The Photodegradation of Lignin Methoxyl C Promotes Fungal Decomposition of Lignin Aromatic C Measured with 13C-CPMAS NMR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. 13C CPMAS NMR Spectroscopy
2.4. Enzyme Activity Assays
2.5. Statistical Analyses
3. Results
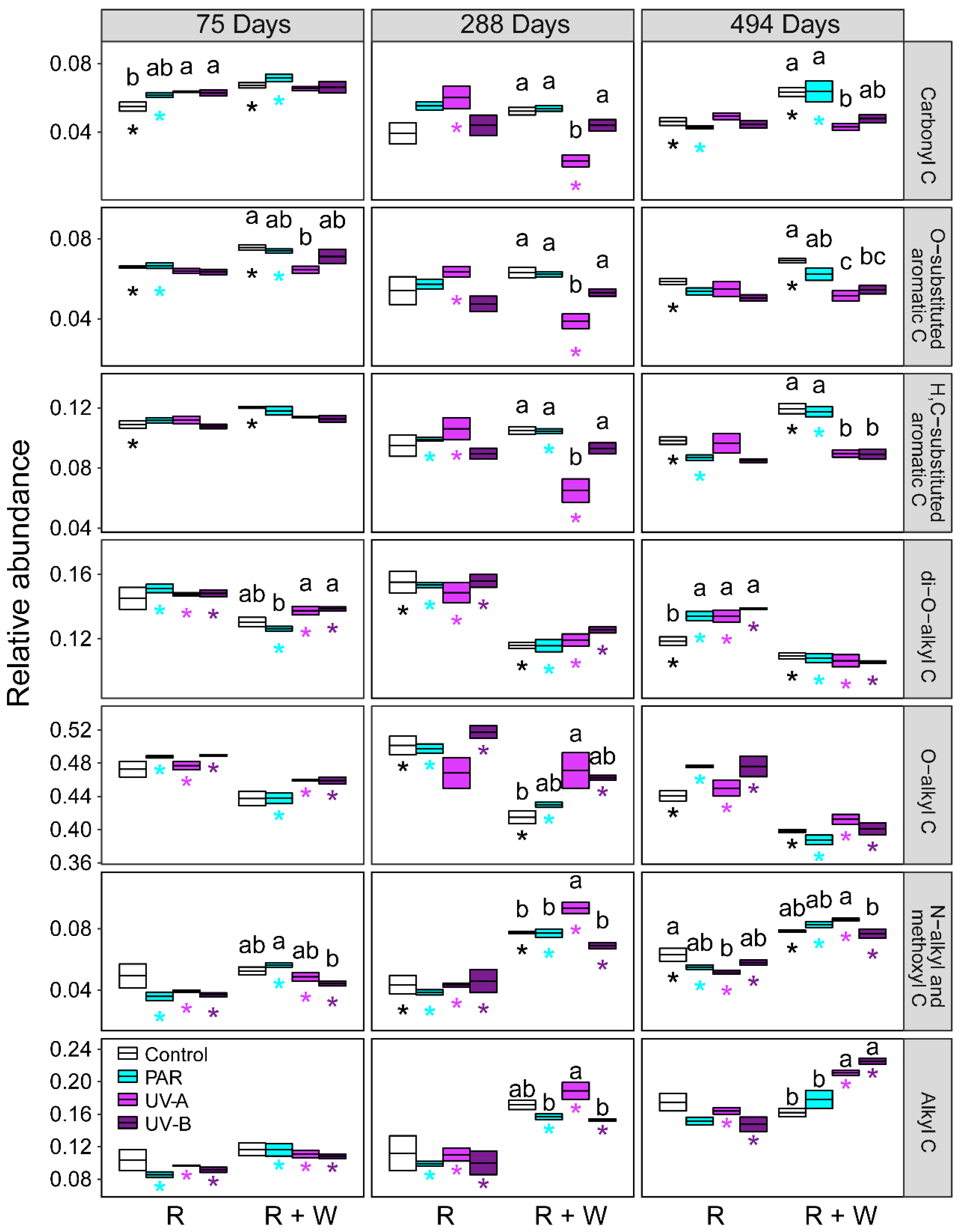
3.1. Changes in Litter Organic C under Exposure to Radiation Interacting with Water Pulses
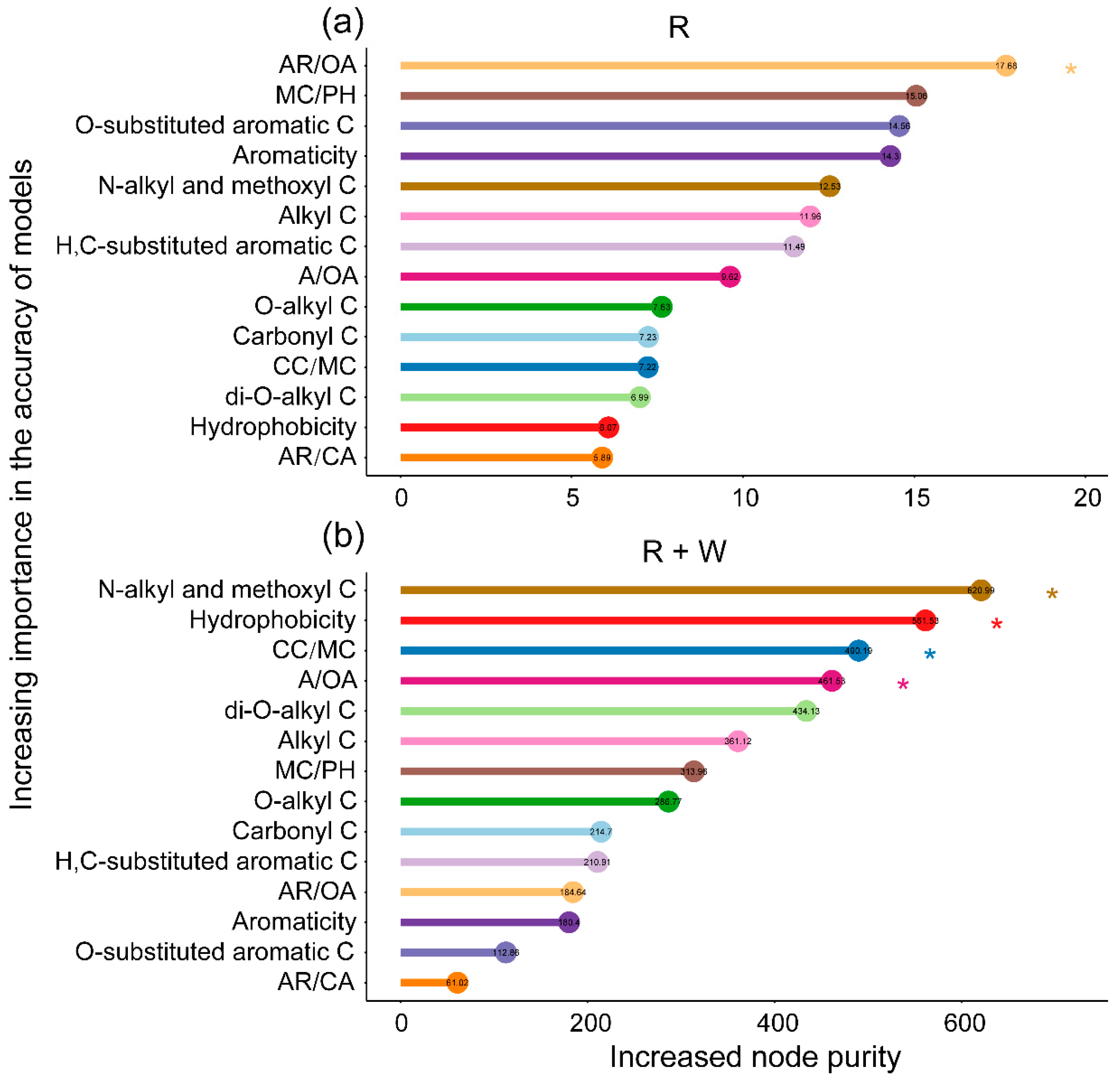
3.2. The Importance of Chemical Components as Predictors of Litter Mass Loss
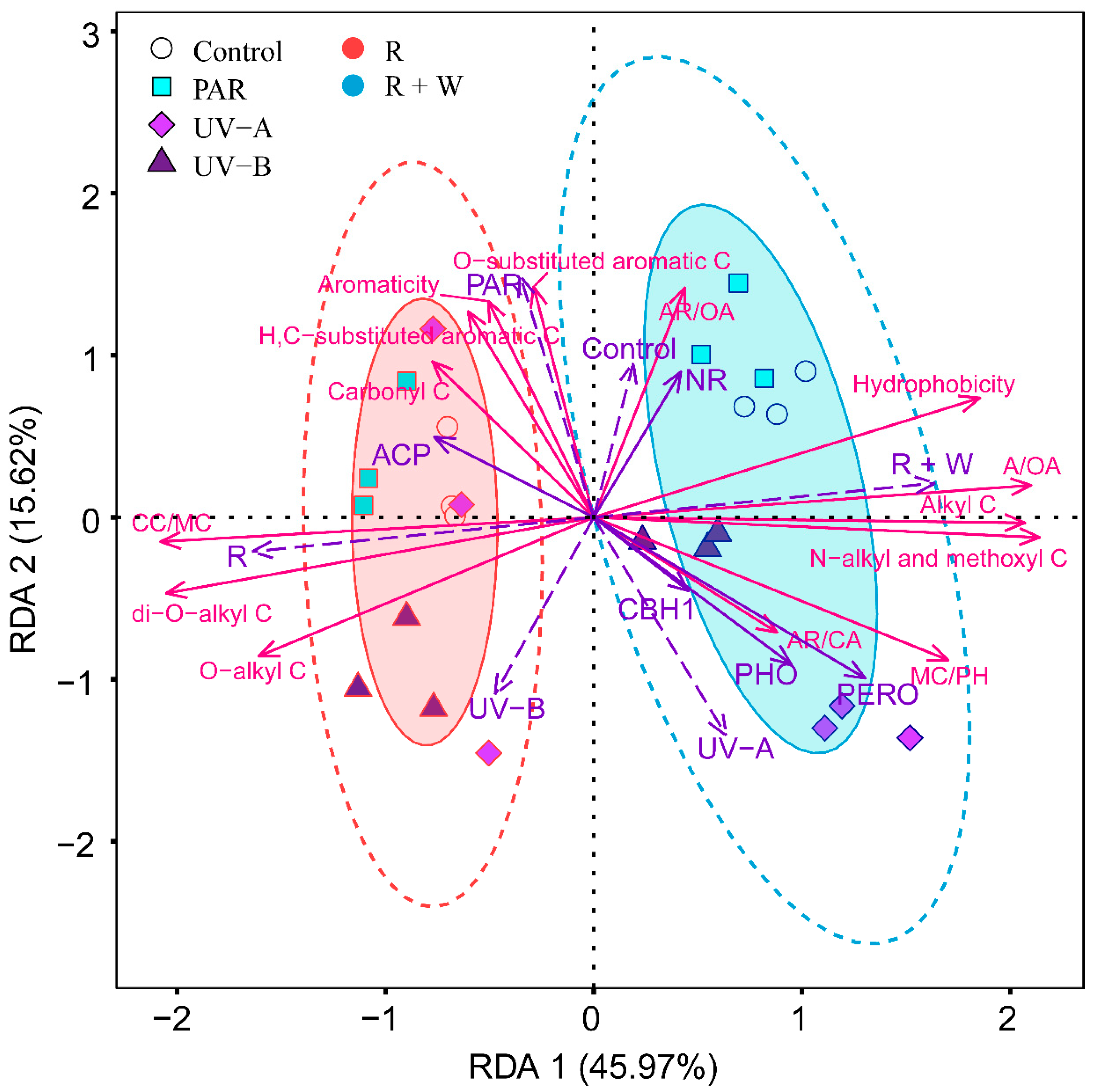
3.3. Relative Contributions of Abiotic and Biotic Drivers to Litter Decomposition
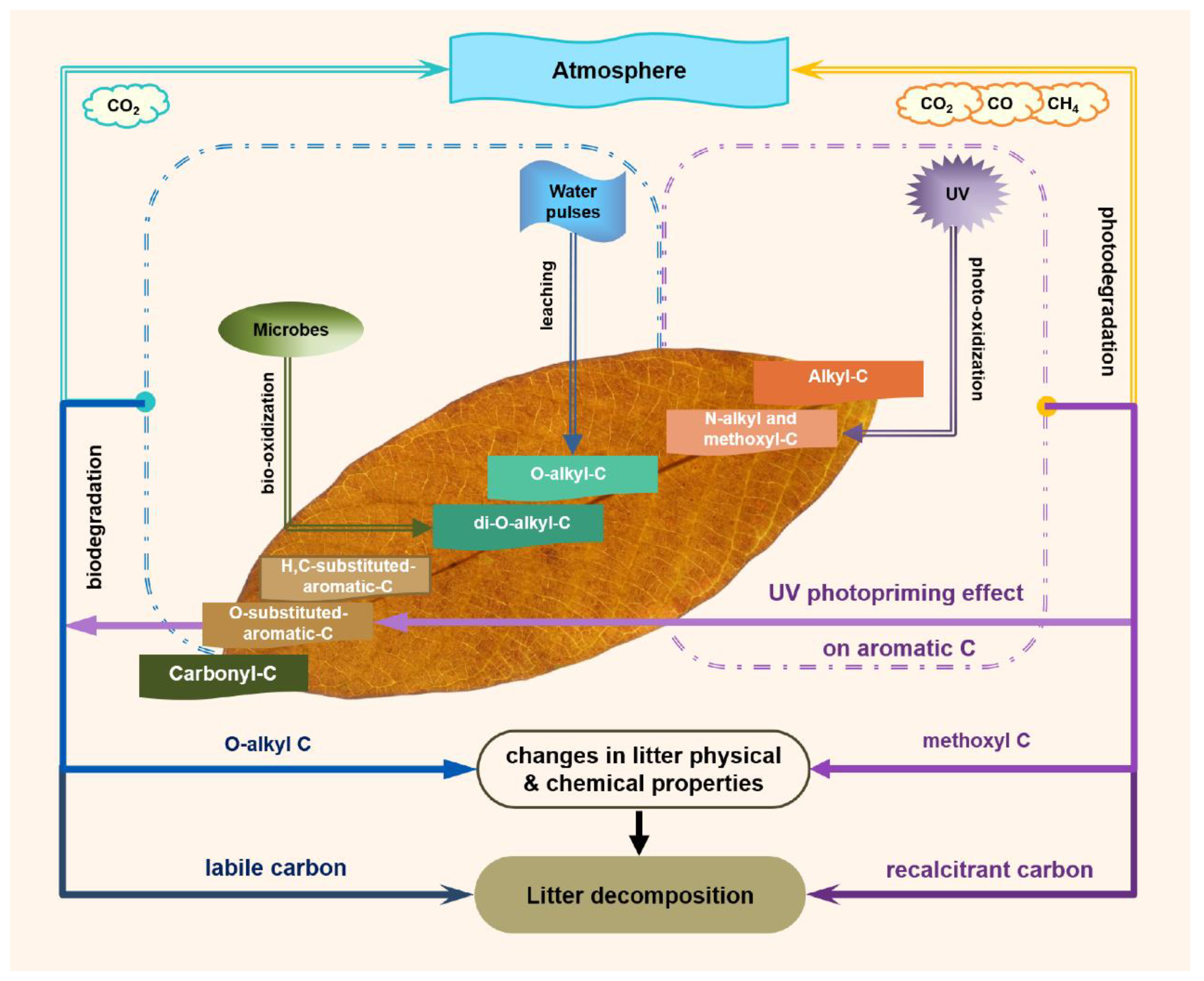
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suseela, V.; Tharayil, N.; Xing, B.; Dukes, J.S. Labile compounds in plant litter reduce the sensitivity of decomposition to warming and altered precipitation. New Phytol. 2013, 200, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.A.; Warren, R.J., II; Baldrian, P.; Crowther, T.W.; Maynard, D.S.; Oldfield, E.E.; Wieder, W.R.; Wood, S.A.; King, J.R. Climate fails to predict wood decomposition at regional scales. Nat. Clim. Change 2014, 4, 625–630. [Google Scholar] [CrossRef]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Adair, E.C.; Parton, W.J.; King, J.Y.; Brandt, L.A.; Lin, Y. Accounting for photodegradation dramatically improves prediction of carbon losses in dryland systems. Ecosphere 2017, 8, e01892. [Google Scholar] [CrossRef]
- King, J.Y.; Brandt, L.A.; Adair, E.C. Shedding light on plant litter decomposition: Advances, implications and new directions in understanding the role of photodegradation. Biogeochemistry 2012, 111, 57–81. [Google Scholar] [CrossRef]
- Bradford, M.A.; Veen, G.F.; Bonis, A.; Bradford, E.M.; Classen, A.T.; Cornelissen, J.H.C.; Crowther, T.W.; De Long, J.R.; Freschet, G.T.; Kardol, P.; et al. A test of the hierarchical model of litter decomposition. Nat. Ecol. Evol. 2017, 1, 1836–1845. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 2006, 442, 555–558. [Google Scholar] [CrossRef]
- Lin, Y.; King, J.Y. Effects of UV Exposure and Litter Position on Decomposition in a California Grassland. Ecosystems 2014, 17, 158–168. [Google Scholar] [CrossRef]
- Huang, G.; Zhao, H.-M.; Li, Y. Litter decomposition in hyper-arid deserts: Photodegradation is still important. Sci. Total Environ. 2017, 601, 784–792. [Google Scholar] [CrossRef]
- Day, T.A.; Urbine, J.M.; Bliss, M.S. Supplemental precipitation accelerates decay but only in photodegraded litter and implications that sunlight promotes leaching loss. Biogeochemistry 2022, 158, 113–129. [Google Scholar] [CrossRef]
- Predick, K.I.; Archer, S.R.; Aguillon, S.M.; Keller, D.A.; Throop, H.L.; Barnes, P.W. UV-B radiation and shrub canopy effects on surface litter decomposition in a shrub-invaded dry grassland. J. Arid Environ. 2018, 157, 13–21. [Google Scholar] [CrossRef]
- Marinho, O.A.; Martinelli, L.A.; Duarte-Neto, P.J.; Mazzi, E.A.; King, J.Y. Photodegradation influences litter decomposition rate in a humid tropical ecosystem, Brazil. Sci. Total Environ. 2020, 715, 136601. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, H.; Jiang, H.; Peng, C. Combination of nitrogen deposition and ultraviolet-B radiation decreased litter decomposition in subtropical China. Plant Soil 2014, 380, 349–359. [Google Scholar] [CrossRef]
- Wang, Q.-W.; Robson, T.M.; Pieristè, M.; Kenta, T.; Zhou, W.; Kurokawa, H. Canopy structure and phenology modulate the impacts of solar radiation on C and N dynamics during litter decomposition in a temperate forest. Sci. Total Environ. 2022, 820, 153185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-W.; Pieriste, M.; Liu, C.; Kenta, T.; Robson, T.M.; Kurokawa, H. The contribution of photodegradation to litter decomposition in a temperate forest gap and understorey. New Phytol. 2021, 229, 2625–2636. [Google Scholar] [CrossRef]
- Pieriste, M.; Chauvat, M.; Kotilainen, T.K.; Jones, A.G.; Aubert, M.; Robson, T.M.; Forey, E. Solar UV-A radiation and blue light enhance tree leaf litter decomposition in a temperate forest. Oecologia 2019, 191, 191–203. [Google Scholar] [CrossRef]
- Pieristè, M.; Neimane, S.; Solanki, T.; Nybakken, L.; Jones, A.G.; Forey, E.; Chauvat, M.; Ņečajeva, J.; Robson, T.M. Ultraviolet radiation accelerates photodegradation under controlled conditions but slows the decomposition of senescent leaves from forest stands in southern Finland. Plant Physiol. Biochem. 2020, 146, 42–54. [Google Scholar] [CrossRef]
- Pieriste, M.; Forey, E.; Sahraoui, A.L.-H.; Meglouli, H.; Laruelle, F.; Delporte, P.; Robson, T.M.; Chauvat, M. Spectral Composition of Sunlight Affects the Microbial Functional Structure of Beech Leaf Litter During the Initial Phase of Decomposition. Plant Soil 2020, 451, 515–530. [Google Scholar] [CrossRef]
- Austin, A.T.; Soledad Mendez, M.; Ballare, C.L. Photodegradation alleviates the lignin bottleneck for carbon turnover in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2016, 113, 4392–4397. [Google Scholar] [CrossRef]
- Berenstecher, P.; Vivanco, L.; Perez, L.I.; Ballare, C.L.; Austin, A.T. Sunlight Doubles Aboveground Carbon Loss in a Seasonally Dry Woodland in Patagonia. Curr. Biol. 2020, 30, 3243–3251.E3. [Google Scholar] [CrossRef]
- Brandt, L.A.; Bohnet, C.; King, J.Y. Photochemically induced carbon dioxide production as a mechanism for carbon loss from plant litter in arid ecosystems. J. Geophys. Res.-Biogeosci. 2009, 114. [Google Scholar] [CrossRef]
- Day, T.A.; Bliss, M.S. A spectral weighting function for abiotic photodegradation based on photochemical emission of CO2 from leaf litter in sunlight. Biogeochemistry 2019, 146, 173–190. [Google Scholar] [CrossRef]
- Brandt, L.A.; King, J.Y.; Milchunas, D.G. Effects of ultraviolet radiation on litter decomposition depend on precipitation and litter chemistry in a shortgrass steppe ecosystem. Glob. Chang. Biol. 2007, 13, 2193–2205. [Google Scholar] [CrossRef]
- Smith, W.K.; Gao, W.; Steltzer, H.; Wallenstein, M.D.; Tree, R. Moisture availability influences the effect of ultraviolet-B radiation on leaf litter decomposition. Glob. Chang. Biol. 2010, 16, 484–495. [Google Scholar] [CrossRef]
- Almagro, M.; Martinez-Lopez, J.; Maestre, F.T.; Rey, A. The Contribution of Photodegradation to Litter Decomposition in Semiarid Mediterranean Grasslands Depends on its Interaction with Local Humidity Conditions, Litter Quality and Position. Ecosystems 2017, 20, 527–542. [Google Scholar] [CrossRef]
- Araujo, P.I.; Austin, A.T. A shady business: Pine afforestation alters the primary controls on litter decomposition along a precipitation gradient in Patagonia, Argentina. J. Ecol. 2015, 103, 1408–1420. [Google Scholar] [CrossRef]
- Gaxiola, A.; Armesto, J.J. Understanding litter decomposition in semiarid ecosystems: Linking leaf traits, UV exposure and rainfall variability. Front. Plant Sci. 2015, 6, 140. [Google Scholar] [CrossRef]
- Austin, A.T.; Ballare, C.L. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2010, 107, 4618–4622. [Google Scholar] [CrossRef]
- de Graaff, M.-A.; Classen, A.T.; Castro, H.F.; Schadt, C.W. Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytol. 2010, 188, 1055–1064. [Google Scholar] [CrossRef]
- Preston, C.M.; Nault, J.R.; Trofymow, J.A. Chemical Changes During 6 Years of Decomposition of 11 Litters in Some Canadian Forest Sites. Part 2. 13C Abundance, Solid-State 13C NMR Spectroscopy and the Meaning of “Lignin”. Ecosystems 2009, 12, 1078–1102. [Google Scholar] [CrossRef]
- Henry, H.A.L.; Brizgys, K.; Field, C.B. Litter decomposition in a california annual grassland: Interactions between photodegradation and litter layer thickness. Ecosystems 2008, 11, 545–554. [Google Scholar] [CrossRef]
- Day, T.A.; Zhang, E.T.; Ruhland, C.T. Exposure to solar UV-B radiation accelerates mass and lignin loss of Larrea tridentata litter in the Sonoran Desert. Plant Ecol. 2007, 193, 185–194. [Google Scholar] [CrossRef]
- Rozema, J.; Tosserams, M.; Nelissen, H.J.M.; van Heerwaarden, L.; Broekman, R.A.; Flierman, N. Stratospheric ozone reduction and ecosystem processes: Enhanced UV-B radiation affects chemical quality and decomposition of leaves of the dune grassland species Calamagrostis epigeios. Plant Ecol. 1997, 128, 284–294. [Google Scholar]
- Song, X.; Peng, C.; Jiang, H.; Zhu, Q.; Wang, W. Direct and Indirect Effects of UV-B Exposure on Litter Decomposition: A Meta-Analysis. PLoS ONE 2013, 8, e68858. [Google Scholar] [CrossRef]
- Keiser, A.D.; Warren, R.; Filley, T.; Bradford, M.A. Signatures of an abiotic decomposition pathway in temperate forest leaf litter. Biogeochemistry 2021, 153, 177–190. [Google Scholar] [CrossRef]
- Brandt, L.A.; King, J.Y.; Hobbie, S.E.; Milchunas, D.G.; Sinsabaugh, R.L. The Role of Photodegradation in Surface Litter Decomposition Across a Grassland Ecosystem Precipitation Gradient. Ecosystems 2010, 13, 765–781. [Google Scholar] [CrossRef]
- Erdenebileg, E.; Ye, X.; Wang, C.; Huang, Z.; Liu, G.; Cornelissen, J.H.C. Positive and negative effects of UV irradiance explain interaction of litter position and UV exposure on litter decomposition and nutrient dynamics in a semi-arid dune ecosystem. Soil Biol. Biochem. 2018, 124, 245–254. [Google Scholar] [CrossRef]
- Talbot, J.M.; Treseder, K.K. Interactions among lignin, cellulose, and nitrogen drive litter chemistry-decay relationships. Ecology 2012, 93, 345–354. [Google Scholar] [CrossRef]
- Mao, J.; Cao, X.; Olk, D.C.; Chu, W.; Schmidt-Rohr, K. Advanced solid-state NMR spectroscopy of natural organic matter. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 100, 17–51. [Google Scholar] [CrossRef]
- Wang, Q.-W.; Pieriste, M.; Kotilainen, T.K.; Forey, E.; Chauvat, M.; Kurokawa, H.; Robson, T.M.; Jones, A.G. The crucial role of blue light as a driver of litter photodegradation in terrestrial ecosystems. Plant Soil 2022. [Google Scholar] [CrossRef]
- De Marco, A.; Spaccini, R.; Vittozzi, P.; Esposito, F.; Berg, B.; De Santo, A.V. Decomposition of black locust and black pine leaf litter in two coeval forest stands on Mount Vesuvius and dynamics of organic components assessed through proximate analysis and NMR spectroscopy. Soil Biol. Biochem. 2012, 51, 1–15. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Giannino, F.; Mingo, A.; Lanzotti, V.; Mazzoleni, S. Litter quality assessed by solid state 13C NMR spectroscopy predicts decay rate better than C/N and Lignin/N ratios. Soil Biol. Biochem. 2013, 56, 40–48. [Google Scholar] [CrossRef]
- Chavez-Vergara, B.; Merino, A.; Vazquez-Marrufo, G.; Garcia-Oliva, F. Organic matter dynamics and microbial activity during decomposition of forest floor under two native neotropical oak species in a temperate deciduous forest in Mexico. Geoderma 2014, 235, 133–145. [Google Scholar] [CrossRef]
- Tang, W.; Yang, K.; Qin, J.; Min, M. Development of a 50-year daily surface solar radiation dataset over China. Sci. China-Earth Sci. 2013, 56, 1555–1565. [Google Scholar] [CrossRef]
- Tang, W. Daily average solar radiation dataset of 716 weather stations in China (1961–2010). Natl. Tibet. Plateau Data Cent. 2019. [Google Scholar] [CrossRef]
- Angst, S.; Cajthaml, T.; Angst, G.; Simackova, H.; Brus, J.; Frouz, J. Retention of dead standing plant biomass (marcescence) increases subsequent litter decomposition in the soil organic layer. Plant Soil 2017, 418, 571–579. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, J.; Ding, W.; Gunina, A.; Chen, Z.; Bol, R.; Luo, J.; Bolan, N. Characterization of organic carbon in decomposing litter exposed to nitrogen and sulfur additions: Links to microbial community composition and activity. Geoderma 2017, 286, 116–124. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Barile, E.; Capodilupo, M.; Antignani, V.; Mingo, A.; Lanzotti, V.; Scala, F.; Mazzoleni, S. Phytotoxicity, not nitrogen immobilization, explains plant litter inhibitory effects: Evidence from solid-state 13C NMR spectroscopy. New Phytol. 2011, 191, 1018–1030. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Abd El-Gawad, A.M.; Cesarano, G.; Sarker, T.C.; Saulino, L.; Lanzotti, V.; Saracino, A.; Rego, F.C.; Mazzoleni, S. Comparing chemistry and bioactivity of burned vs. decomposed plant litter: Different pathways but same result? Ecology 2018, 99, 158–171. [Google Scholar] [CrossRef]
- Mathers, N.J.; Jalota, R.K.; Dalal, R.C.; Boyd, S.E. 13C-NMR analysis of decomposing litter and fine roots in the semi-arid Mulga Lands of southern Queensland. Soil Biol. Biochem. 2007, 39, 993–1006. [Google Scholar] [CrossRef]
- Pu, G.; Du, J.; Ma, X.; Lv, Y.; Jia, Y.; Jia, X.; Tian, X. Contribution of ambient atmospheric exposure to Typha angustifolia litter decomposition in aquatic environment. Ecol. Eng. 2014, 67, 144–149. [Google Scholar] [CrossRef]
- George, B.; Suttie, E.; Merlin, A.; Deglise, X. Photodegradation and photo stabilisation of wood—the state of the art. Polym. Degrad. Stab. 2005, 88, 268–274. [Google Scholar] [CrossRef]
- Hewins, D.B.; Lee, H.; Barnes, P.W.; McDowell, N.G.; Pockman, W.T.; Rahn, T.; Throop, H.L. Early exposure to UV radiation overshadowed by precipitation and litter quality as drivers of decomposition in the northern Chihuahuan Desert. PLoS ONE 2019, 14, e0210470. [Google Scholar] [CrossRef]
- Lee, H.; Rahn, T.; Throop, H.L. An accounting of C-based trace gas release during abiotic plant litter degradation. Glob. Chang. Biol. 2012, 18, 1185–1195. [Google Scholar] [CrossRef]
- Frouz, J.; Cajthaml, T.; Mudrak, O. The effect of lignin photodegradation on decomposability of Calamagrostis epigeios grass litter. Biodegradation 2011, 22, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; King, J.Y.; Karlen, S.D.; Ralph, J. Using 2D NMR spectroscopy to assess effects of UV radiation on cell wall chemistry during litter decomposition. Biogeochemistry 2015, 125, 427–436. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Chapple, C. The Genetics of Lignin Biosynthesis: Connecting Genotype to Phenotype. Annu. Rev. Genet. 2010, 44, 337–363. [Google Scholar] [CrossRef]
- Talbot, J.M.; Yelle, D.J.; Nowick, J.; Treseder, K.K. Litter decay rates are determined by lignin chemistry. Biogeochemistry 2012, 108, 279–295. [Google Scholar] [CrossRef]
- McKee, G.A.; Soong, J.L.; Calderon, F.; Borch, T.; Cotrufo, M.F. An integrated spectroscopic and wet chemical approach to investigate grass litter decomposition chemistry. Biogeochemistry 2016, 128, 107–123. [Google Scholar] [CrossRef]
- Suseela, V.; Tharayil, N.; Xing, B.; Dukes, J.S. Warming alters potential enzyme activity but precipitation regulates chemical transformations in grass litter exposed to simulated climatic changes. Soil Biol. Biochem. 2014, 75, 102–112. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, A.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Wang, X.; Chen, Y. The interaction between abiotic photodegradation and microbial decomposition under ultraviolet radiation. Glob. Chang. Biol. 2015, 21, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Schimel, J.P.; Janssens, I.A.; Song, X.; Song, C.; Yu, G.; Sinsabaugh, R.L.; Tang, D.; Zhang, X.; Thornton, P.E. Global pattern and controls of soil microbial metabolic quotient. Ecol. Monogr. 2017, 87, 429–441. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Ballare, C.L.; Bornman, J.F.; Flint, S.D.; Bjorn, L.O.; Teramura, A.H.; Kulandaivelu, G.; Tevini, M. Terrestrial ecosystems increased solar ultraviolet radiation and interactions with other climatic change factors. Photochem. Photobiol. Sci. 2003, 2, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, F.; Azam, F. Detection of DNA damage in prokaryotes by terminal deoxyribonucleotide transferase-mediated dUTP nick end labeling. Appl. Environ. Microbiol. 2000, 66, 1001–1006. [Google Scholar] [CrossRef]
- Hughes, K.A.; Lawley, B.; Newsham, K.K. Solar UV-B radiation inhibits the growth of antarctic terrestrial fungi. Appl. Environ. Microbiol. 2003, 69, 1488–1491. [Google Scholar] [CrossRef]
- Day, T.A.; Bliss, M.S. Solar Photochemical Emission of CO2 From Leaf Litter: Sources and Significance to C Loss. Ecosystems 2019, 23, 1344–1361. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, B.; Zeng, X.; Pang, L.; Kong, X.; Tian, K.; Ji, Y.; Sun, S.; Tian, X. The Photodegradation of Lignin Methoxyl C Promotes Fungal Decomposition of Lignin Aromatic C Measured with 13C-CPMAS NMR. J. Fungi 2022, 8, 900. https://doi.org/10.3390/jof8090900
Yao B, Zeng X, Pang L, Kong X, Tian K, Ji Y, Sun S, Tian X. The Photodegradation of Lignin Methoxyl C Promotes Fungal Decomposition of Lignin Aromatic C Measured with 13C-CPMAS NMR. Journal of Fungi. 2022; 8(9):900. https://doi.org/10.3390/jof8090900
Chicago/Turabian StyleYao, Bei, Xiaoyi Zeng, Lu Pang, Xiangshi Kong, Kai Tian, Yanli Ji, Shucun Sun, and Xingjun Tian. 2022. "The Photodegradation of Lignin Methoxyl C Promotes Fungal Decomposition of Lignin Aromatic C Measured with 13C-CPMAS NMR" Journal of Fungi 8, no. 9: 900. https://doi.org/10.3390/jof8090900
APA StyleYao, B., Zeng, X., Pang, L., Kong, X., Tian, K., Ji, Y., Sun, S., & Tian, X. (2022). The Photodegradation of Lignin Methoxyl C Promotes Fungal Decomposition of Lignin Aromatic C Measured with 13C-CPMAS NMR. Journal of Fungi, 8(9), 900. https://doi.org/10.3390/jof8090900






