Ophiostomatales Associated with Mediterranean Pine Engraver, Orthotomicus erosus (Coleoptera, Curculionidae) in Dalmatia, Croatia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Collection of Samples
2.2. Isolation of Fungi from Bark Beetles
2.3. Isolation of Fungi from Woody Tissues and Galleries
2.4. Identification of Fungi
2.5. DNA Extraction, PCR, and Sequencing
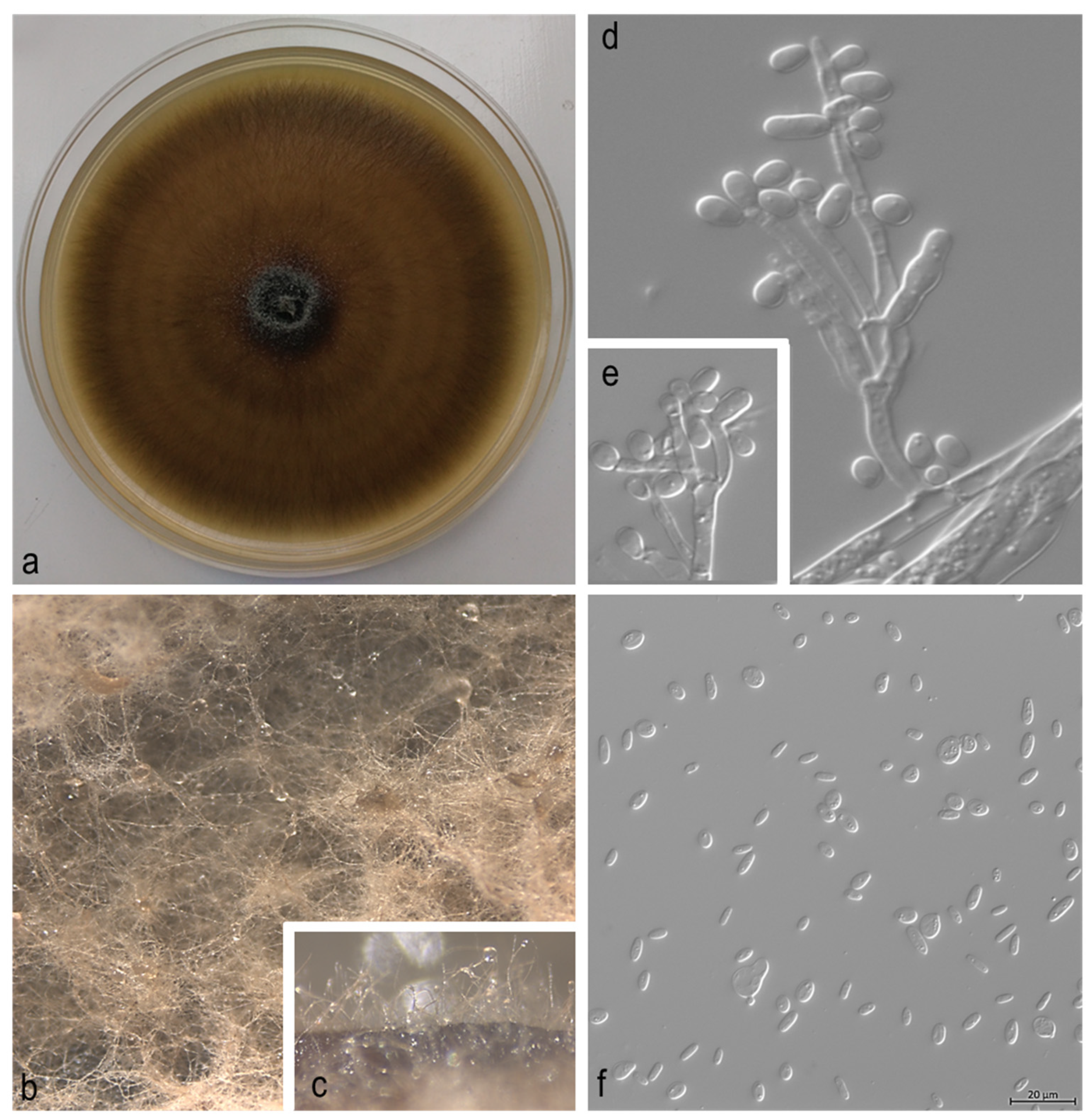
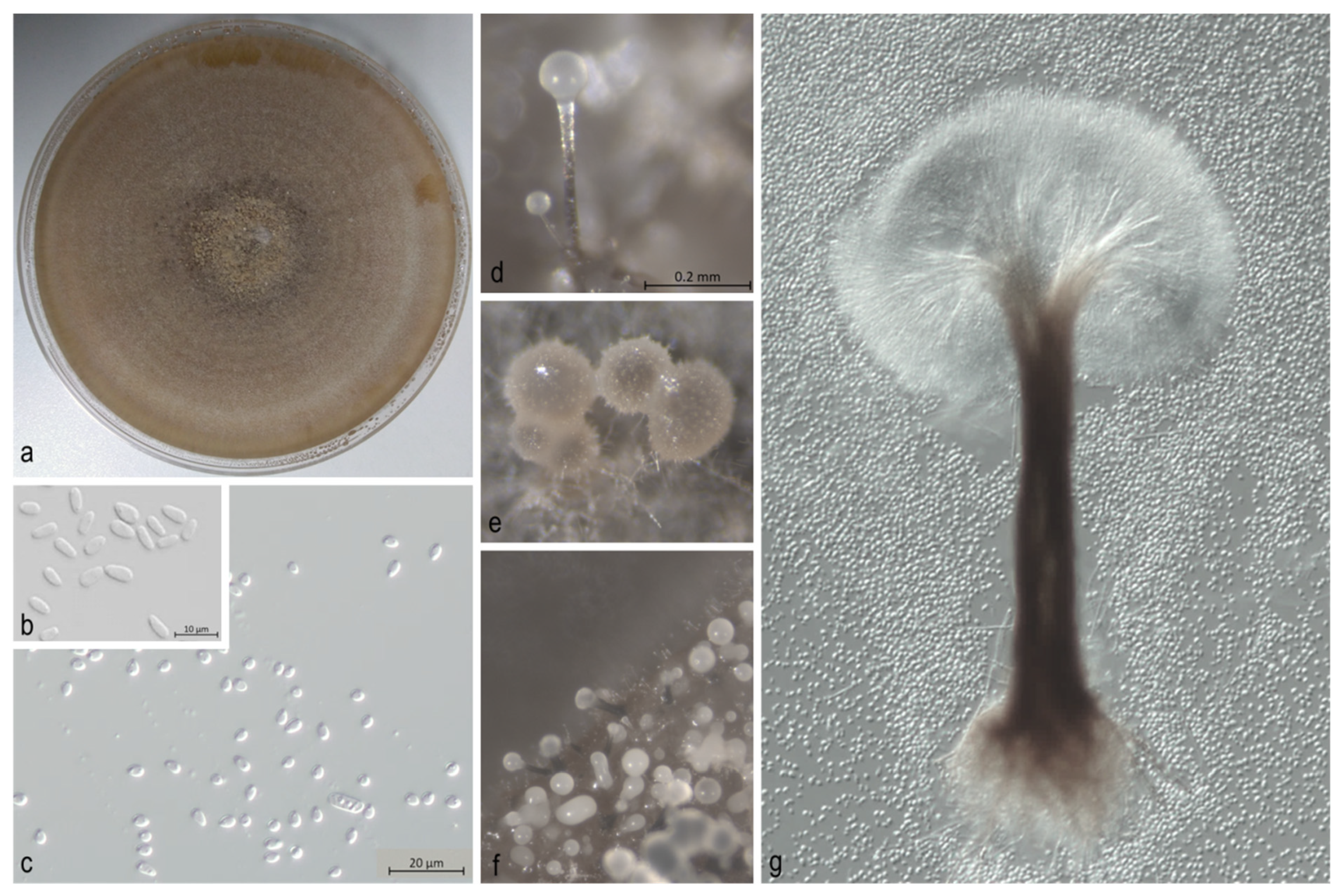
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrington, T.C.; McNew, D.; Steimel, J.; Hofstra, D.; Farrell, R. Phylogeny and taxonomy of the Ophiostoma piceae complex and the Dutch elm disease fungi. Mycologia 2001, 93, 111–136. [Google Scholar] [CrossRef]
- Kirisits, T. Dutch Elm Disease and Other Ophiostoma Diseases; CAB International: Wallingford, CT, USA, 2013; pp. 256–282. [Google Scholar]
- Wingfield, M.J.; Barnes, I.; de Beer, Z.W.; Roux, J.; Wingfield, B.D.; Taerum, S.J. Novel associations between ophiostomatoid fungi, insects and tree hosts: Current status-future prospects. Biol. Invasions 2017, 19, 3215–3228. [Google Scholar] [CrossRef]
- Bentz, B.; Logan, J.; MacMahon, J.; Allen, C.D.; Ayres, M.P.; Berg, E.; Carroll, A.; Hansen, M.; Hicke, J.; Joyce, L.; et al. Bark Beetle Outbreaks in Western North. America: Causes and Consequences; University of Utah: Salt Lake City, UT, USA, 2009; 42p. [Google Scholar]
- de Groot, M.; Ogris, N.; Kobler, A. The effects of a large-scale ice storm event on the drivers of bark beetle outbreaks and associated management practices. For. Ecol. Manag. 2018, 408, 195–201. [Google Scholar] [CrossRef]
- Hlásny, T.; König, L.; Krokene, P.; Lindner, M.; Montagné-Huck, C.; Müller, J.; Qin, H.; Raffa, K.F.; Schelhaas, M.-J.; Svoboda, M.; et al. Bark Beetle Outbreaks in Europe: State of Knowledge and Ways Forward for Management. Curr. For. Rep. 2021, 7, 138–165. [Google Scholar] [CrossRef]
- Schelhaas, M.J.; Nabuurs, G.J.; Schuck, A. Natural disturbances in the European forests in the 19th and 20th centuries. Glob. Chang. Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Rosenberger, R.S.; Bell, L.A.; Champ, P.A.; White, E.M. Estimating the economic value of recreation losses in Rocky Mountain National Park due to a mountain pine beetle outbreak. West. Econ. Forum 2013, 12, 31–39. [Google Scholar]
- Brasier, C. Dual origin of recent Dutch elm disease outbreaks in Europe. Nature 1979, 281, 78–80. [Google Scholar] [CrossRef]
- Redfern, D.B.; Stoakley, J.T.; Steele, H.; Minter, D.W. Dieback and death of larch caused by Ceratocystis laricicola sp. nov. following attack by Ips cembrae. Plant Pathol. 1987, 36, 467–480. [Google Scholar] [CrossRef]
- Montecchio, L.; Vettorazzo, M.; Faccoli, M. Thousand cankers disease in Europe: An overview. EPPO Bull. 2016, 46, 335–340. [Google Scholar] [CrossRef]
- Six, D.L.; Wingfield, M.J. The role of phytopathogenicity in bark beetle-fungus symbioses: A challenge to the classic paradigm. Annu. Rev. Entomol. 2011, 56, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Otrosina, W.J.; Hess, N.J.; Zarnoch, S.J.; Perry, T.J.; Jones, J.P. Blue-stain fungi associated with roots of southern pine trees attacked by the southern pine beetle, Dendroctonus frontalis. Plant Dis. 1997, 81, 942–945. [Google Scholar] [CrossRef]
- Hyde, K.D.; Norphanphoun, C.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Jones, E.B.G.; Bundhun, D.; Chen, Y.J.; Bao, D.F.; Boonmee, S.; Calabon, M.S.; et al. Refined families of Sordariomycetes. Mycosphere 2020, 11, 305–1059. [Google Scholar] [CrossRef]
- Kirisits, T. Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi. In Bark and Wood Boring Insects in Living Trees in Europe, A Synthesis; Lieutier, F., Day, K.R., Battisti, A., Gregoire, J.C., Evans, H.F., Eds.; Kluwer Academic: Dordrecht, The Netherland, 2004; pp. 181–236. [Google Scholar]
- Lieutier, F.; Yart, A.; Salle, A. Stimulation of tree defenses by Ophiostomatoid fungi can explain attack success of bark beetles on conifers. Ann. For. Sci. 2009, 66, 801. [Google Scholar] [CrossRef]
- Christiansen, E.; Solheim, H. The bark beetle-associated blue stain fungus Ophiostoma polonicum can kill various spruces and Douglas fir. Eur. J. For. Pathol. 1990, 20, 436–446. [Google Scholar] [CrossRef]
- Solheim, H.; Långström, B.; Hellqvist, C. Pathogenicity of the blue-stain fungi Leptographium wingfieldii and Ophiostoma minus to Scots pine: Effect of tree pruning and inoculum density. Can. J. For. Res. 1993, 23, 1438–1443. [Google Scholar] [CrossRef]
- Repe, A.; Bojović, S.; Jurc, M. Pathogenicity of ophiostomatoid fungi on Picea abies in Slovenia. For. Pathol. 2015, 45, 290–297. [Google Scholar] [CrossRef]
- Seifert, K.A.; De Beer, Z.W.; Wingfeld, M.J. The Ophiostomatoid Fungi: Expanding Frontiers, CBS Biodiversity Series; CBS-KNAW Biodiversity Centre: Utrecht, The Netherlands, 2013; Volume 12. [Google Scholar]
- Kirisits, T.; Diminić, D.; Hrašovec, B.; Pernek, M.; Barić, B. First reports of Silver fir blue staining Ophiostomatoid fungi associated with Pityokteines spinidens. Croat. J. For. Eng. 2009, 30, 51–57. [Google Scholar]
- Wingfield, M.J.; Seifert, K.A.; Webber, J.F. Ophiostoma and Ceratocystis: Taxonomy, Ecology and Pathogenicity; APS Press: St. Paul, MN, USA, 1993; p. 293. [Google Scholar]
- Pernek, M.; Hrašovec, B.; Matošević, D.; Pilaš, I.; Kirisits, T.; Moser, J.C. Phoretic mites of three bark beetles (Pityokteines spp.) on Silver fir. J. Pest Sci. 2008, 81, 35–42. [Google Scholar] [CrossRef]
- Wood, S.L.; Bright, D.E. A catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: Taxonomic index. Volume A & B. Great Basin Nat. Mem. 1992, 13, 1–1557. [Google Scholar]
- Haack, R.A. Orthotomicus erosus: A new pine-infesting bark beetle in the United States. Newsl. Mich. Entomol. Soc. 2004, 49, 3. [Google Scholar]
- Mendel, Z.; Halperin, J. The biology and behaviour of Orthotomicus erosus in Israel. Phytoparasitica 1982, 10, 169–181. [Google Scholar] [CrossRef]
- Walter, A.J.; Venette, R.C.; Kells, S.A. Acceptance and suitability of novel trees for Orthotomicus erosus, an exotic bark beetle in North America. Biol. Invasions 2009, 12, 1133–1144. [Google Scholar] [CrossRef]
- Sarıkaya, O.; Ibis, H.M.; Toprak, Ö. The flight activity and population density of Orthotomicus erosus (Wollaston, 1857) in the Brutian pine (Pinus brutia Ten.) forests of İzmir Province, Turkey. Int. J. Sci. Basic Appl. 2013, 12, 208–219. [Google Scholar]
- Lieutier, F.; Paine, T.D. Responses of Mediterranean Forest Phytophagous Insects to Climate Change. In Insects and Diseases of Mediterranean Forest Systems, Paine, D.T., Lieutier, F., Eds.; Springer: Basel, Switzerland, 2016; pp. 801–858. [Google Scholar]
- Pernek, M.; Lacković, N.; Lukić, I.; Zorić, N.; Matošević, D. Outbreak of Orthotomicus erosus (Coleoptera, Curculionidae) on Aleppo Pine in the Mediterranean Region in Croatia. South-East Eur. For. 2019, 10, 19–27. [Google Scholar] [CrossRef]
- Mendel, Z.; Madar, Z.; Golan, Y. Hymenopterous parasitoids of pine bark beetles in Israel. Hasadeh 1986, 66, 1899–1901. [Google Scholar]
- Mendel, Z. The relation of bast scale and bark beetle outbreaks to management of pine plantations in Israel. In XVII International Congress of Entomology, Proceedings of the IUFRO Working Party, Vancouver, BC, Canada, 4 July 1988; Payne, T.L., Saarenmaa, H., Eds.; College of Agriculture and Life Science, V.P.I and State University: Blacksburg, VA, USA, 1988; pp. 329–335. [Google Scholar]
- Ben Jamaa, M.L.; Lieutier, F.; Yart, A.; Jerraya, A.; Khouja, M.L. The virulence of phytopathogenic fungi associated with the bark beetles Tomicus piniperda and Orthotomicus erosus in Tunisia. For. Pathol. 2007, 37, 51–63. [Google Scholar] [CrossRef]
- Pernek, M. Sušenje borova u Park šumi Marjan sa mjerama integrirane zaštite šuma za sprječavanje širenja i suzbijanje mediteranskog potkornjaka Orthotomicus erosus (Woll.). Elaborat 2018, 1–83. (In Croatian) [Google Scholar]
- Hlávková, D.; Doležal, P. Cambioxylophagous Pests of Scots Pine: Ecological Physiology of European Populations—A Review. Front. For. Glob. Chang. 2022, 5, 864651. [Google Scholar] [CrossRef]
- Mendel, Z. Seasonal history of Orthotomicus erosus (Coleoptera:Scolytidae) in Israel. Phytoparasitica 1983, 11, 13–24. [Google Scholar] [CrossRef]
- Mendel, Z.; Madar, Z.; Golan, Y. Comparison of the seasonal occurrence and behavior of seven pine bark beetles (Coleoptera, Scolytidae) in Israel. Phytoparasitica 1985, 13, 21–32. [Google Scholar] [CrossRef]
- Pfeffer, A. Zentral- und Westpaläarktische Borkenund Kernkäfer; Pro Entomologia, Naturhistorisches Museum: Basel, Switzerland, 1995; 310p. [Google Scholar]
- Zhou, X.D.; de Beer, Z.W.; Ahumada, R.; Wingfield, B.D.; Wingfield, M.J. Ophiostoma and Ceratocystiopsis spp. associated with two pine-infesting bark beetles in Chile. Fungal Divers. 2004, 15, 261–274. [Google Scholar]
- Harrington, T.C. Ecology and evolution of mycophagous bark beetles and their fungal partners. In Insect-Fungal Associations: Ecology and Evolution; Vega, F.E., Blackwell, M., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 257–291. [Google Scholar]
- Suh, D.Y.; Hyun, M.W.; Kim, J.J.; Son, S.Y.; Kim, S.H. Ophiostoma ips from pinewood nematode vector, Japanese pine sawyer beetle (Monochamus alternatus), in Korea. Mycobiology 2013, 41, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Z.; Liu, F.; Xu Wu, C.; Fang Zhang, S.; Kong, X.B.; Decock, C.; Lu, Q.; Zhang, Z. Diferential patterns of ophiostomatoid fungal communities associated with three sympatric Tomicus species infesting pines in south-western China, with a description of four new species. MycoKeys 2019, 50, 93–133. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Gardes, M.; White, T.J.; Fortin, J.A.; Bruns, T.D.; Taylor, J.W. Identification of indigenous and introduced symbiotic fungi in ectomycorrhizae by amplification of nuclear and mitochondrial ribosomal DNA. Can. J. Bot. 1991, 69, 180–190. [Google Scholar] [CrossRef]
- O’Donnel, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Marincowitz, S.; Duong, T.A.; De Beer, Z.W.; Wingfield, M.J. Cornuvesica: A little known mycophilic genus with a unique biology and unexpected new species. Fungal Biol. 2015, 119, 615–630. [Google Scholar] [CrossRef]
- Jacobs, K.; Bergdahl, D.R.; Wingfield, M.J.; Halik, S.; Seifert, K.A.; Bright, D.E.; Wingfield, B.D. Leptographium wingfieldii introduced into North America and found associated with exotic Tomicus piniperda and native bark beetles. Mycol. Res. 2004, 108, 411–418. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Caroll, A.L.; Taylor, S.W.; Regniere, J.; Safranyik, I. Effects on climate change on range expansion by the mountain pine beetle in British Columbia. In Mountain Pine Beetle Symposium: Challenges and Solutions, Information Report BC-X-399; Shore, T.L., Brooks, J.E., Stone, J.E., Eds.; Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2004; pp. 223–232. [Google Scholar]
- Pureswaran, D.S.; Roques, A.; Battisti, A. Forest insects and climate change. Curr For. Rep. 2018, 4, 35–50. [Google Scholar] [CrossRef]
- Pernek, M.; Kovač, M.; Lacković, N. Testing of biological effectiveness of pheromones and traps for catch of mediterranean bark beetle Orthotomicus erosus (Coleoptera, Curculionidae). Sumar. List 2020, 144, 339–350. [Google Scholar]
- Wingfield, M.J.; Marasas, W.F.O. Ceratocystis ips associated with Orthotomicus erosus (Coleoptera: Scolytidae) on Pinus spp. in the Cape Province of South Africa. Phytophylactica 1980, 12, 65–68. [Google Scholar]
- Zhou, X.D.; De Beer, Z.W.; Wingfield, B.; Wingfield, M. Ophiostomatoid fungi associated with three pine-infesting bark beetles in South Africa. Sydowia 2001, 53, 290–300. [Google Scholar]
- Dori-Bachash, M.; Avrahami-Moyal, L.; Protasov, A.; Mendel, Z.; Freeman, S. The occurrence and pathogenicity of Geosmithia spp. and common blue-stain fungi associated with pine bark beetles in planted forests in Israel. Eur. J. Plant Pathol. 2015, 143, 627–639. [Google Scholar] [CrossRef]
- Human, Z.R.; Slippers, B.; De Beer, Z.W.; Wingfield, M.J.; Venter, S.N. Antifungal actinomycetes associated with the pine bark beetle, Orthotomicus erosus, in South Africa. S. Afr. J. Sci. 2017, 113, 34–40. [Google Scholar] [CrossRef]
- Jankowiak, R. Ophiostomatoid fungi associated with Ips sexdentatus on Pinus sylvestris in Poland. Dendrobiology 2012, 68, 43–54. [Google Scholar]
- Romón, P.; Zhou, X.D.; Iturrondobeitia, J.C.; Wingfield, M.J.; Goldarazena, A. Ophiostoma species (Ascomycetes: Ophiostomatales) associated with bark beetles (Coleoptera: Scolytinae) colonizing Pinus radiata in northern Spain. Can. J. Microbiol. 2007, 53, 756–767. [Google Scholar] [CrossRef] [PubMed]
- De Beer, Z.W.; Duong, T.A.; Wingfield, M.J. The divorce of Sporothrix and Ophiostoma: Solution to a problematic relationship. Stud. Mycol. 2016, 83, 165–191. [Google Scholar] [CrossRef]
- Jankowiak, R.; Strzałka, B.; Bilański, P.; Kacprzyk, M.; Lukášová, K.; Linnakoski, R.; Matwiejczuk, S.; Misztela, M.; Rossa, R. Diversity of Ophiostomatales species associated with conifer infesting beetles in the Western Carpathians. Eur. J. For. Res. 2017, 136, 939–956. [Google Scholar] [CrossRef]
- Trollip, C.; Carnegie, A.J.; Dinh, Q.; Kaur, J.; Smith, D.; Mann, R.; Edwards, J. Ophiostomatoid fungi associated with pine bark beetles and infested pines in south-eastern Australia, including Graphilbum ipis-grandicollis sp. nov. IMA Fungus 2021, 12, 1–27. [Google Scholar]
- Chang, R.; Duong, T.A.; Taerum, S.J.; Wingfield, M.J.; Zhou, X.; de Beer, Z.W. Ophiostomatoid fungi associated with conifer-infesting beetles and their phoretic mites in Yunnan, China. MycoKeys 2017, 28, 19–64. [Google Scholar] [CrossRef] [PubMed]
- Repe, A.; Kirisits, T.; Piškur, B.; De Groot, M.; Kump, B.; Jurc, M. Ophiostomatoid fungi associated with three spruce-infesting bark beetles in Slovenia. Ann. For. Sci. 2013, 70, 717–727. [Google Scholar] [CrossRef][Green Version]
- Kwaśna, H.; Mazur, A.; Łabędzki, A.; Kuźmiński, R.; Łakomy, P. Communities of fungi in decomposed wood of oak and pine. Leśne Pract. Badaw. 2016, 77, 261–275. [Google Scholar] [CrossRef]
- Jankowiak, R.; Szewczyk, G.; Bilański, P.; Jazłowiecka, D.; Harabin, B.; Linnakoski, R. Blue-stain fungi isolated from freshly felled Scots pine logs in Poland, including Leptographium sosnaicola sp. nov. For. Pathol. 2021, 51, e12672. [Google Scholar] [CrossRef]

| Year | Forest Office | Management Unit | Tree Species | WGS Coordinates | Bark Beetle Infestation Level in 2018 | ||
|---|---|---|---|---|---|---|---|
| Area [ha] | Wood Mass [m3] | Intensity [%] | |||||
| 2017 2018 2019 | SPLIT | Marjan | Pinus halepensis Pinus pinea | 43°30′33″ N, 16°24′38″ E | 196 | 5.900 | 21–40 |
| 2018 | KORČULA | Ošjak | Pinus halepensis | 42°57′40″ N, 16°41′7″ E | 18 | 1.800 | 21–40 |
| 2018 | ZADAR | Sukošan | Pinus halepensis | 44°02′54.0″ N, 15°19′22.1″ E | 70 | 150 | 1–20 |
| 2019 | DUBROVNIK | Lokrum | Pinus halepensis | 42°37′38.7″ N, 18°07′15.9″ E | 70 | 100 | 1–20 |
| Isolate ID | Host Tree | Isolation Source | Year of Sampling | Locality | Identified Fungi |
|---|---|---|---|---|---|
| 1OE1 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 2OE2 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 3OE3 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 4OE4 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Sporothrix pseudoabietina |
| 5OE5 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Pezizomycotina |
| 6OE6 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 7OE7 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ceratocystiopsis cf. minuta |
| 8BS1 | Pinus halepensis | blue stained wood | 2018 | Marjan | Sporothrix pseudoabietina |
| 9OE8 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Sporothrix pseudoabietina |
| 10OE9 | Pinus pinea | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 11BS2 | Pinus pinea | blue stained wood | 2018 | Marjan | Ophiostoma ips |
| 12OE10 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 13OE11 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 14OE12 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Graphilbum cf. rectangulosporium |
| 15OE13 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Graphilbum cf. rectangulosporium |
| 16OE14 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 18OE15 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Sporothrix pseudoabietina |
| 19OE16 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Sporothrix pseudoabietina |
| 20BS3 | Pinus halepensis | blue stained wood | 2018 | Ošjak | Pezizomycotina |
| 20BS4 | Pinus halepensis | blue stained wood | 2018 | Ošjak | Pezizomycotina |
| 21BS5 | Pinus halepensis | blue stained wood | 2018 | Ošjak | Pezizomycotina |
| 22OE17 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 23OE18 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 24OE19 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 25OE20 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 26BS6 | Pinus pinea | blue stained wood | 2018 | Marjan | Ophiostoma ips |
| 27BS7 | Pinus halepensis | blue stained wood | 2018 | Ošjak | Pezizomycotina |
| 28OE21 | Pinus pinea | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 29G1 | Pinus pinea | gallery | 2018 | Marjan | Ophiostoma ips |
| 30G2 | Pinus pinea | gallery | 2018 | Marjan | Ophiostoma ips |
| 32OE22 | Pinus pinea | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 34OE23 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 35OE24 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 36OE25 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Sporothrix pseudoabietina |
| 37OE26 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Sporothrix pseudoabietina |
| 38OE27 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Sporothrix pseudoabietina |
| 39BS9 | Pinus halepensis | blue stained wood | 2019 | Marjan | Ophiostoma piceae |
| 40OE28 | Pinus pinea | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 41OE29 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 42BS10 | Pinus halepensis | blue stained wood | 2019 | Marjan | Graphilbum cf. rectangulosporium |
| 42BS11 | Pinus halepensis | blue stained wood | 2019 | Marjan | Graphilbum cf. rectangulosporium |
| 43OE30 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 44OE31 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 45OE32 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Ophiostoma ips |
| 46BS11 | Pinus pinea | blue stained wood | 2018 | Marjan | Ophiostoma ips |
| 47BS12 | Pinus pinea | blue stained wood | 2018 | Marjan | Ophiostoma ips |
| 48OE33 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Graphilbum cf. rectangulosporium |
| 49OE34 | Pinus halepensis | Orthotomicus erosus | 2018 | Marjan | Sporothrix pseudoabietina |
| 50BS13 | Pinus halepensis | blue stained wood | 2017 | Marjan | Ophiostoma ips |
| 51BS14 | Pinus halepensis | blue stained wood | 2017 | Marjan | Ophiostoma floccosum |
| 52BS15 | Pinus halepensis | blue stained wood | 2017 | Marjan | Ophiostoma floccosum |
| 53BS16 | Pinus halepensis | blue stained wood | 2017 | Marjan | Ophiostoma floccosum |
| 54BS17 | Pinus halepensis | blue stained wood | 2017 | Marjan | Ophiostoma piceae |
| 55BS18 | Pinus halepensis | blue stained wood | 2018 | Sukošan | Ophiostoma ips |
| 56BS19 | Pinus halepensis | blue stained wood | 2018 | Sukošan | Ophiostoma ips |
| 57BS20 | Pinus halepensis | blue stained wood | 2018 | Sukošan | Sarocladium strictum |
| 58BS21 | Pinus halepensis | blue stained wood | 2019 | Lokrum | Ophiostoma piceae |
| 59BS22 | Pinus halepensis | blue stained wood | 2019 | Lokrum | Ophiostoma piceae |
| 60BS23 | Pinus halepensis | blue stained wood | 2019 | Lokrum | Ophiostoma piceae |
| 61BS24 | Pinus halepensis | blue stained wood | 2019 | Lokrum | Ophiostoma piceae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovač, M.; Rigling, D.; Pernek, M. Ophiostomatales Associated with Mediterranean Pine Engraver, Orthotomicus erosus (Coleoptera, Curculionidae) in Dalmatia, Croatia. J. Fungi 2022, 8, 788. https://doi.org/10.3390/jof8080788
Kovač M, Rigling D, Pernek M. Ophiostomatales Associated with Mediterranean Pine Engraver, Orthotomicus erosus (Coleoptera, Curculionidae) in Dalmatia, Croatia. Journal of Fungi. 2022; 8(8):788. https://doi.org/10.3390/jof8080788
Chicago/Turabian StyleKovač, Marta, Daniel Rigling, and Milan Pernek. 2022. "Ophiostomatales Associated with Mediterranean Pine Engraver, Orthotomicus erosus (Coleoptera, Curculionidae) in Dalmatia, Croatia" Journal of Fungi 8, no. 8: 788. https://doi.org/10.3390/jof8080788
APA StyleKovač, M., Rigling, D., & Pernek, M. (2022). Ophiostomatales Associated with Mediterranean Pine Engraver, Orthotomicus erosus (Coleoptera, Curculionidae) in Dalmatia, Croatia. Journal of Fungi, 8(8), 788. https://doi.org/10.3390/jof8080788








