Identification and Functional Characterization of Adenosine Deaminase in Mucor circinelloides: A Novel Potential Regulator of Nitrogen Utilization and Lipid Biosynthesis
Abstract
:1. Introduction
2. Results
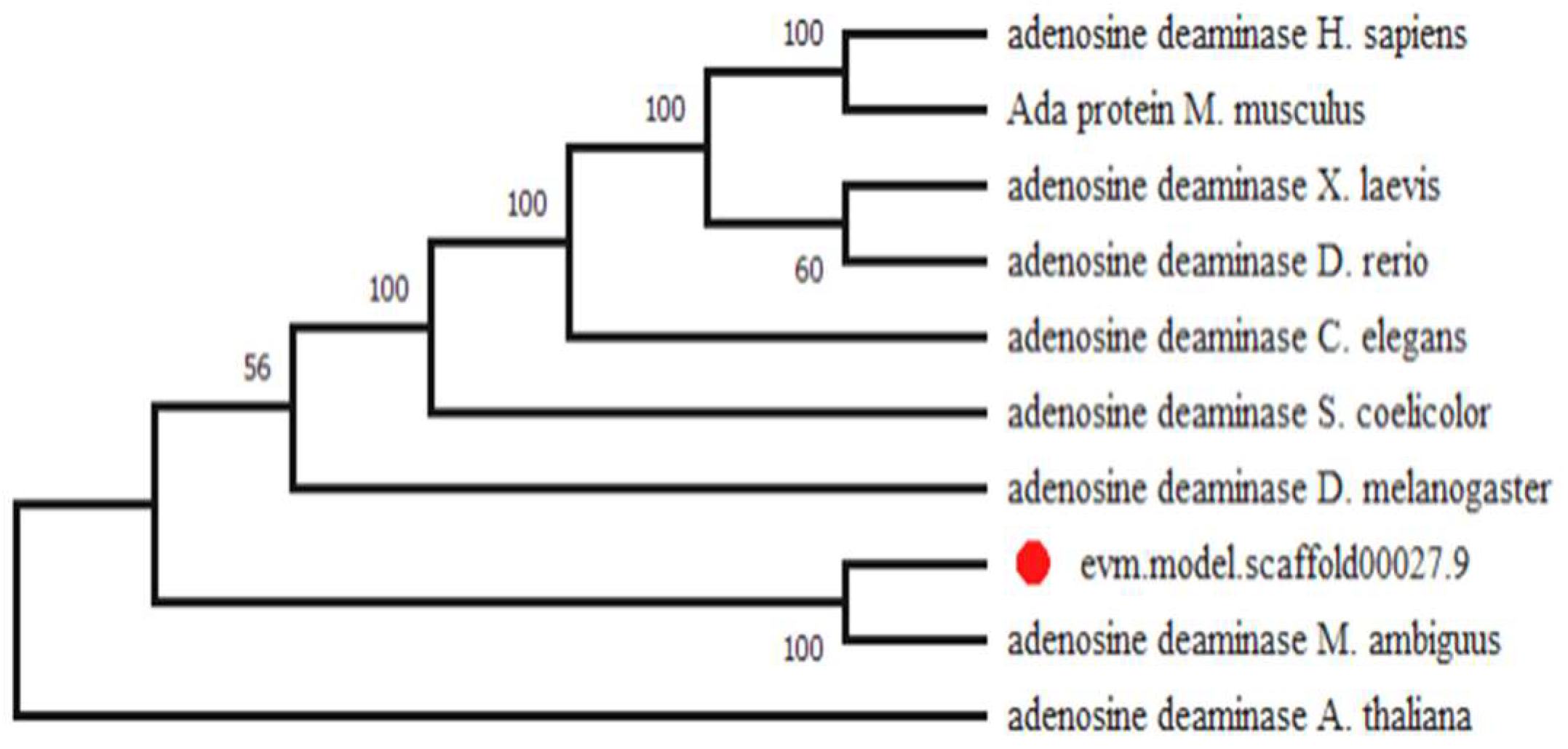
2.1. Identification of Gene Coding for the ADA in M. circinelloides
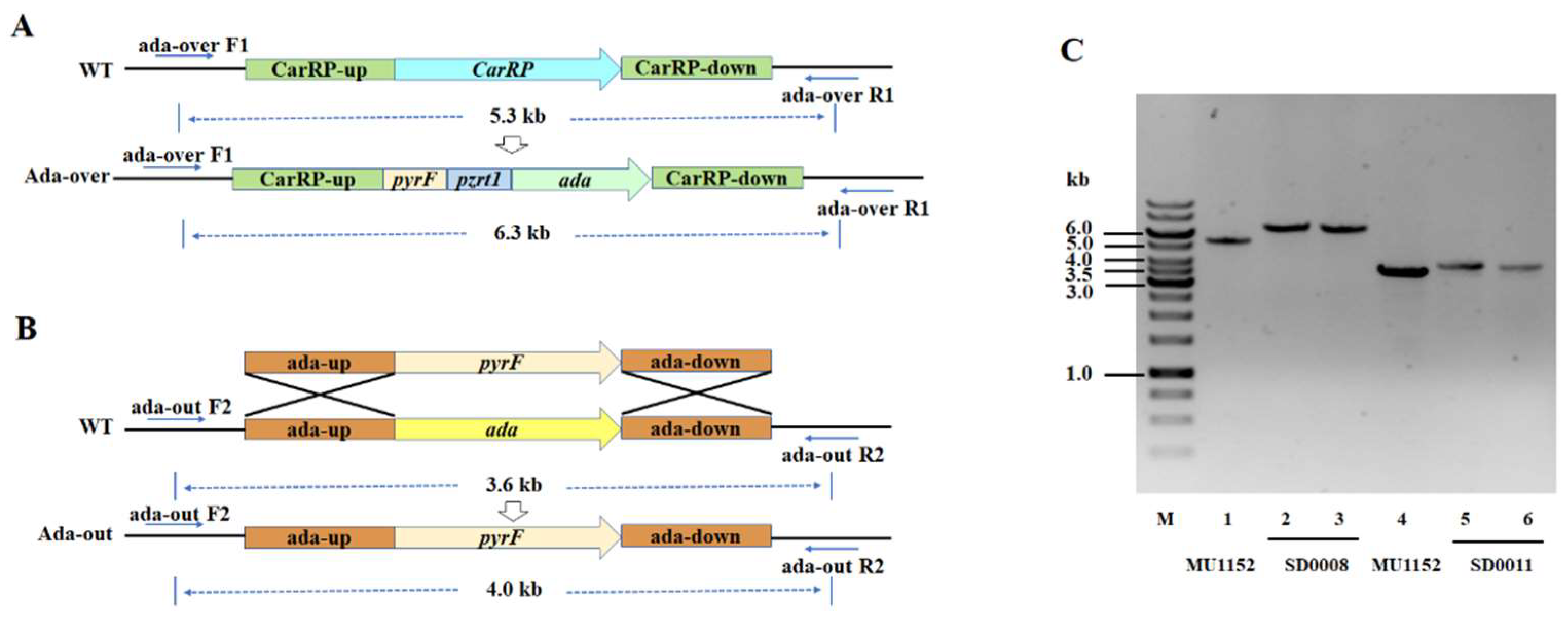
2.2. Generation of Ada Overexpressing and Knockout Transformants of M. circinelloides
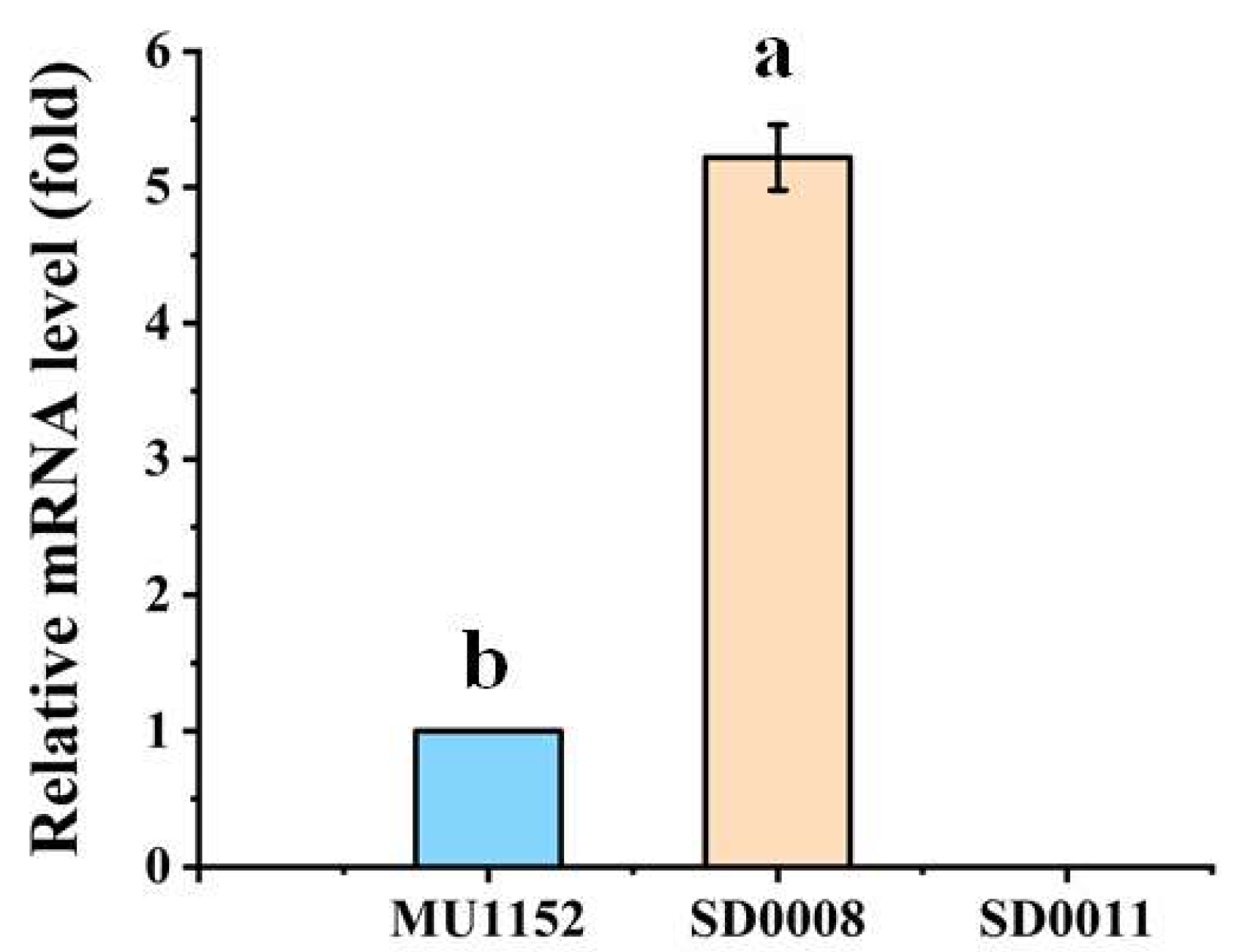
2.3. Analysis of Ada Expression in the Transformants
2.4. Ada Regulated Nitrogen Metabolism and Affected Cell Growth and Lipid Accumulation
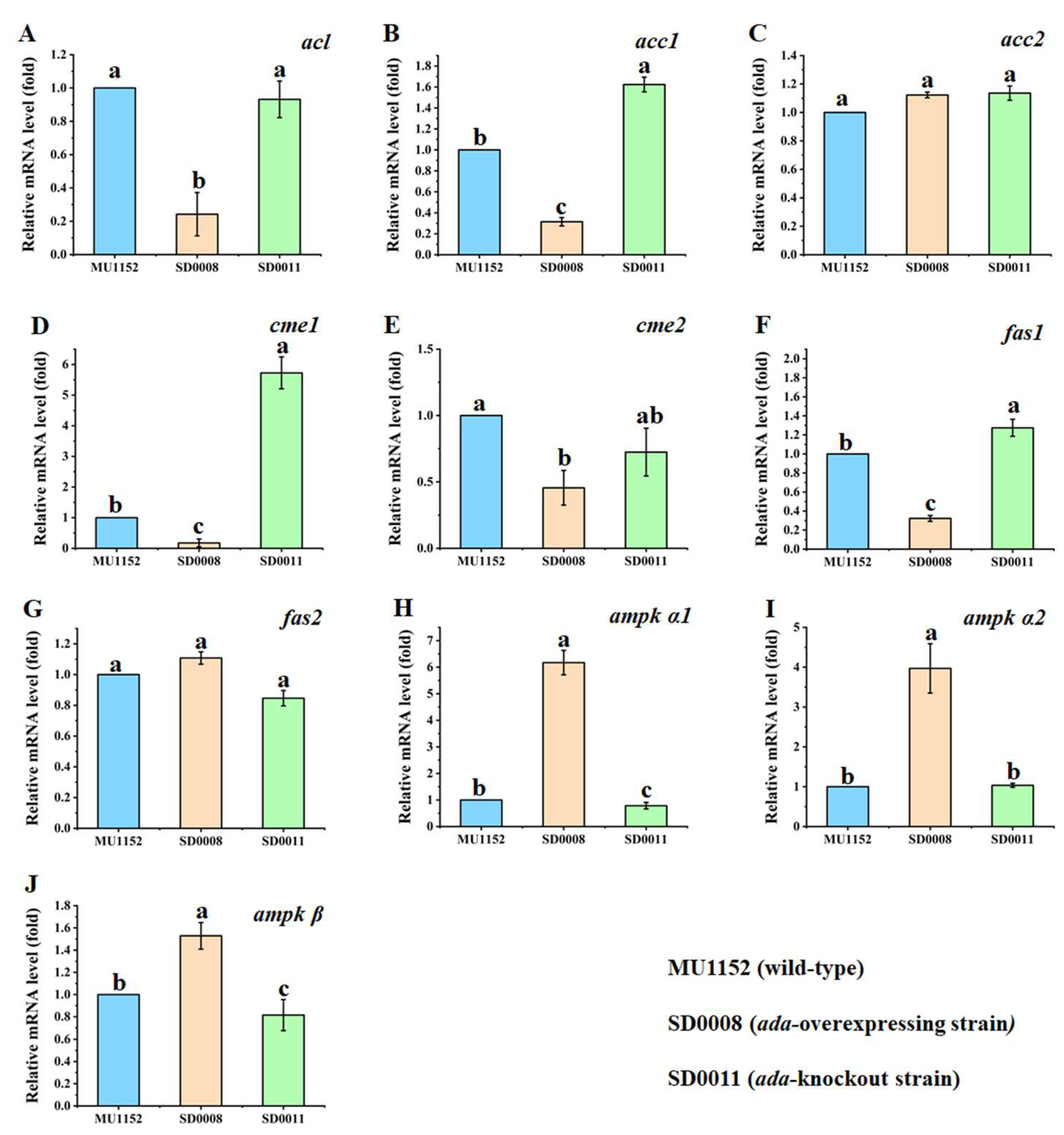
2.5. Impacts of Ada Gene Manipulation on the Expression Levels of the Key Genes for Fatty Acid Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Strains, Transformation, and Fermentation Conditions
4.2. Identification and Bioinformatics Analysis of ADA Gene in M. circinelloides
4.3. Plasmid Construction
4.4. Biochemical Analysis of the Fermentation Process
4.5. Determination of Lipid Accumulation in Transformants
4.6. RNA Isolation and Gene Expression Analysis by qRT-PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papanikolaou, S.; Rontou, M.; Belka, A.; Athenaki, M.; Gardeli, C.; Mallouchos, A.; Kalantzi, O.; Koutinas, A.A.; Kookos, I.K.; Zeng, A.P.; et al. Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng. Life Sci. 2017, 17, 262–281. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Amaro, R.; Nicaud, J.-M. Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog. Lipid Res. 2016, 61, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.S.; Yilmaz, J.L.; Green, A.G.; Stymne, S.; Hofvander, P. Replacing fossil oil with fresh oil—With what and for what? Eur. J. Lipid Sci. Technol. 2011, 113, 812–831. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Wan, X.; Jiang, M.; Hu, C.; Hu, H.; Huang, F. Metabolic engineering of microorganisms to produce omega-3 very long-chain polyunsaturated fatty acids. Prog. Lipid Res. 2014, 56, 19–35. [Google Scholar] [CrossRef]
- Park, Y.K.; Nicaud, J.M. Metabolic Engineering for Unusual Lipid Production in Yarrowia lipolytica. Microorganisms 2020, 8, 1937. [Google Scholar] [CrossRef]
- Czerwiec, Q.; Idrissitaghki, A.; Imatoukene, N.; Nonus, M.; Thomasset, B.; Nicaud, J.M.; Rossignol, T. Optimization of cyclopropane fatty acids production in Yarrowia lipolytica. Yeast 2019, 36, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Morin-Sardin, S.; Nodet, P.; Coton, E.; Jany, J.-L. Mucor: A Janus-faced fungal genus with human health impact and industrial applications. Fungal Biol. Rev. 2017, 31, 12–32. [Google Scholar] [CrossRef]
- Rodrigues Reis, C.E.; Bento, H.B.S.; Carvalho, A.K.F.; Rajendran, A.; Hu, B.; De Castro, H.F. Critical applications of Mucor circinelloides within a biorefinery context. Crit. Rev. Biotechnol. 2019, 39, 555–570. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Yang, J.; Yang, W.; Wang, X.; Wu, C.; Song, Y. Improved gamma-Linolenic Acid Production from Cellulose in Mucor circinelloides via Coexpression of Cellobiohydrolase and Delta-6 Desaturase. J. Agric. Food Chem. 2022, 70, 4373–4381. [Google Scholar] [CrossRef]
- Nordin, N.; Yusof, N.; Maeda, T.; Mustapha, N.A.; Mohd Yusoff, M.Z.; Raja Khairuddin, R.F. Mechanism of carbon partitioning towards starch and triacylglycerol in Chlorella vulgaris under nitrogen stress through whole-transcriptome analysis. Biomass Bioenergy 2020, 138, 105600. [Google Scholar] [CrossRef]
- Ratledg, C. Regulation of lipid accumulation in oleaginous micro-organisms. Biochem. Soc. 2002, 30, 1047–1050. [Google Scholar] [CrossRef] [Green Version]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Adams, I.P.; Ratledge, C. Malic enzyme: The controlling activity for lipid production? Overexpression of malic enzyme in Mucor circinelloides leads to a 2.5-fold increase in lipid accumulation. Microbiology (Reading) 2007, 153, 2013–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.A.; Hameed, A.; Khan, M.A.K.; Zhang, Y.; Zhang, H.; Garre, V.; Song, Y. Engineering of Fatty Acid Synthases (FASs) to Boost the Production of Medium-Chain Fatty Acids (MCFAs) in Mucor circinelloides. Int. J. Mol. Sci. 2019, 20, 786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Mohamed, H.; Bao, Y.; Wu, C.; Shi, W.; Song, Y.; Yang, J. Heterologous Expression of Two Malate Transporters From an Oleaginous Fungus Mucor circinelloides Improved the Lipid Accumulation in Mucor lusitanicus. Front. Microbiol. 2021, 12, 774825. [Google Scholar] [CrossRef]
- Tang, X.; Chen, H.; Gu, Z.; Zhang, H.; Chen, Y.Q.; Song, Y.; Chen, W. Role of g6pdh and leuB on Lipid Accumulation in Mucor circinelloides. J. Agric. Food Chem. 2020, 68, 4245–4251. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Song, Y. Identification and Characterization of Diacylglycerol Acyltransferase from Oleaginous Fungus Mucor circinelloides. J. Agric. Food Chem. 2018, 66, 674–681. [Google Scholar] [CrossRef]
- Yang, J.; Cánovas-Márquez, J.T.; Li, P.; Li, S.; Niu, J.; Wang, X.; Nazir, Y.; López-García, S.; Garre, V.; Song, Y. Deletion of Plasma Membrane Malate Transporters Increased Lipid Accumulation in the Oleaginous Fungus Mucor circinelloides WJ11. J. Agric. Food Chem. 2021, 69, 9632–9641. [Google Scholar] [CrossRef]
- Gao, Z.W.; Wang, X.; Zhang, H.Z.; Lin, F.; Liu, C.; Dong, K. The roles of adenosine deaminase in autoimmune diseases. Autoimmun. Rev. 2021, 20, 102709. [Google Scholar] [CrossRef]
- Challa, A.; Johnson, S.; Robertson, K.; Gumasekaran, M. Properties of adenosine deaminase from Candida albicans. J. Basic Microbiol. 1998, 39, 97–101. [Google Scholar] [CrossRef]
- Wolfenden, R.; Sharpless, T.K.; Allan, R. Substrate Binding by Adenosine Deaminase. J. Biol. Chem. 1967, 242, 977–983. [Google Scholar] [CrossRef]
- Lin, J.; Liao, X.; Du, G.; Chen, J. Enhancement of glutathione production in a coupled system of adenosine deaminase-deficient recombinant Escherichia coli and Saccharomyces cerevisiae. Enzym. Microb. Technol. 2009, 44, 269–273. [Google Scholar] [CrossRef]
- Erkilic, K.; Evereklioglu, C.; Cekmen, M.; Ozkiris, A.; Duygulu, F.; Dogan, H. Adenosine deaminase enzyme activity is increased and negatively correlates with catalase, superoxide dismutase and glutathione peroxidase in patients with Behcet’s disease: Original contributions/clinical and laboratory investigations. Mediat. Inflamm 2003, 12, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gitanjali, G.; Sudeep, G.; Neerja, G.; Deepak, A.; Priyanka, S. The effect of Hyperglycemia on some biochemical parameters in diabetes mellitus. J. Clin. Diag Res. 2010, 4, 3181–3186. [Google Scholar]
- Havilah, P.; Pandit, V.B.; Durga, P.K. Adenosine Deaminase Activity in Type-2 Diabetes Mellitus—An Independent Marker of Glycemic Status and Stimulator of Lipid Peroxidation. Int. J. Chem. Life Sci. 2013, 2, 1175–1178. [Google Scholar]
- Niraula, A.; Thapa, S.; Kunwar, S.; Lamsal, M.; Baral, N.; Maskey, R. Adenosine deaminase activity in type 2 diabetes mellitus: Does it have any role? BMC Endocr. Disord. 2018, 18, 58. [Google Scholar] [CrossRef]
- Wilson, D.K.; Rudolph, F.B.; Quiocho, F.A.R. Atomic Structure of Adenosine Deaminase Complexed with a Transition-State Analog: Understanding Catalysis and Immunodeficiency Mutations. Science 1991, 252, 1278–1284. [Google Scholar] [CrossRef]
- Franco, R.; Mallol, J.; Casadó, V.; Lluis, C.; Canela, E.I.; Saura, C.; Blanco, J.; Ciruela, F. Ecto-Adenosine Deaminase: An Ecto-Enzyme and a Costimulatory Protein Acting On a Variety of Cell Surface Receptors. Drug Dev. Res. 1998, 45, 261–268. [Google Scholar] [CrossRef]
- Rosemberg, D.B.; Rico, E.P.; Guidoti, M.R.; Dias, R.D.; Souza, D.O.; Bonan, C.D.; Bogo, M.R. Adenosine deaminase-related genes: Molecular identification, tissue expression pattern and truncated alternative splice isoform in adult zebrafish (Danio rerio). Life Sci. 2007, 81, 1526–1534. [Google Scholar] [CrossRef]
- Rodriguez-Frometa, R.A.; Gutierrez, A.; Torres-Martinez, S.; Garre, V. Malic enzyme activity is not the only bottleneck for lipid accumulation in the oleaginous fungus Mucor circinelloides. Appl. Microbiol. Biotechnol. 2013, 97, 3063–3072. [Google Scholar] [CrossRef]
- Yang, Z.K.; Ma, Y.H.; Zheng, J.W.; Yang, W.D.; Liu, J.S.; Li, H.Y. Proteomics to reveal metabolic network shifts towards lipid accumulation following nitrogen deprivation in the diatom Phaeodactylum tricornutum. J. Appl. Phycol. 2014, 26, 73–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Zan, X.; Zhao, L.; Chen, H.; Chen, Y.Q.; Chen, W.; Song, Y.; Ratledge, C. Proteomics analysis of high lipid-producing strain Mucor circinelloides WJ11: An explanation for the mechanism of lipid accumulation at the proteomic level. Microb. Cell Fact. 2016, 15, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, J.S.; Sugden, P.H. Stimulation of protein synthesis, glucose uptake and lactate output by insulin and adenosine deaminase in the rat heart. Fed. Eur. Biochem. Soc. 1986, 201, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Saghiri, R.; Ghashghai, N.; Movaseghi, S.; Poursharifi, P.; Jalilfar, S.; Bidhendi, M.A.; Ghazizadeh, L.; Ebrahimi-Rad, M. Serum adenosine deaminase activity in patients with systemic lupus erythematosus: A study based on ADA1 and ADA2 isoenzymes pattern. Rheumatol. Int. 2012, 32, 1633–1638. [Google Scholar] [CrossRef]
- Kose, K.; Yazici, C.; Ascioglu, O. The evaluation of lipid peroxidation and adenosine deaminase activity in patients with Behcet’s disease. Clin. Biochem. 2001, 34, 125–129. [Google Scholar] [CrossRef]
- Koupenova, M.; Ravid, K. Adenosine, adenosine receptors and their role in glucose homeostasis and lipid metabolism. J. Cell Physiol. 2013, 228, 1703–1712. [Google Scholar] [CrossRef]
- Johansson, S.M.; Lindgren, E.; Yang, J.N.; Herling, A.W.; Fredholm, B.B. Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue-interactions with insulin. Eur. J. Pharmacol. 2008, 597, 92–101. [Google Scholar] [CrossRef]
- Botham, P.A.; Ratledge, C. A Biochemical Explanation for Lipid Accumulation in Candida 107 and Other Oleaginous Micro-organisms. J. Gen. Microbiol. 1979, 114, 361–375. [Google Scholar] [CrossRef] [Green Version]
- Larijani, B.; Heshmat, R.; Ebrahimi-Rad, M.; Khatami, S.; Valadbeigi, S.; Saghiri, R. Diagnostic Value of Adenosine Deaminase and Its Isoforms in Type II Diabetes Mellitus. Enzyme Res. 2016, 2016, 9526593. [Google Scholar] [CrossRef]
- Namiot, Z.; Barańczuk, E.; Marcinkiewicz, M.; Wójcik, B. Adenosine deaminase activity in various parts of the gastrointestinal tract of streptozotocin treated rats. Die Pharm. 1993, 48, 950. [Google Scholar]
- Zurovec, M.; Dolezal, T.; Gazi, M.; Pavlova, E.; Bryant, P.J. Adenosine deaminase-related growth factors stimulate cell proliferation in Drosophila by depleting extracellular adenosine. Proc. Natl. Acad. Sci. USA 2002, 99, 4403–4408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcelino, H.; Nogueira, V.C.; Santos, C.R.A.; Quelhas, P.; Carvalho, T.M.A.; Fonseca-Gomes, J.; Tomas, J.; Diogenes, M.J.; Sebastiao, A.M.; Cascalheira, J.F. Adenosine inhibits human astrocyte proliferation independently of adenosine receptor activation. J. Neurochem. 2020, 153, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Yamada, K.; Masuoka, K.; Tsuboi, I.; Itoh, K.; Nonaka, K.; Oizumi, K. Elevated adenosine deaminase activity in the serum of patients with diabetes mellitus. Diabetes Res. Clin. Pract. 1994, 25, 97–102. [Google Scholar] [CrossRef]
- Johansson, S.M.; Yang, J.N.; Lindgren, E.; Fredholm, B.B. Eliminating the antilipolytic adenosine A1 receptor does not lead to compensatory changes in the antilipolytic actions of PGE2 and nicotinic acid. Acta Physiol. 2007, 190, 87–96. [Google Scholar] [CrossRef]
- Cheng, J.T.; Liu, I.M.; Chi, T.C.; Shinozuka, K.; Lu, F.H.; Wu, T.J.; Chang, C.J. Role of Adenosine in Insulin-Stimulated Release of Leptin From Isolated White Adipocytes of Wistar Rats. Diabetes 2000, 49, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.J.; Sacramento, J.F.; Gallego-Martin, T.; Olea, E.; Melo, B.F.; Guarino, M.P.; Yubero, S.; Obeso, A.; Conde, S.V. High fat diet blunts the effects of leptin on ventilation and on carotid body activity. J. Physiol. 2018, 596, 3187–3199. [Google Scholar] [CrossRef]
- Ratledge, C.; Boulyon, C.A. ATP: Citrate Lyase—The Regulatory Enzyme for Lipid Biosynthesis in Lipornyces starkeyi? J. Gen. Microbiol. 1981, 127, 423–426. [Google Scholar] [CrossRef] [Green Version]
- Ratledge, C.; Wynn, J.P. Malic enzyme is a major source of NADPH for lipid accumulation by Aspergillus nidulans. Microbiology 1997, 143, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, L.; Chen, H.; Chen, Y.Q.; Chen, W.; Song, Y.; Ratledge, C. Enhanced lipid accumulation in the yeast Yarrowia lipolytica by over-expression of ATP:citrate lyase from Mus musculus. J. Biotechnol. 2014, 192, 78–84. [Google Scholar] [CrossRef]
- Dulermo, T.; Lazar, Z.; Dulermo, R.; Rakicka, M.; Haddouche, R.; Nicaud, J.M. Analysis of ATP-citrate lyase and malic enzyme mutants of Yarrowia lipolytica points out the importance of mannitol metabolism in fatty acid synthesis. Biochim. Biophys. Acta 2015, 1851, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Sun, H.; Mo, X.; Li, X.; Xu, B.; Tian, P. Overexpression of malic enzyme (ME) of Mucor circinelloides improved lipid accumulation in engineered Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97, 4927–4936. [Google Scholar] [CrossRef] [PubMed]
- Ruenwai, R.; Cheevadhanarak, S.; Laoteng, K. Overexpression of acetyl-CoA carboxylase gene of Mucor rouxii enhanced fatty acid content in Hansenula polymorpha. Mol. Biotechnol. 2009, 42, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, R.; Wang, R.; Haque, M.E.; Liu, A. Overexpression of ACC gene from oleaginous yeast Lipomyces starkeyi enhanced the lipid accumulation in Saccharomyces cerevisiae with increased levels of glycerol 3-phosphate substrates. Biosci. Biotechnol. Biochem. 2016, 80, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.S.; Solbiati, J.; Cronan, J.E., Jr. Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J. Biol. Chem. 2000, 275, 28593–28598. [Google Scholar] [CrossRef] [Green Version]
- Klaus, D.; Ohlrogge, J.B.; Neuhaus, H.E.; Dormann, P. Increased fatty acid production in potato by engineering of acetyl-CoA carboxylase. Planta 2004, 219, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Visser, B.; Roelofs, D.; Hahn, D.A.; Teal, P.E.; Marien, J.; Ellers, J. Transcriptional changes associated with lack of lipid synthesis in parasitoids. Genome Biol. Evol. 2012, 4, 752–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajiwara, S.; Oura, T.; Shishido, K. Cloning of a fatty acid synthase component FAS1 gene from Saccharomyces kluyveri and its functional complementation of S. cerevisiae fas1 mutant. Yeast Seq. Rep. 2001, 18, 1339–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, C.A.; Trub, A.G.; Hirschey, M.D. Sensing Mitochondrial Acetyl-CoA to Tune Respiration. Trends Endocrinol. Metab. 2019, 30, 1–3. [Google Scholar] [CrossRef]
- Boone, A.; Brownsey, R.; Elliott, J.; Kulpa, J.; Lee, W. Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 2006, 34, 223–227. [Google Scholar] [CrossRef]
- Seip, J.; Jackson, R.; He, H.; Zhu, Q.; Hong, S.P. Snf1 is a regulator of lipid accumulation in Yarrowia lipolytica. Appl. Environ. Microbiol. 2013, 79, 7360–7370. [Google Scholar] [CrossRef] [Green Version]
- Nosheen, S.; Naz, T.; Yang, J.; Hussain, S.A.; Fazili, A.B.A.; Nazir, Y.; Li, S.; Mohamed, H.; Yang, W.; Mustafa, K.; et al. Role of Snf-beta in lipid accumulation in the high lipid-producing fungus Mucor circinelloides WJ11. Microb. Cell Fact. 2021, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, C.; Yoo, J.Y.; Tagne, J.B.; Kacherovsky, N.; Lee, T.I.; Young, E.T. Combined global localization analysis and transcriptome data identify genes that are directly coregulated by Adr1 and Cat8. Mol. Cell Biol. 2005, 25, 2138–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, K.; Wu, F.; Chen, G.; Dong, H.; Li, J.; Zhao, Y.; Xu, L.; Zou, X.; Lu, F. Diosgenin ameliorates palmitic acid-induced lipid accumulation via AMPK/ACC/CPT-1A and SREBP-1c/FAS signaling pathways in LO2 cells. BMC Complement. Altern. Med. 2019, 19, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimann, E.; Nyman, M.; Degerman, E. Propionic acid and butyric acid inhibit lipolysis and de novo lipogenesis and increase insulin-stimulated glucose uptake in primary rat adipocytes. Adipocyte 2015, 4, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.; Mottillo, E.P. Adipocyte lipolysis: From molecular mechanisms of regulation to disease and therapeutics. Biochem. J. 2020, 477, 985–1008. [Google Scholar] [CrossRef]
- Hanahan, D. Studies on Transformation of Escherichia coli with Plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef]
- Nicolas, F.E.; Navarro-Mendoza, M.I.; Perez-Arques, C.; Lopez-Garcia, S.; Navarro, E.; Torres-Martinez, S.; Garre, V. Molecular Tools for Carotenogenesis Analysis in the Mucoral Mucor circinelloides. Methods Mol. Biol 2018, 1852, 221–237. [Google Scholar] [CrossRef]
- Kendrick, A.; Ratledge, C. Desaturation of polyunsaturated fatty acids in Mucor circinelloides and the involvement of a novel membrane-bound malic enzyme. Eur. J. Biochem. 1992, 209, 667–673. [Google Scholar] [CrossRef]
- Yang, J.; Khan, M.A.K.; Lopez-Garcia, S.; Nosheen, S.; Nazir, Y.; Zhang, H.; Garre, V.; Song, Y. Improved SDA Production in High Lipid Accumulating Strain of Mucor circinelloides WJ11 by Genetic Modification. Am. J. Biochem. Biotechnol. 2020, 16, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yang, J.; Mohamed, H.; Wang, X.; Pang, S.; Wu, C.; López-García, S.; Song, Y. Identification and Functional Characterization of Adenosine Deaminase in Mucor circinelloides: A Novel Potential Regulator of Nitrogen Utilization and Lipid Biosynthesis. J. Fungi 2022, 8, 774. https://doi.org/10.3390/jof8080774
Li S, Yang J, Mohamed H, Wang X, Pang S, Wu C, López-García S, Song Y. Identification and Functional Characterization of Adenosine Deaminase in Mucor circinelloides: A Novel Potential Regulator of Nitrogen Utilization and Lipid Biosynthesis. Journal of Fungi. 2022; 8(8):774. https://doi.org/10.3390/jof8080774
Chicago/Turabian StyleLi, Shaoqi, Junhuan Yang, Hassan Mohamed, Xiuwen Wang, Shuxian Pang, Chen Wu, Sergio López-García, and Yuanda Song. 2022. "Identification and Functional Characterization of Adenosine Deaminase in Mucor circinelloides: A Novel Potential Regulator of Nitrogen Utilization and Lipid Biosynthesis" Journal of Fungi 8, no. 8: 774. https://doi.org/10.3390/jof8080774






