | Percentage |
| FB1 × FB2 | a1 b1 × a2 b2 | 32/35 | 91 |
| UMD12 × UMD16 | a1 b1 ∆mes1 × a2 b2 ∆mes1 | 0/47 b | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cánovas, D. The Fungal Protein Mes1 Is Required for Morphogenesis and Virulence in the Dimorphic Phytopathogen Ustilago maydis. J. Fungi 2022, 8, 759. https://doi.org/10.3390/jof8080759
Cánovas D. The Fungal Protein Mes1 Is Required for Morphogenesis and Virulence in the Dimorphic Phytopathogen Ustilago maydis. Journal of Fungi. 2022; 8(8):759. https://doi.org/10.3390/jof8080759
Chicago/Turabian StyleCánovas, David. 2022. "The Fungal Protein Mes1 Is Required for Morphogenesis and Virulence in the Dimorphic Phytopathogen Ustilago maydis" Journal of Fungi 8, no. 8: 759. https://doi.org/10.3390/jof8080759






