Increased Attraction and Stability of Beauveria bassiana-Formulated Microgranules for Aedes aegypti Biocontrol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mosquito Source and Rearing Conditions
2.2. B. bassiana Culture and Mass Production
2.3. Formulations Production
2.3.1. Viability of Conidia in Combination with Additives
2.3.2. Conidia Viability in Microgranular Formulations
2.3.3. Additives Effectiveness in MGF
2.3.3.1. Attractiveness Test of MGF in Ae. aegypti Adults
2.3.3.2. Ae. aegypti Trap Mortality by MGF with B. bassiana Conidia Active Ingredient
2.4. Effect of Spirulina sp. as Ae. aegypti Attractant or Conidia Stabilizer
2.4.1. Attraction of Ae. aegypti Adults by Spirulina sp. in MGFs
2.4.1.1. Evaluation of Attractiveness Spirulina sp. in Granular Formulation
2.4.2. B. bassiana Conidia Viability on a Granular Formulation with Spirulina sp.
2.5. Evaluation of Granular and Solid Formulations
2.5.1. Conidia Viability on MGFs with Spirulina sp. and Solid Formulation (SF) with Coco Fiber
2.5.2. Aedes aegypti Adult Infection by Microgranular Formulations and Solid Formulations
3. Results
3.1. B. bassiana Conidial Viability after Exposure to Additives
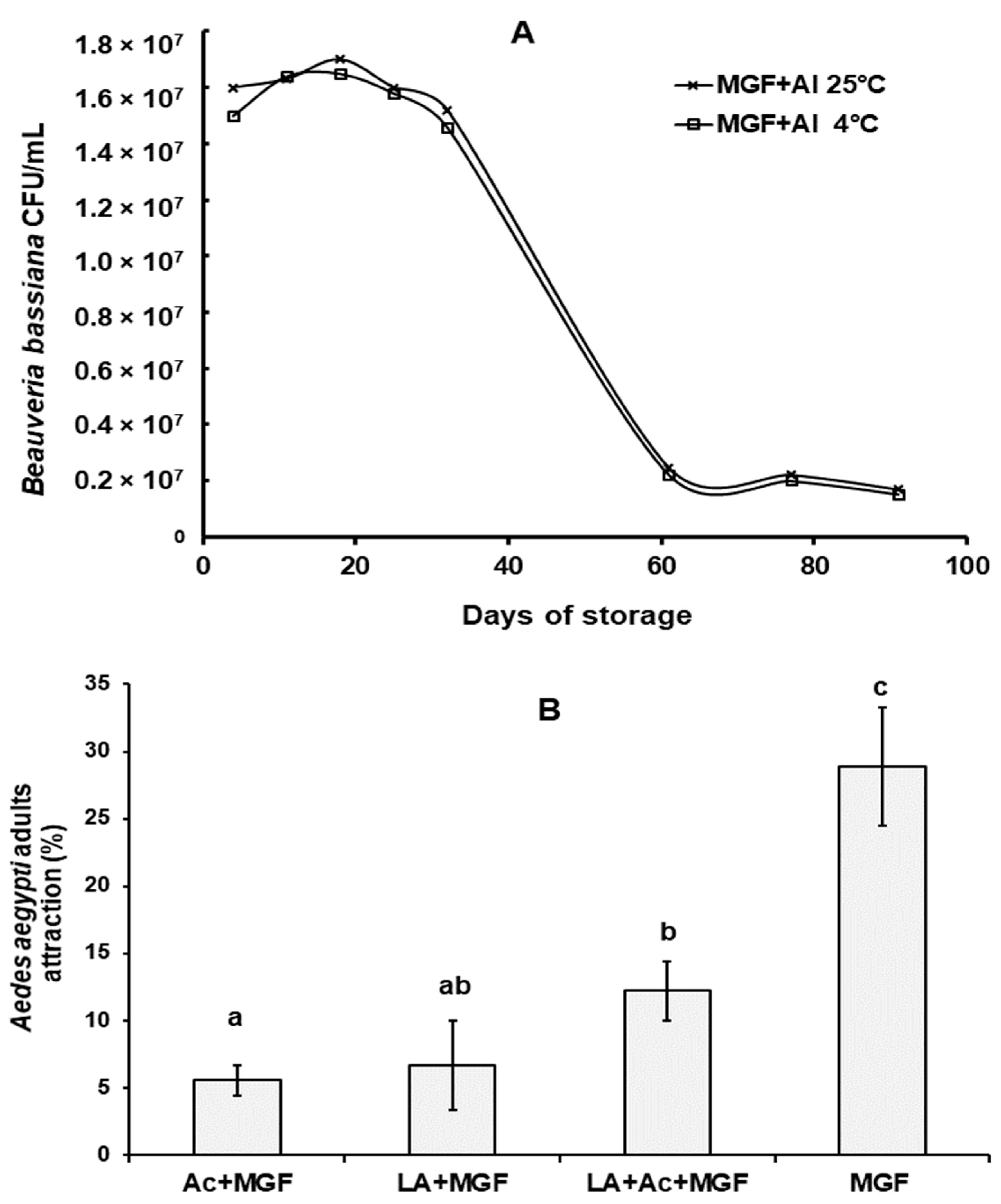
3.2. B. bassiana Conidial Viability after Exposure to MGF
3.3. Ae. aegypti Attraction Efficacy by Microganule-Formulated Additives
3.4. Ae. aegypti Attraction and Mortality by MGFs with B. bassiana
3.5. Ae. aegypti Attraction by MGFs with Spirulina sp.
3.6. B. bassiana Conidia Viability in MGFs with Spirulina sp.
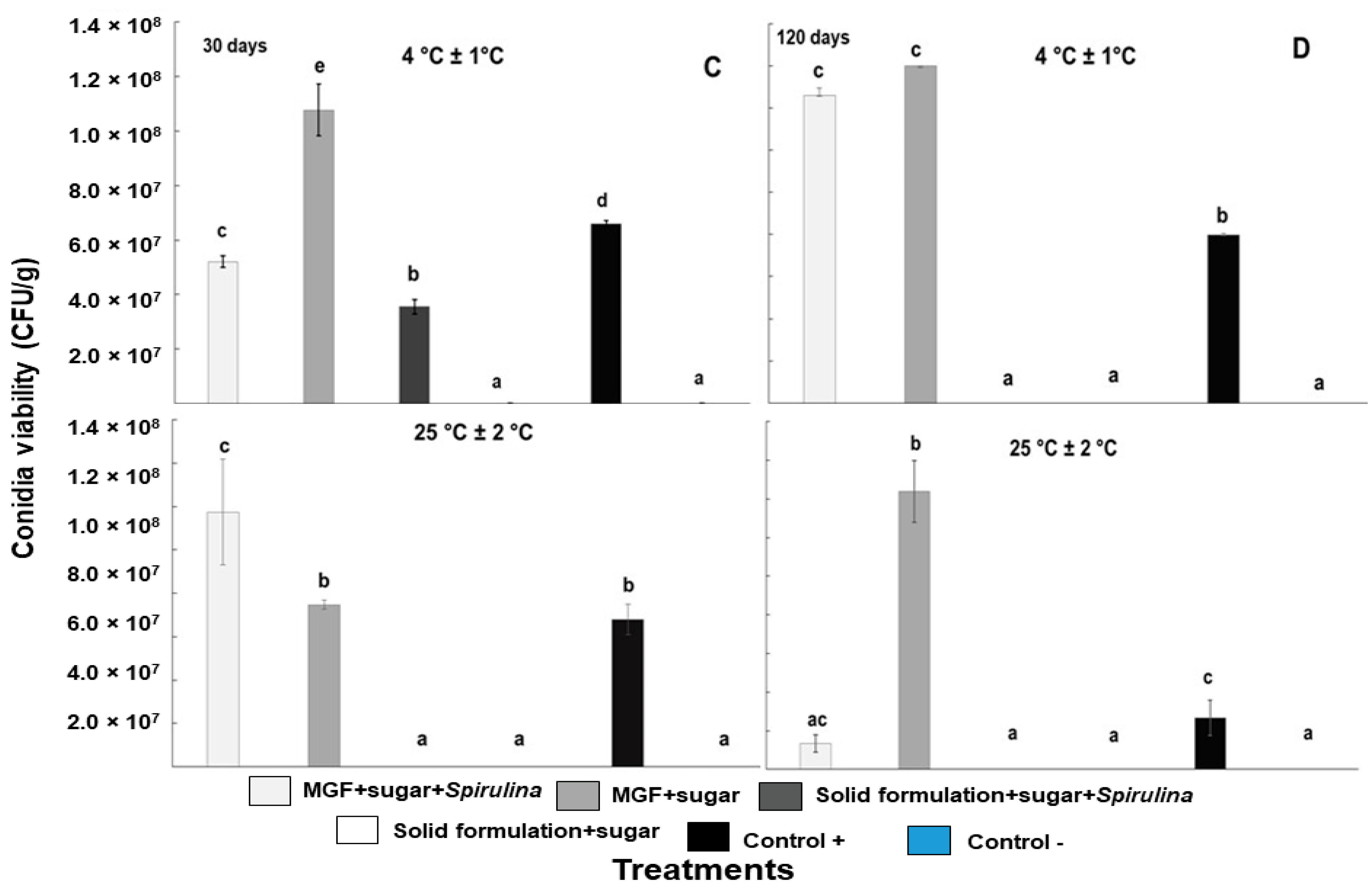
3.7. B. bassiana Conidia Viability in MGFs and Solid Formulations in Two Storage Conditions (25 °C and 4 °C)
3.8. Aedes aegypti Biocontrol by MGF and FS with B. bassiana Conidia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu-Helmersson, J.; Brännström, Å.; Sewe, M.O.; Semenza, J.C.; Rocklöv, J. Estimating past, present, and future trends in the global distribution and abundance of the arbovirus vector Aedes aegypti under climate change scenarios. Front. Public Health 2019, 7, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garjito, T.A.; Hidajat, M.C.; Kinansi, R.R.; Setyaningsih, R.; Anggraeni, Y.M.; Trapsilowati, W.; Jastal; Ristiyanto; Baskoro, T.; Satoto, T.; et al. Stegomyia indices and risk of dengue transmission: A lack of correlation. Front. Public Health 2020, 8, 328. [Google Scholar] [CrossRef]
- Benelli, G. Managing mosquitoes and ticks in a rapidly changing world–facts and trends. Saudi J. Biol. Sci. 2019, 26, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drack, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Bardach, A.E.; García-Perdomo, H.A.; Alcaraz, A.; Tapia-López, E.; Gándara, R.A.R.; Ruvinsky, S.; Ciapponi, A. Interventions for the control of Aedes aegypti in Latin America and the Caribbean: Systematic review and meta-analysis. Trop. Med. Int. Health 2019, 24, 530–552. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, A.P.; Paiva, M.H.S.; Cabral, A.M.; Cavalcanti, A.E.H.D.; Pessoa, L.F.F.; Diniz, D.F.A.; Helvecio, E.; Da Silva, E.V.G.; da Silva, N.M.; Anastácio, D.B.; et al. Screening Aedes aegypti (Diptera: Culicidae) populations from Pernambuco, Brazil for resistance to temephos, diflubenzuron, and cypermethrin and characterization of potential resistance mechanisms. J. Insect Sci. 2019, 19, 16. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.E.I.; Paula, A.R.; Ribeiro, A.; Butt, T.M.; Silva, C.P.; Samuels, R.I. A new method of deploying entomopathogenic fungi to control adult Aedes aegypti mosquitoes. J. Appl. Entomol. 2017, 142, 59–66. [Google Scholar] [CrossRef] [Green Version]
- García-Munguía, A.M.; Garza-Hernández, J.A.; Rebollar-Tellez, E.A.; Rodríguez-Pérez, M.A.; Reyes-Villanueva, F. Transmission of Beauveria bassiana from male to female Aedes aegypti mosquitoes. Parasites Vectors 2011, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Shahid, A.A.; Rao, A.Q.; Bakhsh, A.; Husnain, T. Entomopathogenic fungi as biological controllers: New insights into their virulence and pathogenicity. Arch. Biol. Sci. 2012, 64, 21–42. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, P.; Malik, A. Effect of temperature and humidity on pathogenicity of native Beauveria bassiana isolate against Musca domestica L. J. Parasit. Dis. 2015, 39, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, T.; Takken, W.; Koenraadt, C.J. Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasites Vectors 2011, 4, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmidevi, P.; Gopalakrishnan, C.; Antony, R.S. Bio-formulation based on plant oil of the future Beauveria bassiana (Balsamo) for fruit borer control in tomatoes. J. Entomol. Zool. Stud. 2020, 8, 200–206. [Google Scholar]
- Rodrigues, J.; Catão, A.M.L.; dos Santos, A.S.; Paixão, F.R.S.; Santos, T.R.; Martinez, J.M.; Marreto, R.N.; Mascarin, G.M.; Fernandes, K.K.; Humber, R.A.; et al. Relative humidity impacts development and activity against Aedes aegypti adults by granular formulations of Metarhizium humberi microsclerotia. Appl. Microbiol. Biotechnol. 2021, 105, 2725–2736. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jaronski, S.; Reddy, G.V. Impact of granular carriers to improve the efficacy of entomopathogenic fungi against wireworms in spring wheat. J. Pest Sci. 2020, 93, 275–290. [Google Scholar] [CrossRef]
- Vemmer, M.; Patel, A.V. Review of encapsulation methods suitable for microbial biological control agents. Biol. Control 2013, 67, 380–389. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of grape polyphenols using maltodextrin and gum Arabic as two alternative coating materials: Development and characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef]
- Perlatti, B.; de Souza Bergo, P.L.; Fernandes, J.B.; Forim, M.R. Polymeric nanoparticle-based insecticides: A controlled release purpose for agrochemicals. In Insecticides—Development of Safer and More Effective Technologies; Trdan, S., Ed.; IntechOpen: London, UK, 2013; pp. 521–540. [Google Scholar]
- Preininger, C.; Sauer, U.; Bejarano, A.; Berninger, T. Concepts and applications of foliar spray for microbial inoculants. Appl. Microbiol. Biotechnol. 2018, 102, 7265–7282. [Google Scholar] [CrossRef]
- Karimi, J.; Dara, S.K.; Arthurs, S. Microbial insecticides in Iran: History, current status, challenges and perspective. J. Invertebr. Pathol. 2019, 165, 67–73. [Google Scholar] [CrossRef]
- Hudson, B.J.; Karis, I.G. The lipids of the alga Spirulina. J. Sci. Food Agric. 1974, 25, 759–763. [Google Scholar] [CrossRef]
- Berry, S.; Bolychevtseva, Y.V.; Rögner, M.; Karapetyan, N.V. Photosynthetic and respiratory electron transport in the alkaliphilic cyanobacterium Arthrospira (Spirulina) platensis. Photosynth. Res. 2003, 78, 67–76. [Google Scholar] [CrossRef]
- Zwiebel, L.J.; Takken, W. Olfactory regulation of mosquito–host interactions. Insect Biochem. Mol. Biol. 2004, 34, 645–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logan, J.G.; Birkett, M.A.; Clark, S.J.; Powers, S.; Seal, N.J.; Wadhams, L.J.; Mordue, A.J.; Pickett, J.A. Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J. Chem. Ecol. 2008, 34, 308. [Google Scholar] [CrossRef] [PubMed]
- Bernier, U.R.; Kline, D.L.; Posey, K.H.; Booth, M.M.; Yost, R.A.; Barnard, D.R. Synergistic attraction of Aedes aegypti (L.) to binary blends of L-lactic acid and acetone, dichloromethane, or dimethyl disulfide. J. Med. Entomol. 2003, 40, 653–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logan, J.G.; Birkett, M.A. Semiochemicals for biting fly control: Their identification and exploitation. Pest Manag. Sci. 2007, 63, 647–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasser, S.; da Costa, M.P.M.; de Mello Ferreira, I.L.; Lima, J.B.P. k-Carrageenan-Bacillus thuringiensis israelensis hydrogels: A promising material to combat larvae of the Aedes aegypti mosquito. Carbohydr. Polym. Technol. Appl. 2021, 2, 100125. [Google Scholar] [CrossRef]
- Scholte, E.J.; Takken, W.; Knols, B.G. Infection of adult Aedes aegypti and Ae. albopictus mosquitoes with the entomopathogenic fungus Metarhizium anisopliae. Acta Trop. 2007, 102, 151–158. [Google Scholar] [CrossRef]
- de Paula, A.R.; Brito, E.S.; Pereira, C.R.; Carrera, M.P.; Samuels, R.I. Susceptibility of adult Aedes aegypti (Diptera: Culicidae) to infection by Metarhizium anisopliae and Beauveria bassiana: Prospects for Dengue vector control. Biocontrol Sci. Technol. 2008, 18, 1017–1025. [Google Scholar] [CrossRef]
- Carolino, A.T.; Paula, A.R.; Silva, C.P.; Butt, T.M.; Samuels, R.I. Monitoring persistence of the entomopathogenic fungus Metarhizium anisopliae under simulated field conditions with the aim of controlling adult Aedes aegypti (Diptera: Culicidae). Parasites Vectors 2014, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hamed, A.M.; El-Sherbini, M.S.; Abdeltawab, M.S. Eco-friendly mosquito-control strategies: Advantages and disadvantages. Egypt. Acad. J. Biol. Sci. E Med. Entomol. Parasitol. 2022, 14, 17–31. [Google Scholar] [CrossRef]
- Cabrera, M.; Jaffe, K. An aggregation pheromone modulates lekking behavior in the vector mosquito Aedes aegypti (Diptera: Culicidae). J. Am. Mosq. Control Assoc. 2007, 23, 1–10. [Google Scholar] [CrossRef]
- Castrejón-Antonio, J.E.; Nuñez-Mejia, G.; Iracheta, M.M.; Gomez-Flores, R.; Tamayo-Mejia, F.; Ocampo-Hernandez, J.A.; Tamez-Guerra, P. Beauveria bassiana blastospores produced in selective medium reduce survival time of Epilachna varivestis Mulsant larvae. Southwest. Entomol. 2017, 42, 203–220. [Google Scholar] [CrossRef]
- Inglis, G.D.; Enkerli, J.; Goettel, M.S. Laboratory techniques used for entomopathogenic fungi: Hypocreales. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 189–254. [Google Scholar]
- SPSS, Version 17.0; SPSS Inc.: Chicago, IL, USA, 2008.
- Jaronski, S.T.; Jackson, M.A. Mass production of entomopathogenic Hypocreales. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 255–284. [Google Scholar]
- Gámez, M.D. Efectividad de un Bio-Insecticida Fúngico con Adición de Atrayentes, Aplicado en Trampas Para el Control de Aedes aegypti (L.). Ph.D. Thesis, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Mexico, 2020. [Google Scholar]
- de Paula, A.R.; Silva, L.E.I.; Ribeiro, A.; da Silva, G.A.; Silva, C.P.; Butt, T.M.; Samuels, R.I. Metarhizium anisopliae blastospores are highly virulent to adult Aedes aegypti, an important arbovirus vector. Parasites Vectors 2021, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tamez-Guerra, P.; Tamayo-Mejia, F.; Gomez-Flores, R.; Rodriguez-Padilla, C.; Damas, G.; Tamez-Guerra, R.; Ek-Ramos, M.; Williams, T. Increased efficacy and extended shelf life of spinosad formulated in phagostimulant granules against Spodoptera frugiperda. Pest Manag. Sci. 2018, 14, 100–110. [Google Scholar] [CrossRef]
- Tamayo-Mejía, F.; Tamez-Guerra, P.; Guzmán-Franco, A.W.; Gomez-Flores, R. Developmental stage affects survival of the ectoparasitoid Tamarixia triozae exposed to the fungus Beauveria bassiana. Biol. Control 2016, 93, 30–36. [Google Scholar] [CrossRef]
- Morato, V.C.; Teixeira, M.D.G.; Gomes, A.C.; Bergamaschi, D.P.; Barreto, M.L. Infestation of Aedes aegypti estimated by oviposition traps in Brazil. Rev. Saude Publica 2005, 39, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Khachatourians, G.G.; Qazi, S.S. Entomopathogenic fungi: Biochemistry and molecular biology. In Human and Animal Relationship, Mycota VI; Brakhage, A.A., Zipfel, P.F., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2008; p. 66. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jackson, M.A.; Kobori, N.N.; Behle, R.W.; Dunlap, C.A.; Júnior, Í.D. Glucose concentration alters dissolved oxygen levels in liquid cultures of Beauveria bassiana and affects formation and bioefficacy of blastospores. Appl. Microbiol. Biotechnol. 2015, 99, 6653–6665. [Google Scholar] [CrossRef]
- Luo, X.; Keyhani, N.O.; Yu, X.; He, Z.; Luo, Z.; Pei, Y.; Zhang, Y. The MAP kinase Bbslt2 controls growth, conidiation, cell wall integrity, and virulence in the insect pathogenic fungus Beauveria bassiana. Fungal Genet. Biol. 2012, 49, 544–555. [Google Scholar] [CrossRef]
- Kramer, K.J.; Muthukrishnan, S. Insect chitinases: Molecular biology and potential use as biopesticides. Insect Biochem. Mol. Biol. 1997, 27, 887–900. [Google Scholar] [CrossRef]
- Buckner, E.A.; Williams, K.F.; Marsicano, A.L.; Latham, M.D.; Lesser, C.R. Evaluating the vector control potential of the In2Care® mosquito trap against Aedes aegypti and Aedes albopictus under semifield conditions in Manatee County, Florida. J. Am. Mosq. Control Assoc. 2017, 33, 193–200. [Google Scholar] [CrossRef]
- Leemon, D.M.; Jonsson, N.N. Laboratory studies on Australian isolates of Metarhizium anisopliae as a biopesticide for the cattle tick Boophilus microplus. J. Invertebr. Pathol. 2008, 97, 40–49. [Google Scholar] [CrossRef]
- Corrêa, E.B.; Sutton, J.C.; Bettiol, W. Formulation of Pseudomonas chlororaphis strains for improved shelf life. Biol. Control 2015, 80, 50–55. [Google Scholar] [CrossRef]
- Hynes, R.K.; Boyetchko, S.M. Research initiatives in the art and science of biopesticide formulations. Soil Biol. Biochem. 2006, 38, 845–849. [Google Scholar] [CrossRef]
- Lyn, M.E.; Burnett, D.; Garcia, A.R.; Gray, R. Interaction of water with three granular biopesticide formulations. J. Agric. Food Chem. 2010, 58, 1804–1814. [Google Scholar] [CrossRef]
- Kim, J.S.; Je, Y.H.; Woo, E.O.; Park, J.S. Persistence of Isaria fumosorosea (Hypecreales: Cordycipitaceae) SFP-198 conidia in corn oil-based suspension. Mycopathologia 2011, 171, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.R.; Wang, B.; Way, H.Z.; Lin, H.F.; Huang, B.; Li, Z.Z. Protection of ZnO nanoparticles to Beauveria bassiana conidia from ultraviolet radiation and their biocompatibility. Chem. J. Chin. Univ. 2010, 31, 2322–2328. [Google Scholar]
- Darbro, J.M.; Johnson, P.H.; Thomas, M.B.; Ritchie, S.A.; Kay, B.H.; Ryan, P.A. Effects of Beauveria bassiana on survival, blood-feeding success, and fecundity of Aedes aegypti in laboratory and semi-field conditions. Am. J. Trop. Med. Hyg. 2012, 86, 656. [Google Scholar] [CrossRef] [Green Version]
- Côté, J.C.; Vincent, C.; Son, K.H.; Bok, S. Persistence of insecticidal activity of novel bio-encapsulated formulations of Bacillus thuringiensis var. kurstaki against Choristoneura rosaceana [Lepidoptera: Tortricidae]. Phytoprotection 2001, 82, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Llopis, V.; Ayala, I.; Sanchis, J.; Primo, J.; Moya, P. Field efficacy of a Metarhizium anisopliae-based attractant–contaminant device to control Ceratitis capitata (Diptera: Tephritidae). J. Econ. Entomol. 2015, 108, 1570–1578. [Google Scholar] [CrossRef]
 gray bar) or without (
gray bar) or without (  white bar) Beauveria bassiana as active ingredient (AI). (A) Attraction percentage and (B) mortality percentage after exposure to BBPTG4 on MGF. Data represent mean + SEM of triplicate determinations from three independent experiments. Same letter on each column indicates that treatments are not significantly different (Student t test; p ˂ 0.05).
white bar) Beauveria bassiana as active ingredient (AI). (A) Attraction percentage and (B) mortality percentage after exposure to BBPTG4 on MGF. Data represent mean + SEM of triplicate determinations from three independent experiments. Same letter on each column indicates that treatments are not significantly different (Student t test; p ˂ 0.05).
 gray bar) or without (
gray bar) or without (  white bar) Beauveria bassiana as active ingredient (AI). (A) Attraction percentage and (B) mortality percentage after exposure to BBPTG4 on MGF. Data represent mean + SEM of triplicate determinations from three independent experiments. Same letter on each column indicates that treatments are not significantly different (Student t test; p ˂ 0.05).
white bar) Beauveria bassiana as active ingredient (AI). (A) Attraction percentage and (B) mortality percentage after exposure to BBPTG4 on MGF. Data represent mean + SEM of triplicate determinations from three independent experiments. Same letter on each column indicates that treatments are not significantly different (Student t test; p ˂ 0.05).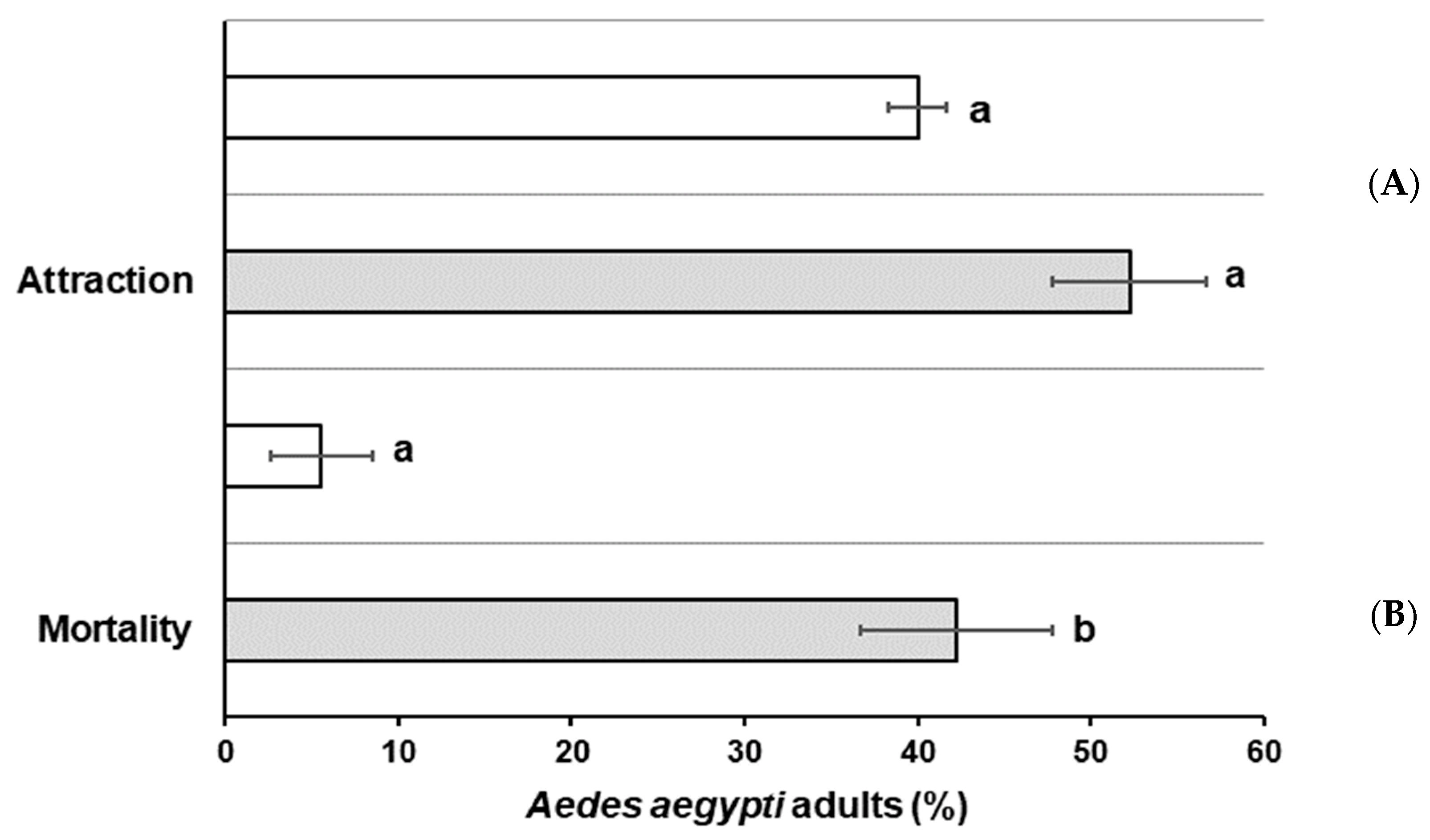


| Ingredients | MGF | MGF + Sp | SF | SF + Sp |
|---|---|---|---|---|
| B. bassiana (AI; 1 × 108 conidia/g) | 0.9 g | 0.9 g | 0.9 g | 0.9 g |
| Nixtamalized corn flour | 21.5 g | 8.14 g | - | - |
| Cornstarch | 21.5 g | 8.14 g | - | - |
| Purified water | 30 mL | 30 mL | - | - |
| Corn oil | 16 mL | 16 mL | - | - |
| Sucrose (1%) | - | 0.9 | 0.9 g | 0.9 g |
| Vegetable grease (25%) | - | 22.5 g | 22.5 g | 22.5 g |
| Spirulina (3.8%) | - | 3.4 g | - | 3.4 g |
| Coconut fiber | - | - | 62.3 g | 58.9 g |
| Total | 90 g | |||
| Treatments | Treatment Codes and Storage Temperatures 25 °C ± 2 °C and 4 °C ± 1 °C |
|---|---|
| MGF + AI + sugar + Spirulina | MGF + sugar + Spirulina |
| MGF + AI + sugar | MGF + sugar |
| FS + AI + sugar + Spirulina + coconut fiber | Solid formulation + sugar + Spirulina |
| FS + AI + sugar + coconut fiber | Solid formulation + sugar |
| Positive control (0.5% INEX-A + AI) | Positive control |
| Negative control (0.5% INEX-A) | Negative control |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamora-Avilés, N.; Orozco-Flores, A.A.; Gomez-Flores, R.; Domínguez-Gámez, M.; Rodríguez-Pérez, M.A.; Tamez-Guerra, P. Increased Attraction and Stability of Beauveria bassiana-Formulated Microgranules for Aedes aegypti Biocontrol. J. Fungi 2022, 8, 828. https://doi.org/10.3390/jof8080828
Zamora-Avilés N, Orozco-Flores AA, Gomez-Flores R, Domínguez-Gámez M, Rodríguez-Pérez MA, Tamez-Guerra P. Increased Attraction and Stability of Beauveria bassiana-Formulated Microgranules for Aedes aegypti Biocontrol. Journal of Fungi. 2022; 8(8):828. https://doi.org/10.3390/jof8080828
Chicago/Turabian StyleZamora-Avilés, Norma, Alonso A. Orozco-Flores, Ricardo Gomez-Flores, Maribel Domínguez-Gámez, Mario A. Rodríguez-Pérez, and Patricia Tamez-Guerra. 2022. "Increased Attraction and Stability of Beauveria bassiana-Formulated Microgranules for Aedes aegypti Biocontrol" Journal of Fungi 8, no. 8: 828. https://doi.org/10.3390/jof8080828
APA StyleZamora-Avilés, N., Orozco-Flores, A. A., Gomez-Flores, R., Domínguez-Gámez, M., Rodríguez-Pérez, M. A., & Tamez-Guerra, P. (2022). Increased Attraction and Stability of Beauveria bassiana-Formulated Microgranules for Aedes aegypti Biocontrol. Journal of Fungi, 8(8), 828. https://doi.org/10.3390/jof8080828






