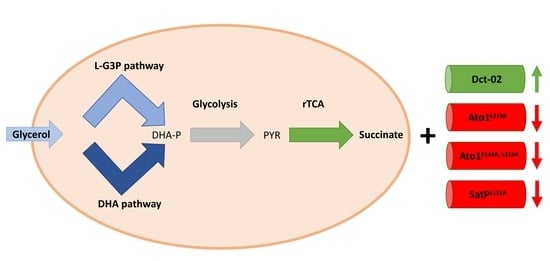
The Dicarboxylate Transporters from the AceTr Family and Dct-02 Oppositely Affect Succinic Acid Production in S. cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids and Maintenance
2.2. General Molecular Biology Techniques
2.3. General Strategies for Genomic Integrations Via CRISPR-Cas9
2.4. Construction of the SA Strain
2.5. Construction of SA-Dct-02, SA-Ato1 (S), SA-Ato1 (HS), SA-SatP (HS), and SA-COX7SatP (HS) Strains
2.6. Isolation of Genomic DNA from S. cerevisiae Transformants and Diagnostic PCR
2.7. Media and Cultivation Conditions for the Production of Succinic Acid from Glycerol
2.8. Metabolite Analysis by HPLC
3. Results
3.1. Construction of the Baseline SA Strain
3.2. Impact of Dct-02 Expression on Extracellular Succinic Acid Accumulation in the Baseline Strain SA
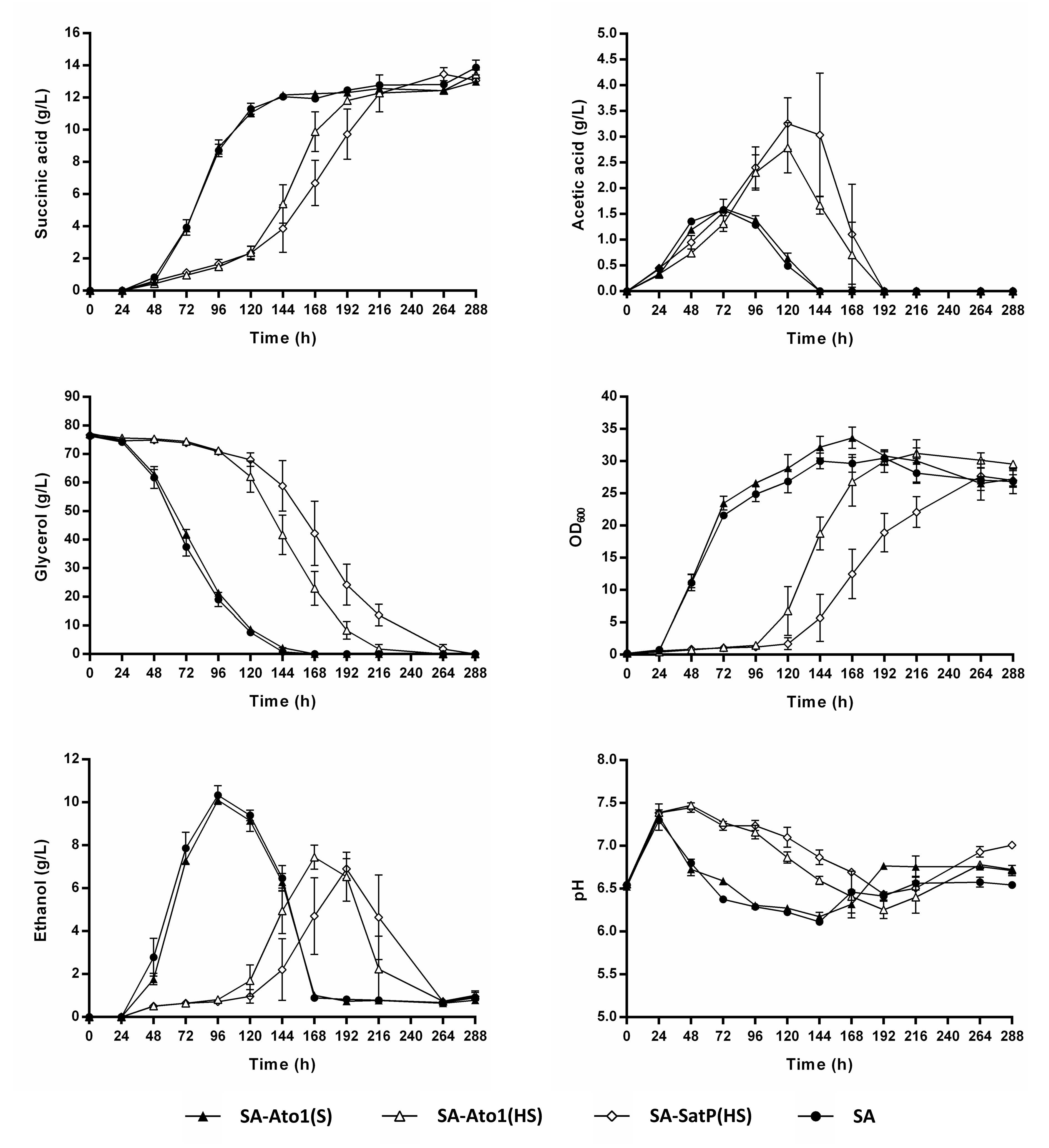
3.3. Impact of Engineered AceTr Homologues on Succinic Acid Production of the Strain SA
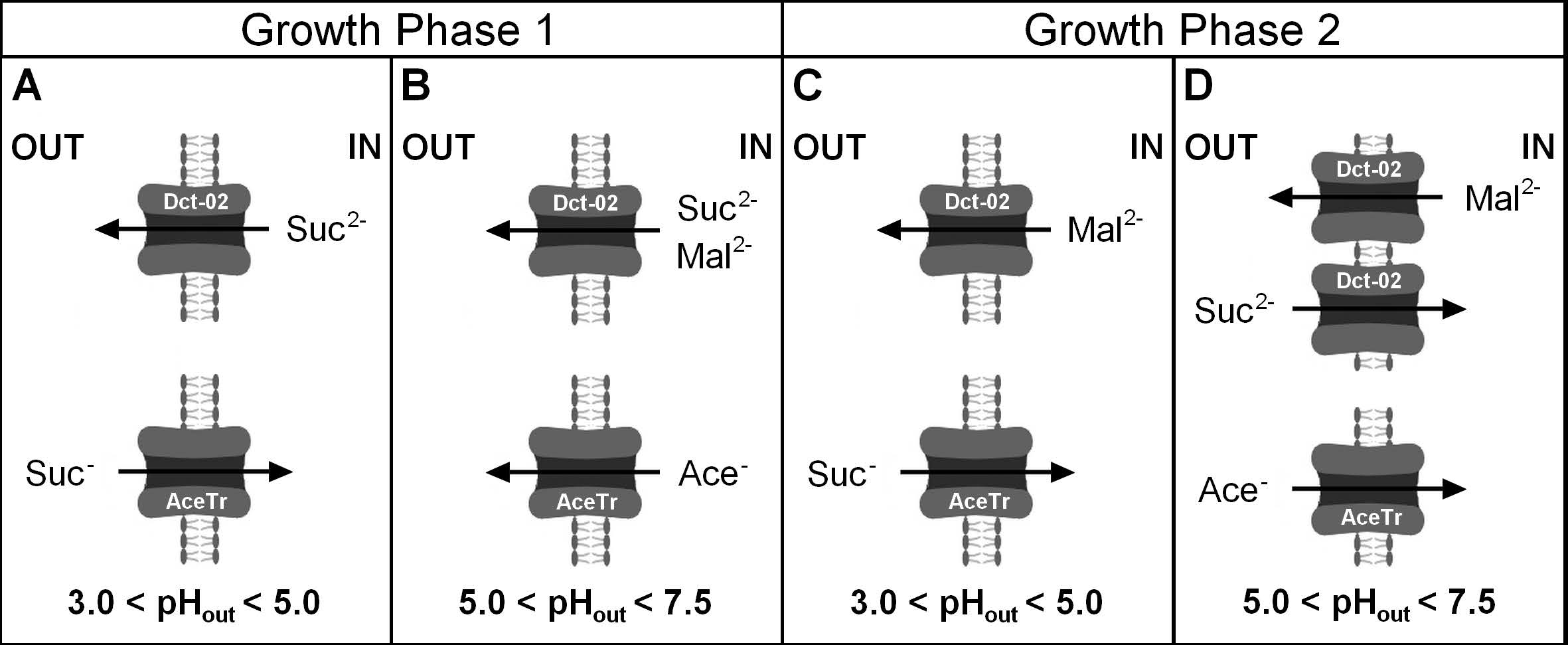
3.4. External pH Modulated the Activity of Engineered AceTr Homologues
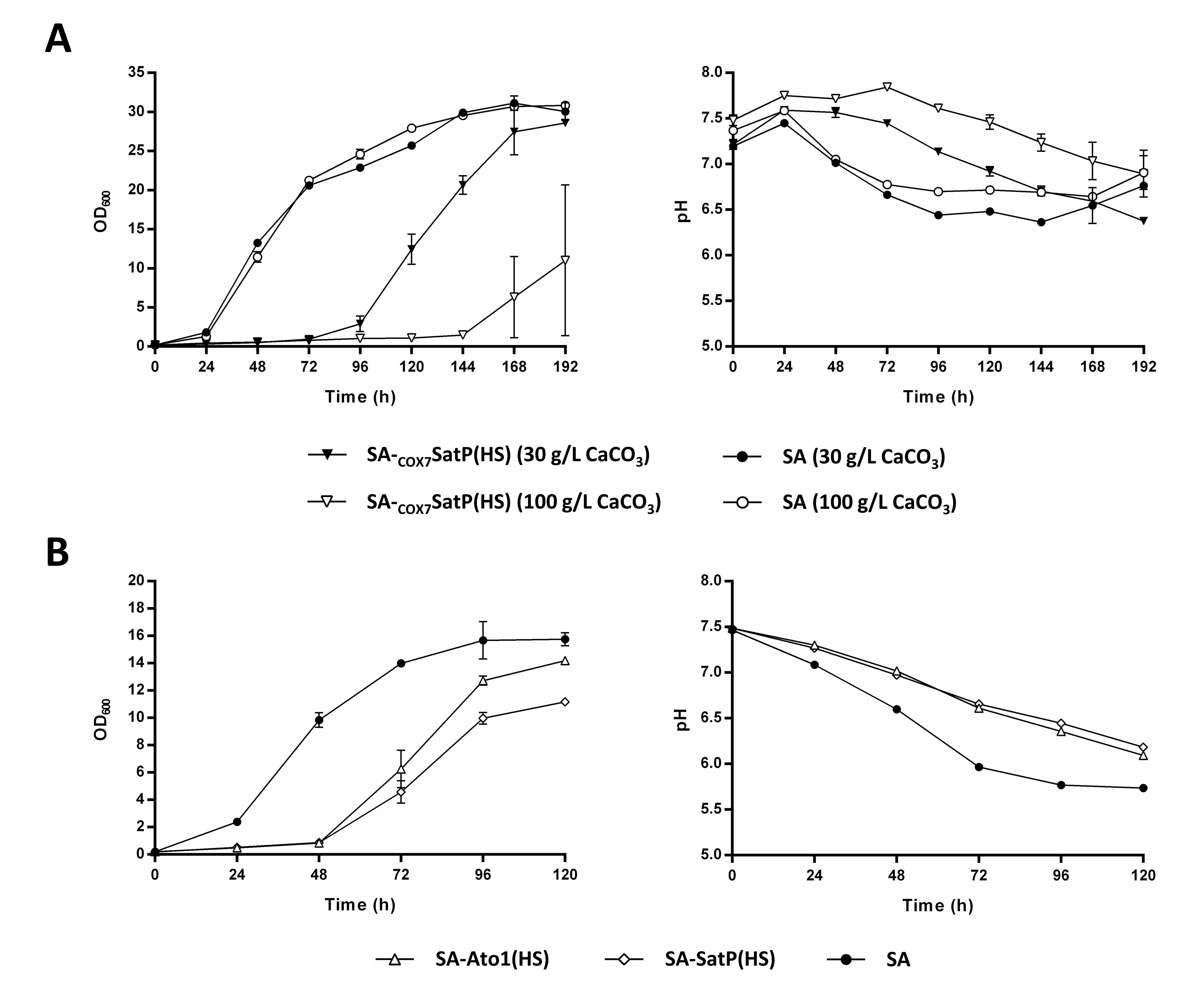
3.5. Understanding the Prolonged Lag Phase of the Strains SA-Ato1 (HS) and SA-SatP (HS) in CaCO3-Buffered Shake Flask Cultivations
4. Discussion
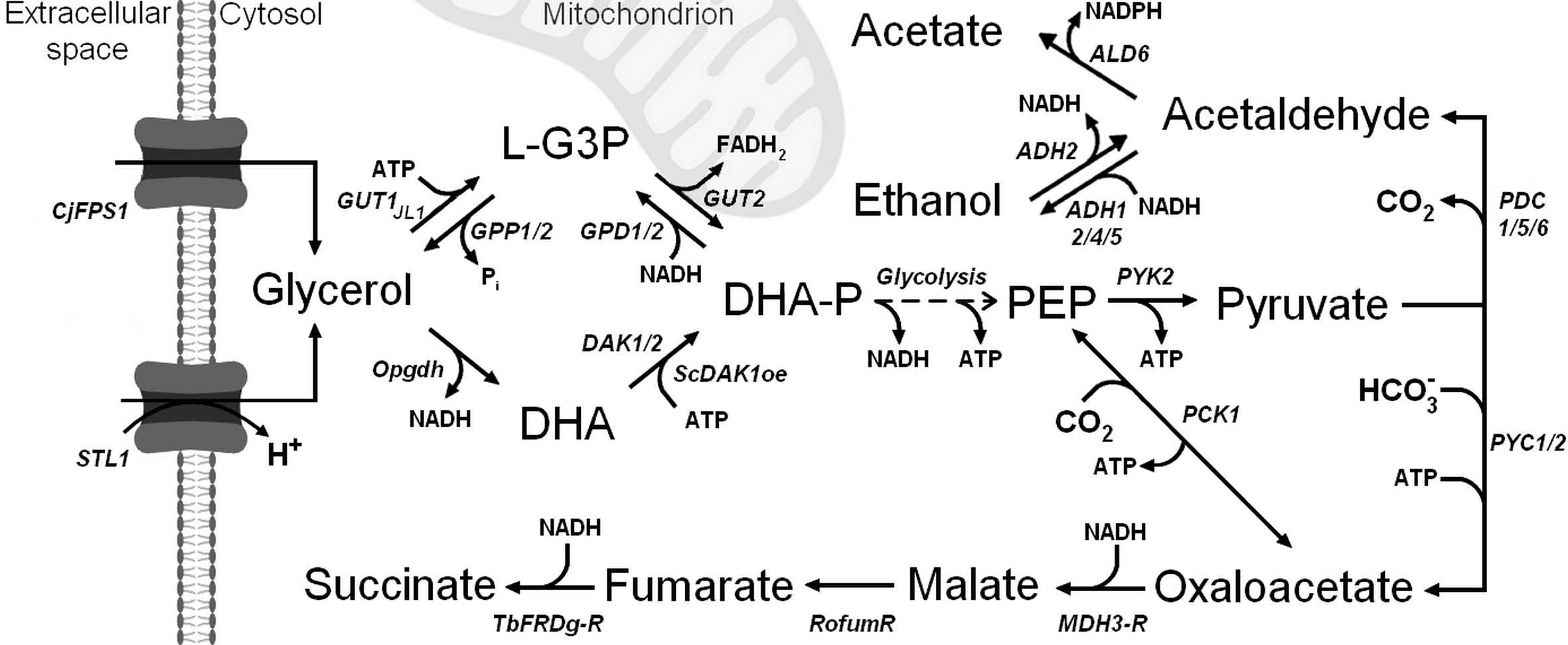
4.1. Succinic Acid Export by the SA Strain
4.2. New Insights into the Effects of Dct-02 Expression in Yeast Cell Factories
4.3. Influence of External PH on Succinic Acid Production by the Strains Expressing Ato1L219A, Ato1E144A, L219A, and SatPL131A
4.4. Why Do Dct-02 and AceTr Members Display Opposite Effects on Succinic Acid Production?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soares-Silva, I.; Ribas, D.; Sousa-Silva, M.; Azevedo-Silva, J.; Rendulić, T.; Casal, M. Membrane transporters in the bioproduction of organic acids: State of the art and future perspectives for industrial applications. FEMS Microbiol. Lett. 2020, 367, fnaa118. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Jang, Y.S.; Lee, S.Y. Production of succinic acid by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–66. [Google Scholar] [CrossRef]
- Kell, D.B.; Swainston, N.; Pir, P.; Oliver, S.G. Membrane transporter engineering in industrial biotechnology and whole cell biocatalysis. Trends Biotechnol. 2015, 33, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Borodina, I. Understanding metabolite transport gives an upper hand in strain development. Microb. Biotechnol. 2019, 12, 69–70. [Google Scholar] [CrossRef]
- Casal, M.; Paiva, S.; Queirós, O.; Soares-Silva, I. Transport of carboxylic acids in yeasts. FEMS Microbiol. Rev. 2008, 32, 974–994. [Google Scholar] [CrossRef]
- Abbott, D.A.; Zelle, R.M.; Pronk, J.T.; van Maris, A.J. Metabolic engineering of Saccharomyces cerevisiae for production of carboxylic acids: Current status and challenges. FEMS Yeast Res. 2009, 9, 1123–1136. [Google Scholar] [CrossRef]
- Grobler, J.; Bauer, F.; Subden, R.E.; Van Vuuren, H.J. The mae1 gene of Schizosaccharomyces pombe encodes a permease for malate and other C4 dicarboxylic acids. Yeast 1995, 11, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; Heijnen, J.; Verwaal, R. Process for Preparing Dicarboxylic Acids Employing Fungal Cells. WO Patent 2013/004670, 10 January 2013. [Google Scholar]
- Darbani, B.; Stovicek, V.; van der Hoek, S.A.; Borodina, I. Engineering energetically efficient transport of dicarboxylic acids in yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2019, 116, 19415–19420. [Google Scholar] [CrossRef]
- Verwall, R.; Wu, L.; Damveld, R.A.; Sagt, C.M.J. Succinic Acid Production in a Eukaryotic Cell. WO 2009/065778 A1, 28 May 2009. [Google Scholar]
- Xiberras, J.; Klein, M.; de Hulster, E.; Mans, R.; Nevoigt, E. Engineering Saccharomyces cerevisiae for Succinic Acid Production From Glycerol and Carbon Dioxide. Front. Bioeng. Biotechnol. 2020, 8, 566. [Google Scholar] [CrossRef]
- Malubhoy, Z.; Bahia, F.M.; de Valk, S.C.; de Hulster, E.; Rendulić, T.; Ortiz, J.P.R.; Xiberras, J.; Klein, M.; Mans, R.; Nevoigt, E. Carbon dioxide fixation via production of succinic acid from glycerol in engineered Saccharomyces cerevisiae. Microb. Cell Fact. 2022, 21, 102. [Google Scholar] [CrossRef]
- Sá-Pessoa, J.; Paiva, S.; Ribas, D.; Silva, I.J.; Viegas, S.C.; Arraiano, C.M.; Casal, M. SATP (YaaH), a succinate–acetate transporter protein in Escherichia coli. Biochem. J. 2013, 454, 585–595. [Google Scholar] [CrossRef]
- Ribas, D.; Soares-Silva, I.; Vieira, D.; Sousa-Silva, M.; Sá-Pessoa, J.; Azevedo-Silva, J.; Viegas, S.; Arraiano, C.; Diallinas, G.; Paiva, S.; et al. The acetate uptake transporter family motif “NPAPLGL (M/S)” is essential for substrate uptake. Fungal Genet. Biol. 2019, 122, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Sousa-Silva, M.; Vieira, D.; Soares, P.; Chebaro, Y.; Lorenz, M.; Casal, M.; Soares-Silva, I.; Paiva, S. Carboxylic Acid Transporters in Candida Pathogenesis. MBio 2020, 11, e00156-20. [Google Scholar] [CrossRef]
- Rendulić, T.; Alves, J.; Azevedo-Silva, J.; Soares-Silva, I.; Casal, M. New insights into the acetate uptake transporter (AceTr) family: Unveiling amino acid residues critical for specificity and activity. Comput. Struct. Biotechnol. J. 2021, 19, 4412–4425. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Silva, M.; Soares, P.; Alves, J.; Vieira, D.; Casal, M.; Soares-Silva, I. Uncovering Novel Plasma Membrane Carboxylate Transporters in the Yeast Cyberlindnera jadinii. J. Fungi 2022, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Paiva, S.; Devaux, F.; Barbosa, S.; Jacq, C.; Casal, M. Ady2p is essential for the acetate permease activity in the yeast Saccharomyces cerevisiae. Yeast 2004, 21, 201–210. [Google Scholar] [CrossRef]
- Qiu, B.; Xia, B.; Zhou, Q.; Lu, Y.; He, M.; Hasegawa, K.; Ma, Z.; Zhang, F.; Gu, L.; Mao, Q.; et al. Succinate-acetate permease from Citrobacter koseri is an anion channel that unidirectionally translocates acetate. Cell Res. 2018, 28, 644–654. [Google Scholar] [CrossRef]
- Sun, P.; Li, J.; Zhang, X.; Guan, Z.; Xiao, Q.; Zhao, C.; Song, M.; Zhou, Y.; Mou, L.; Ke, M. Crystal structure of the bacterial acetate transporter SatP reveals that it forms a hexameric channel. J. Biol. Chem. 2018, 293, 19492–19500. [Google Scholar] [CrossRef]
- Taymaz-Nikerel, H.; Jamalzadeh, E.; Borujeni, A.E.; Verheijen, P.; van Gulik, W.; Heijnen, J.J. A thermodynamic analysis of dicarboxylic acid production in microorganisms. In Biothermodynamics, 1st ed.; von Stockar, U., van der Wielen, L.A.M., Eds.; EPFL Press: Lausanne, Switzerland, 2013; Volume 16, p. 34. [Google Scholar]
- de Kok, S.; Kozak, B.U.; Pronk, J.T.; van Maris, A.J. Energy coupling in Saccharomyces cerevisiae: Selected opportunities for metabolic engineering. FEMS Yeast Res. 2012, 12, 387–397. [Google Scholar] [CrossRef]
- Bahia, F.M.; Perpelea, A.; Klein, M.; Branduardi, P.; Nevoigt, E. School of Science, Jacobs University Bremen, Bremen, Germany. 2022; in preparation. [Google Scholar]
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Carrillo, M.; Xiberras, J.; Islam, Z.U.; Swinnen, S.; Nevoigt, E. Towards the exploitation of glycerol’s high reducing power in Saccharomyces cerevisiae-based bioprocesses. Metab. Eng. 2016, 38, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Jessop-Fabre, M.M.; Jakociunas, T.; Stovicek, V.; Dai, Z.; Jensen, M.K.; Keasling, J.D.; Borodina, I. EasyClone-MarkerFree: A vector toolkit for marker-less integration of genes into Saccharomyces cerevisiae via CRISPR-Cas9. Biotechnol. J. 2016, 11, 1110–1117. [Google Scholar] [CrossRef]
- Gietz, R.D.; Schiestl, R.H.; Willems, A.R.; Woods, R.A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 1995, 11, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Flagfeldt, D.B.; Siewers, V.; Huang, L.; Nielsen, J. Characterization of chromosomal integration sites for heterologous gene expression in Saccharomyces cerevisiae. Yeast 2009, 26, 545–551. [Google Scholar] [CrossRef]
- Ho, P.W.; Swinnen, S.; Duitama, J.; Nevoigt, E. The sole introduction of two single-point mutations establishes glycerol utilization in Saccharomyces cerevisiae CEN.PK derivatives. Biotechnol. Biofuels 2017, 10, 10. [Google Scholar] [CrossRef]
- Hoffman, C.S.; Winston, F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 1987, 57, 267–272. [Google Scholar] [CrossRef]
- Verduyn, C.; Postma, E.; Scheffers, W.A.; Van Dijken, J.P. Effect of benzoic acid on metabolic fluxes in yeasts: A continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 1992, 8, 501–517. [Google Scholar] [CrossRef]
- Swinnen, S.; Klein, M.; Carrillo, M.; McInnes, J.; Nguyen, H.T.T.; Nevoigt, E. Re-evaluation of glycerol utilization in Saccharomyces cerevisiae: Characterization of an isolate that grows on glycerol without supporting supplements. Biotechnol. Biofuels 2013, 6, 157. [Google Scholar] [CrossRef]
- Liu, Y.; Esen, O.; Pronk, J.T.; van Gulik, W.M. Uncoupling growth and succinic acid production in an industrial Saccharomyces cerevisiae strain. Biotechnol. Bioeng. 2021, 118, 1576–1586. [Google Scholar] [CrossRef]
- Van De Graaf, M.J.; Valianpoer, F.; Fiey, G.; Delattre, L.; Schulten, E.A.M. Process for the Crystallization of Succinic Acid. U.S. Patent 2015/0057425 A1, 26 February 2015. [Google Scholar]
- Ho, P.W.; Klein, M.; Futschik, M.; Nevoigt, E. Glycerol positive promoters for tailored metabolic engineering of the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2018, 18, foy019. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wang, C.; Zhou, J.; Liu, Y.; Yang, M.; Xing, J. Construction of reductive pathway in Saccharomyces cerevisiae for effective succinic acid fermentation at low pH value. Bioresour. Technol. 2014, 156, 232–239. [Google Scholar] [CrossRef]
- Deng, Y.N.; Kashtoh, H.; Wang, Q.; Zhen, G.X.; Li, Q.Y.; Tang, L.H.; Gao, H.L.; Zhang, C.R.; Qin, L.; Su, M.; et al. Structure and activity of SLAC1 channels for stomatal signaling in leaves. Proc. Natl. Acad. Sci. USA 2021, 115, E1598–E1607. [Google Scholar] [CrossRef]
- Shah, M.V.; van Mastrigt, O.; Heijnen, J.J.; van Gulik, W.M. Transport and metabolism of fumaric acid in Saccharomyces cerevisiae in aerobic glucose-limited chemostat culture. Yeast 2016, 33, 145–161. [Google Scholar] [CrossRef]
- Zelle, R.M.; de Hulster, E.; van Winden, W.A.; de Waard, P.; Dijkema, C.; Winkler, A.A.; Geertman, J.M.; van Dijken, J.P.; Pronk, J.T.; van Maris, A.J. Malic acid production by Saccharomyces cerevisiae: Engineering of pyruvate carboxylation, oxaloacetate reduction, and malate export. Appl. Environ. Microbiol. 2008, 74, 2766–2777. [Google Scholar] [CrossRef]
- Brown, S.H.; Bashkirova, L.; Berka, R.; Chandler, T.; Doty, T.; McCall, K.; McCulloch, M.; McFarland, S.; Thompson, S.; Yaver, D.; et al. Metabolic engineering of Aspergillus oryzae NRRL 3488 for increased production of L-malic acid. Appl. Microbiol. Biotechnol. 2013, 97, 8903–8912. [Google Scholar] [CrossRef]
- Yang, L.; Christakou, E.; Vang, J.; Lubeck, M.; Lubeck, P.S. Overexpression of a C4-dicarboxylate transporter is the key for rerouting citric acid to C4-dicarboxylic acid production in Aspergillus carbonarius. Microb. Cell Factories 2017, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Yan, L.; Li, M.; Liu, X.; Xu, Y.; Xie, Z.; Liu, H. Identification and engineering a C4-dicarboxylate transporter for improvement of malic acid production in Aspergillus niger. Appl. Microbiol. Biotechnol. 2020, 104, 9773–9783. [Google Scholar] [CrossRef] [PubMed]
- Camarasa, C.; Bidard, F.; Bony, M.; Barre, P.; Dequin, S. Characterization of Schizosaccharomyces pombe malate permease by expression in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2001, 67, 4144–4151. [Google Scholar] [CrossRef]
- Flikweert, M.T.; de Swaaf, M.; van Dijken, J.P.; Pronk, J.T. Growth requirements of pyruvate-decarboxylase-negative Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1999, 174, 73–79. [Google Scholar] [CrossRef]
- van Maris, A.J.; Konings, W.N.; van Dijken, J.P.; Pronk, J.T. Microbial export of lactic and 3-hydroxypropanoic acid: Implications for industrial fermentation processes. Metab. Eng. 2004, 6, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Zhan, T.; Xu, H.; Chen, J.; Bi, C.; Fan, F.; Zhang, X. Characterization of JEN family carboxylate transporters from the acid-tolerant yeast Pichia kudriavzevii and their applications in succinic acid production. Microb. Biotechnol. 2021, 14, 1130–1147. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.L.; Lane, S.; Jayakody, L.N.; Zhang, G.C.; Kim, H.; Cho, W.; Jin, Y.S. Deletion of JEN1 and ADY2 reduces lactic acid yield from an engineered Saccharomyces cerevisiae, in xylose medium, expressing a heterologous lactate dehydrogenase. FEMS Yeast Res. 2019, 19, foz050. [Google Scholar] [CrossRef] [PubMed]


| S. cerevisiae Strain | Relevant Genotype | Reference |
|---|---|---|
| CEN 2PW | ubr2::UBR2CBS6412-13A; gut1::GUT1JL1; YGLCτ3::PTEF1-Opgdh-TCYC1-PADH2-ScDAK1-TTPS1-PTDH3-CjFPS1-TRPL15A | [23] |
| SA | ubr2::UBR2CBS6412-13A; gut1::GUT1JL1; YGLCτ3::PTEF1-Opgdh-TCYC1-PADH2-ScDAK1-TTPS1-PTDH3-CjFPS1-TRPL15A; YPRCτ3(21)::PJEN1-ScMDH3-R-TIDP1-PHOR7-RofumR-TDIT1-PFBA1-TbFRD-R-TADH1 | This study |
| SA-Dct-02 | ubr2::UBR2CBS6412-13A; gut1::GUT1JL1; YGLCτ3::PTEF1-Opgdh-TCYC1-PADH2-ScDAK1-TTPS1-PTDH3-CjFPS1-TRPL15A; YPRCτ3(21)::PJEN1-ScMDH3-R-TIDP1-PHOR7-RofumR-TDIT1-PFBA1-TbFRD-R-TADH1; XI-3::PTDH3-AnDCT-02-TCYC1 | This study |
| SA-Ato1(S) | ubr2::UBR2CBS6412-13A; gut1::GUT1JL1; YGLCτ3::PTEF1-Opgdh-TCYC1-PADH2-ScDAK1-TTPS1-PTDH3-CjFPS1-TRPL15A; YPRCτ3(21)::PJEN1-ScMDH3-R-TIDP1-PHOR7-RofumR-TDIT1-PFBA1-TbFRD-R-TADH1; XI-3::PTDH3-ScATO1L219A-TCYC1 | This study |
| SA-Ato1(HS) | ubr2::UBR2CBS6412-13A; gut1::GUT1JL1; YGLCτ3::PTEF1-Opgdh-TCYC1-PADH2-ScDAK1-TTPS1-PTDH3-CjFPS1-TRPL15A; YPRCτ3(21)::PJEN1-ScMDH3-R-TIDP1-PHOR7-RofumR-TDIT1-PFBA1-TbFRD-R-TADH1; XI-3::PTDH3-ScATO1E144A, L219A-TCYC1 | This study |
| SA-SatP(HS) | ubr2::UBR2CBS6412-13A; gut1::GUT1JL1; YGLCτ3::PTEF1-Opgdh-TCYC1-PADH2-ScDAK1-TTPS1-PTDH3-CjFPS1-TRPL15A; YPRCτ3(21)::PJEN1-ScMDH3-R-TIDP1-PHOR7-RofumR-TDIT1-PFBA1-TbFRD-R-TADH1; XI-3::PTDH3-EcSATPL131A-TCYC1 | This study |
| SA-COX7SatP(HS) | ubr2::UBR2CBS6412-13A; gut1::GUT1JL1; YGLCτ3::PTEF1-Opgdh-TCYC1-PADH2-ScDAK1-TTPS1-PTDH3-CjFPS1-TRPL15A; YPRCτ3(21)::PJEN1-ScMDH3-R-TIDP1-PHOR7-RofumR-TDIT1-PFBA1-TbFRD-R-TADH1; XI-3::PCOX7-EcSATPL131A-TCYC1 | This study |
| Plasmid Name | Description | Reference |
|---|---|---|
| pUC18-MDH3 | Template for the amplification of PJEN1-ScMDH3-R-TIDP1 expression cassette. | [12] |
| pUC18-RoFUM | Template for the amplification of PHOR7-RofumR-TDIT1 expression cassette. | [12] |
| pUC18-TbFRD | Template for the amplification of PFBA1-TbFRD-R-TADH1 expression cassette. | [12] |
| pUC18-AnDCT-02 w/o STOP | Codon optimized coding sequence for the dicarboxylic acid transporter Dct-02 from A. niger. | [12] |
| pL219A | Coding sequence for the carboxylic acid transporter variant Ato1L219A from S. cerevisiae | [16] |
| pE144A/L219A | Coding sequence for the carboxylic acid transporter variant Ato1E144A, L219A from S. cerevisiae | [16] |
| pSatP-L131A | Codon optimized coding sequence for the carboxylic acid transporter variant SatPL131A from E. coli. | [16] |
| p414-TEF1p-Cas9-CYC1t-nat1 | CEN6/ARSH4, natMX4, TEF1p-cas9-CYC1t | [25] |
| p414-TEF1p-Cas9-CYC1t-hphMX | CEN6/ARSH4, hphMX, TEF1p-cas9-CYC1t | [12] |
| p426-SNR52p-gRNA.YPRCτ3-SUP4t-hphMX | 2 µm, hphMX, SNR52p-gRNA.YPRCτ3-SUP4t | [11] |
| pCfB3045 | 2 µm, natMX6, SNR52p-gRNA.XI-3-SUP4t | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rendulić, T.; Mendonça Bahia, F.; Soares-Silva, I.; Nevoigt, E.; Casal, M. The Dicarboxylate Transporters from the AceTr Family and Dct-02 Oppositely Affect Succinic Acid Production in S. cerevisiae. J. Fungi 2022, 8, 822. https://doi.org/10.3390/jof8080822
Rendulić T, Mendonça Bahia F, Soares-Silva I, Nevoigt E, Casal M. The Dicarboxylate Transporters from the AceTr Family and Dct-02 Oppositely Affect Succinic Acid Production in S. cerevisiae. Journal of Fungi. 2022; 8(8):822. https://doi.org/10.3390/jof8080822
Chicago/Turabian StyleRendulić, Toni, Frederico Mendonça Bahia, Isabel Soares-Silva, Elke Nevoigt, and Margarida Casal. 2022. "The Dicarboxylate Transporters from the AceTr Family and Dct-02 Oppositely Affect Succinic Acid Production in S. cerevisiae" Journal of Fungi 8, no. 8: 822. https://doi.org/10.3390/jof8080822